Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is characterized by repeated episodes of upper-airway obstruction during sleep leading to significant hypercapnic hypoxic conditions. These conditions are associated with increased levels of proinflammatory cytokines (including interleukin [IL]-6, tumor necrosis factor [TNF]-α, and C-reactive protein [CRP]) and subsequent increased cardiovascular risk. It is unclear whether hypercapnic hypoxia itself causes inflammatory perturbations.
Design:
We evaluated circulating IL-6, TNF- α and CRP in a piglet model of infant OSA, following exposure to acute intermittent hypercapnic hypoxia (IHH). Study groups comprised of treatment (n = 8) and control (n = 8) groups. Treatment was two 90-minute sessions of IHH with arterial blood sampled before and after each IHH session.
Measurements and Results:
IL-6, TNF-α and CRP levels were measured before and after IHH treatment sessions. Results showed an increase in IL-6 following the first session of IHH that was neither sustained, nor repeated, during a subsequent exposure. Using mixed-modelling, TNF-α changed between time points and groups. There were no changes in CRP over the duration of the study.
Conclusion:
These results suggest that acute hypoxia causes a transient increase in IL-6 levels and has implications for the pathogenesis of increased cardiovascular disease in OSA, especially in childhood.
Citation:
Tam CS; Wong M; Tam K et al. The effect of acute intermittent hypercapnic hypoxia treatment on IL-6, TNF-α, and CRP levels in piglets. SLEEP 2007;30(6):723-727.
Keywords: Cytokine, hypoxia, inflammation, obstructive sleep apnea
OBSTRUCTIVE SLEEP APNEA (OSA) HAS A PREVALENCE OF APPROXIMATELY 2% TO 3% IN THE PEDIATRIC POPULATION AND IS CHARACTERIZED BY PERIODS OF partial or complete upper-airway obstruction during sleep, despite continuing respiratory effort.1,2 Patients with OSA have repetitive episodes of hypoxia followed by reoxygenation3 and have increased circulating reactive oxygen species,3 proinflammatory cytokines, including tumor necrosis factor (TNF)-α; interleukin (IL)-1, IL-6, and IL-84–7; and c-reactive protein (CRP),8,9 a powerful marker and predictor of cardiovascular risk.10–12 Because inflammation is a mechanism for atherosclerosis and other cardiovascular diseases, these findings suggest that OSA, and its associated intermittent hypercapnic hypoxia (IHH), may cause cardiovascular complications by activating inflammatory pathways.8,13
To evaluate the mechanisms and characteristics of sequelae of OSA during early development, our laboratory has developed a piglet model of IHH. The use of a piglet model is relevant to the human condition because of the many anatomic and physiologic similarities between piglets and the human infant.10 Importantly, the maturation of respiratory control is equivalent to that of human infants at birth, so that the period up to 30 days of age in a piglet equates to the first 6 months of development in a human infant. 11 IHH treatment is used to mimic OSA because apneas are associated not only with periodic decreases in oxygen (hypoxia), but also with simultaneous increases in arterial carbon dioxide (hypercapnia).2 Therefore, the study of inflammatory changes in response to this IHH treatment on piglets is an excellent model for examining the mechanisms for inflammatory changes in OSA in young children.
The aim of the present study was to determine the effect of acute (IHH) treatment on IL-6, TNF-α, and CRP levels in a piglet model of OSA.
METHODS
Piglet Model of IHH
The experimental procedure and study environment of our piglet model has been described previously.12 Briefly, large white cross with durah breed male piglets (n = 16) were transported from a commercial piggery on day 3.5 ± 2.3 after birth. The piglets weighed 2.1 ± 0.9 kg on arrival. Ethical approval was obtained from the University of Sydney Animal Ethics Committee.
Surgery for Insertion of Catheter
Aseptic surgery was undertaken under general anaesthetic on day 8.6 ± 4.5 when piglets weighed 2.6 ± 0.8 kg. Anesthesia was induced by using a face mask delivering isofluorane (1%–3% halothane with 30%–50% nitrous oxide). Catheters were placed in the left femoral artery and vein, tunnelled subcutaneously to exit in the ipsilateral flank, and protected in the pockets of jackets that were worn from the time of the surgery. Analgesia commenced intraoperatively with paracetamol rectal suppository (Panadol, GlaxoSmithKline, Auckland), 52.5 mg of cephalexin antibiotic (Trilexine 150 suspension, Virbac Pty Limited, Australia), and 1 mg of meloxicam (Metacam, antiinflammatory injection, Boehringer Ingelheim Pty Limited, Australia). Catheter lines were flushed daily with heparin to keep them patent. IHH treatment commenced a minimum of 48 hours (1 full day's rest with analgesics) after surgery to allow for recovery.
IHH Treatment
On the day of the study, piglets were assigned to either control (n = 8) or treatment (n = 8) groups. Piglets were exposed to two 90-minute treatment sessions, separated by 90 minutes of rest. A rest period was included in the experimental protocol because it has been established that the decline in ventilatory response with sustained hypoxia may require up to 1 hour for complete reversal.13 Therefore, the experimental protocol was established with the aim of mimicking brief repeated exposures to hypercapnic hypoxia, similar to the clinical situation of OSA.
IHH treatment consisted of 6 minutes of hypercapnic hypoxia (8% o2, 7% co2/n2) alternating with 6 minutes of air, over 90 minutes' duration. Piglets in the control group breathed fresh air for the duration of the experiment in the same study environment. Piglets were placed in a vinyl hammock within a temperaturecontrolled sealed chamber to maintain their head position, relative to the respiratory-monitoring devices. Gases were delivered via a breathing mask using a gas-tight 3-way tap attached to reservoir bags containing air or the premixed IHH gas. After the study, all animals were killed painlessly with an overdose of pentobarbitone.
Blood Gases
Blood samples, to assess and confirm the relative o2 and co2 saturation reached during hypoxia treatment, were taken at 8 timepoints: (1) before IHH session 1, (2) during the first 6-minute hypercapnic-hypoxia cycle, (3) during the last 6-minute hypercapnic-hypoxia cycle, and (4) after IHH session 1.
Blood-sample collection was repeated in Session 2. Blood-gas tensions, pH, and hemoglobin were measured in an automated blood-gas analyzer (Model 520, ABL, Radiometer, Copenhagen, Denmark). All values were corrected to the rectal temperature of the animal.
Blood Collection
Over the duration of the experiment, 2-mL blood samples (1 mL plasma, 1 mL serum) were collected before and after each IHH session for the measurement of inflammatory markers.
Blood samples were spun immediately in a routine benchtop centrifuge for 5 minutes and plasma/serum samples were aliquotted into a fresh tube and stored at −20°C until analysis.
IL-6, TNF-α, and CRP Assays
All piglet blood samples were analyzed using commercially available enzyme-linked immunosorbent assay kits: IL-6 and TNF-α(R&D Systems, Minneapolis, Minn) and CRP (Phase Range Porcine CRP Assay Kit, Tridelta, Ireland) following the instructions supplied with the kits. All samples were measured in duplicate at the same time. IL-6, TNF-α and CRP measures were read using a plate reader (MultiscanEX, LabSystem, PathTach Company, Australia). According to the manufacturers, the minimum detection limits for IL-6, TNF-α, and CRP are 10 pg/mL, 3.7 pg/mL and 20 ng/mL, respectively. The coefficients of variance for IL-6, TNF-α, and CRP were 2.9%, 4.9%, and 8% for intraassay precision and 8.5%, 8.9%, and 10% for interassay precision.
Statistical Analysis
All analyses were conducted using the SPSS statistical package (SPSS for Windows, V11.5.1, LEAD Chicago, Ill). Independent samples t-tests were used for analyzing normally distributed data and Mann Whitney U for data that were not normally distributed data. We used mixed modelling to examine the effects of time and group (control or treatment) on inflammatory marker levels. Mixed models were used to minimize the effects of missing data points and to take into account the longitudinal nature of the data. All P values reported are 2-tailed with statistical significance set at < 0.05.
RESULTS
Piglet Characteristics
A total of 16 male piglets completed this study. On the day of the study, the treatment (n = 8) and control (n = 8) groups were not different in age (treatment = 15.4 ± 4.1 days; control = 11.3 ± 5.1 days; P = 0.1) or weight (treatment = 2.9 ± 0.9 kg; control = 2.6 ± 0.8 kg; P = 0.58).
Blood-Gas Measurements on the Day of Treatment
The Pao2 and Paco2 levels achieved during the exposure to the inspired hypercapnic hypoxic gas mix during this study were 53.8 ± 21.5 mm Hg and 54.4 ± 4.0 mm Hg, respectively.
Inflammatory Measures
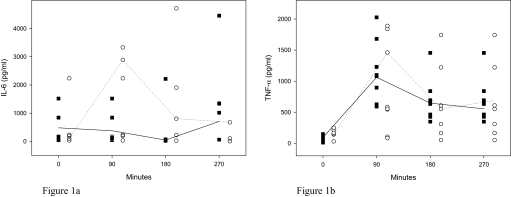
There was a significant rise in IL-6 levels in the treatment group after the first session of IHH (P = 0.005), which fell gradually thereafter. Although still elevated prior to the second session, this was not statistically significant (P = 0.066), and the second session produced no further elevation in IL-6 levels (P = 0.484). In both control and treatment groups, TNF-α concentrations increased after session 1 and then decreased gradually over the rest period and the second IHH session. CRP levels in both control and treatment groups showed no significant differences during the study. Inflammatory measures are shown in Table 1 and Figure 1.
Table 1.
IL-6, TNF-α, and CRP Levels at Baseline and After IHH Treatment Sessions 1 and 2
| Timepoint | IL-6, pg/mL |
TNF-α, pg/mL |
CRP, μg/mL |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Treatment | P Value | Control | Treatment | P Value | Control | Treatment | P Value | |
| Baseline 1 | 172.8 (47.5–1518.3) |
197.2 (35.0–2236.7) |
0.917 | 41.5 (13.8–149.9) |
149.9 (31.6–251.6) |
0.021* | 151.3 (3.5–222.8) |
121.8 (28.3–191.9) |
0.620 |
| Hypoxia 1 | 320.2 (30.2–1437.8) |
2891.1 (406.6–3331.1) |
0.019* | 1089.6 (592.9–2026.9) |
570.9 (84.6–1889.3) |
0.208 | 178.0 (41.2–232.1) |
144.5 (70.2–207.0) |
0.655 |
| Baseline 2 | 72.9 (23.8–6360.0) |
1355.6 (27.5–4714.4) |
0.252 | 647.4 (349.4–1455.3) |
551.6 (52.6–1741.7) |
0.418 | 165.2 (4.9–226.5) |
147.4 (39.4–191.5) |
0.563 |
| Hypoxia 2 | 1177.23 (61.1–4454.4) |
393.3 (4.7–1503.8) |
0.522 | 489.1 (385.8–1180.3) |
525.3 (166.6–1676.7) |
0.908 | 209.8 (22.5–240.5) |
156.8 (75.0–200.5) |
0.482 |
Data are presented as median (range), and P values were derived using Mann Whitney U. IL, interleukin; TNF, tumor necrosis factor; C-reactive protein; IHH, intermittent hypercapnic hypoxia.
P < 0.05.
Figure 1.
IL-6 and TNF-α levels during IHH treatment. In Figure 1a, interleukin (IL)-6 levels increased significantly in the treatment group after the first session of intermittent hypercapnic hypoxia (IHH) treatment, which gradually fell thereafter. The second IHH session produced no further elevation in IL-6 levels. In Figure 1b, TNF-αlevels increased in both control and treatment groups after the IHH Session 1 and continued to decrease gradually over the rest period and the second IHH session. The lines represent the median values for the groups. ■ Controls (n=8); ◦ Treatment (n = 8).
Mixed Models
Using mixed models, we found a significant difference in IL-6 and TNF-α levels between the different time points (P = 0.03 and 0.001, respectively) and a significant group difference for TNF-α (P = 0.005).
DISCUSSION
This is the first study we are aware of to apply IHH stimuli to an animal model to study the acute effects on proinflammatory cytokine production. We found an increase in IL-6 levels following an initial IHH exposure but no further change following a subsequent exposure. TNF-α and CRP showed no significant differences in the control and treatment groups, although TNF-α did increase after IHH treatment in both groups. These results suggest that acute hypoxia causes a transient increase in circulating IL-6. Recurring episodes of such hypoxia may result in persisting increases in IL-6 levels, with implications for the cardiovascular sequelae of OSA, especially in children.
IL-6 is a pleiotropic cytokine that is commonly produced at local tissue sites and released into the circulation in almost all situations of homeostatic perturbation, including trauma and acute infections.14 Results from the present study indicate a 7-fold increase in IL-6 levels after 90 minutes of IHH treatment. Bowen et al found that IL-6 production by placental trophoblast, normal, and preeclamptic cells increased significantly after 48 hours hypoxia ex vivo.15 Klausen et al found human serum concentrations of IL-6 increased steadily 9-fold over baseline during 4 days at altitude, whereas Hartmann et al found a 20-fold increase in IL-6 under the same conditions.16,17 In adults with OSA, plasma IL-6 levels have been shown to increase significantly after an obstructive sleep apnea (Sao2 < 85%).18 These findings suggest that both acute and chronic hypoxia contribute to an increase in proinflammatory cytokine production. To date, there have been no equivalent human studies conducted during early development.
A mechanism by which IL-6 may be produced in hypoxic conditions is the upregulation of transcription factors NF-κB and NF-IL6.19–22 Findings from in vivo studies indicate that acute hypoxia-induced expression of IL-6 is primarily dependent on the upregulation of NF-κB19 or NF-IL6.21,22 Matsuaka et al suggested that NF-κB and NF-IL-6 synergistically activate the expression of IL-6.20 Adults with OSA show markedly elevated monocyte and neutrophil NF-κB activity, which is reversed after continuous positive airway pressure treatment for OSA.23,24
Results from our study indicate that elevated IL-6 levels after an initial hypoxic treatment were refractory to a repeated hypoxic stimulus within 90 minutes of the first, possibly suggesting an adaptation of the organism to hypoxic conditions.25 Meffert et al found repetitive administration of recombinant IL-2 induced significantly lower IL-6 serum peaks, when compared with the initial administration.26 Adaptive responses to stimuli have also been shown in TNF-α, where additional lipopolysaccharide injections administered within 3 days gave peak measures of 15% of the concentration detected after the first injection.27
The refractory nature of IL-6 may also be attributed to its short half-life. After production, the kinetics of IL-6 clearance is biphasic. Initially, IL-6 has a rapid disappearance corresponding to a half-life of 3 minutes and a second slow one corresponding to a half life of about 55 minutes.28 Previous studies have found IL-6 increases at 2 hours and peaks at 24 hours after stimulation with lipopolysaccharide in whole blood, consistent with in vivo studies by Ertel et al.29–31 Blood samples in our study (taken after 90 minutes of hypoxia treatment) may not have been taken at the peaks of IL-6 production. Elucidating the full time course of this phenomenon would require the study of additional samples, including later time points.
After hypoxic treatment, there was an elevation in TNF-α in both control and treatment groups, suggesting that the inflammatory changes were precipitated by a stimulus other than IHH. The most likely cause is a stress response relating to the study environment. This may relate more to the increase in sympathetic activity associated with OSA.32–34 Various forms of psychologic stress are associated with increased IL-6 levels,35,36 although the relationship between IL-6 and environmental stress is not clear. Restraint stress alone is capable of inducing increased TNF-α levels.37 It may be that stimuli elicit different responses in different environments.
CRP, an acute phase reactant, is a marker of systemic inflammation and a strong predictor of cardiovascular risk.38 Mean half lives of serum CRP have been reported at between 19 and 21 hours and peak at about 48 hours, before decreasing.16,39 Hepatic production of CRP is regulated by cytokines, particularly IL-6, even in high-altitude hypoxic conditions. The results herein are consistent with previous observations that increases in IL-6 precede increases in CRP.38,40 Ehl et al suggest that a period of 10 hours must elapse between the initiation of CRP synthesis and the time until CRP levels become measurable in the serum. In this context, our finding that hypoxia treatment causes IL-6 changes, but no changes in CRP, suggests that there may not have been sufficient time for measurable changes to occur.
We studied cytokine responses to a very acute period of IHH. The period of IHH was only for 90 minutes, and our results suggest that we have detected only early cytokine changes. Nonetheless, some clinical studies have examined the response to single apneas.18 Previous studies conducted in humans injected with lipopolysaccharide found that circulating TNF-α levels had increased by 60 minutes with maximal levels at 90 minutes.41 This is also evidenced in both unstimulated and lipopolysaccharidestimulated human PBMC; TNF-α protein levels increase immediately after the start of cultivation, reaching maximal levels at 4 hours42 and 72 hours.43 For CRP, Kenma et al found that circulating levels increased following lipopolysaccharide injection, with maximal levels detected 22 hours after injection.41 Based on results from these studies, we are confident that 90 minutes is sufficient time to allow for measurable TNF-α levels, but, consistent with our results, change in CRP would be expected to follow a longer time course. In future studies it would be interesting to preform lipopolysaccharide injections in piglets to further elucidate the dynamics of cytokine secretion.
There is some evidence suggesting that surgical intervention affects circadian rhythms and thus sleep, both of which can lead to cytokine increases.44 Kakela et al found that even relatively minor surgery delays the onset of nocturnal melatonin secretion postoperatively.44 However, Nishumura et al reported no changes in melatonin levels after invasive surgery.45 In addition to the effects of inflammation and stress, it is possible that the associated dysregulation of circadian rhythms may have affected cytokine levels in this study; however, further studies are needed to examine this.
A potential limitation of the study is the relatively small sample size. Using the results we obtained in this study, power calculations show that we would need 50 to 80 piglets in each group to detect a 25% increase from baseline in TNF-α and CRP. The power of the study to detect a significant difference at the .05 level in IL-6 after 90 minutes of IHH was calculated to be 93%. It would be important to further validate findings in a larger number of piglet.
Another limitation of the study may be the relatively short recovery time after surgery (minimum 48 hours). However, we have previously reported in this piglet model that physiologic parameters, including respiratory and heart rate and blood pressure, return to baseline 24 hours after surgery46 and a study by Dimofte et al found an almost full recovery in TNF-α and IL-6 levels 24 hours after major abdominal operations.47
The period of rest between hypoxic episodes was equivalent to the intervals that would normally occur between bouts of rapid eye movement sleep and not between separate sleep periods. We may not see the refractory period in IL-6 production on second exposure to hypoxia if there was a rest period of 1 day.
In conclusion, the present study is the first model involving neonatal animals demonstrating that even acute hypoxia, as occurs in human OSA, causes increases in IL-6. In chronic intermittent hypoxia, the resultant increase in IL-6 and possibly other proinflammatory mediators may potentially lead to an increased risk of cardiovascular disease in later life. Determination of the effects of chronicity and severity of IHH on inflammatory measures would require further studies.
ACKNOWLEDGMENTS
The authors would like to gratefully acknowledge Dr. Jennifer Peat, Honorary Biostatistician, Research & Development Office, The Children's Hospital at Westmead and Dr. Rita Machaalani, University of Sydney for their statistical advice and critical input. This study was funded by the National Institutes of Health (HL 70784).
Footnotes
Disclosure Statement
This was not an industry supported study. Drs. Charmaine Tam, Wong, Kimberley Tam, Aouad, and Waters have indicated no financial conflicts of interest.
REFERENCES
- 1.Tal A, Bar A, Leiberman A, Tarasiuk A. Sleep characteristics following adenotonsillectomy in children with obstructive sleep apnea syndrome. Chest. 2003;124:948–53. doi: 10.1378/chest.124.3.948. [DOI] [PubMed] [Google Scholar]
- 2.Prabhakar NR. Sleep apneas: an oxidative stress? Am J Respir Crit Care Med. 2002;165:859–60. doi: 10.1164/ajrccm.165.7.2202030c. [DOI] [PubMed] [Google Scholar]
- 3.Golej J, Winter P, Schoffmann G, et al. Impact of extracorporeal membrane oxygenation modality on cytokine release during rescue from infant hypoxia. Shock. 2003;20:110–5. doi: 10.1097/01.shk.0000075571.93053.2c. [DOI] [PubMed] [Google Scholar]
- 4.Mills PJ, Dimsdale JE. Sleep apnea: a model for studying cytokines, sleep, and sleep disruption. Brain Behav Immunol. 2004;18:298–303. doi: 10.1016/j.bbi.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Naldini A, Pucci A, Carraro F. Hypoxia induces the expression and release of interleukin 1 receptor antagonist in mitogen-activated mononuclear cells. Cytokine. 2001;13:334–41. doi: 10.1006/cyto.2001.0842. [DOI] [PubMed] [Google Scholar]
- 6.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906–14. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 7.Tauman R, Ivanenko A, O'Brien LM, Gozal D. Plasma C-reactive protein levels among children with sleep-disordered breathing. Pediatrics. 2004;113:e564–9. doi: 10.1542/peds.113.6.e564. [DOI] [PubMed] [Google Scholar]
- 8.Saito M, Ishimitsu T, Minami J, Ono H, Ohrui M, Matsuoka H. Relations of plasma high-sensitivity C-reactive protein to traditional cardiovascular risk factors. Atherosclerosis. 2003;167:73–9. doi: 10.1016/s0021-9150(02)00380-5. [DOI] [PubMed] [Google Scholar]
- 9.Kofler S, Nickel T, Weis M. Role of cytokines in cardiovascular diseases: a focus on endothelial responses to inflammation. Clin Sci (Lond) 2005;108:205–13. doi: 10.1042/CS20040174. [DOI] [PubMed] [Google Scholar]
- 10.Ewer AK, Al Salti W, Coney AM, Marshall JM, Ramani P, Booth IW. The role of platelet activating factor in a neonatal piglet model of necrotising enterocolitis. Gut. 2004;53:207–13. doi: 10.1136/gut.2003.024521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waters KA, Tinworth KD. Depression of ventilatory responses after daily, cyclic hypercapnic hypoxia in piglets. J Appl Physiol. 2001;90:1065–73. doi: 10.1152/jappl.2001.90.3.1065. [DOI] [PubMed] [Google Scholar]
- 12.Waters KA, Tinworth KD. Habituation of arousal responses after intermittent hypercapnic hypoxia in piglets. Am J Respir Crit Care Med. 2005;171:1305–11. doi: 10.1164/rccm.200405-595OC. [DOI] [PubMed] [Google Scholar]
- 13.Easton PA, Slykerman LJ, Anthonisen NR. Recovery of the ventilatory response to hypoxia in normal adults. J Appl Physiol. 1988;64:521–8. doi: 10.1152/jappl.1988.64.2.521. [DOI] [PubMed] [Google Scholar]
- 14.Abbas AK, Lichtman AH. 5th ed. Philadelphia: Elsevier Science; 2003. Cellular and Molecular Immunology; pp. P262–3. [Google Scholar]
- 15.Bowen RS, Gu Y, Zhang Y, Lewis DF, Wang Y. Hypoxia promotes interleukin-6 and -8 but reduces interleukin-10 production by placental trophoblast cells from preeclamptic pregnancies. J Soc Gynecol Invest. 2005;12:428–32. doi: 10.1016/j.jsgi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Hartmann G, Tschop M, Fischer R, et al. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and Creactive protein. Cytokine. 2000;12:246–52. doi: 10.1006/cyto.1999.0533. [DOI] [PubMed] [Google Scholar]
- 17.Klausen T, Olsen NV, Poulsen TD, Richalet JP, Pedersen BK. Hypoxemia increases serum interleukin-6 in humans. Eur J Appl Physiol Occup Physiol. 1997;76:480–2. doi: 10.1007/s004210050278. [DOI] [PubMed] [Google Scholar]
- 18.Alberti A, Sarchielli P, Gallinella E, et al. Plasma cytokine levels in patients with obstructive sleep apnea syndrome: a preliminary study. J Sleep Res. 2003;12:305–31. doi: 10.1111/j.1365-2869.2003.00361.x. [DOI] [PubMed] [Google Scholar]
- 19.Matsui H, Ihara Y, Fujio Y, et al. Induction of interleukin (IL)-6 by hypoxia is mediated by nuclear factor (NF)-kappa B and NF-IL6 in cardiac myocytes. Cardiovasc Res. 1999;42:104–12. doi: 10.1016/s0008-6363(98)00285-5. [DOI] [PubMed] [Google Scholar]
- 20.Matsusaka T, Fujikawa K, Nishio Y, et al. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci U S A. 1993;90:10193–7. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Semenza GL. Oxygen-regulated transcription factors and their role in pulmonary disease. Respir Res. 2000;1:159–62. doi: 10.1186/rr27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan SF, Tritto I, Pinsky D, et al. Induction of interleukin 6 (IL-6) by hypoxia in vascular cells. Central role of the binding site for nuclear factor-IL-6. J Biol Chem. 1995;270:11463–71. doi: 10.1074/jbc.270.19.11463. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg H, Ye X, Wilson D, Htoo AK, Hendersen T, Liu SF. Chronic intermittent hypoxia activates nuclear factor-κB in cardiovascular tissues in vivo. Biochem Biophys Res Commun. 2006;343:591–6. doi: 10.1016/j.bbrc.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Htoo AK, Greenberg H, Tongia S, et al. Activation of nuclear factor κB in obstructive sleep apnea: a pathway leading to systemic inflammation. Sleep Breath. 2006;10:43–50. doi: 10.1007/s11325-005-0046-6. [DOI] [PubMed] [Google Scholar]
- 25.Angele MK, Schwacha MG, Smail N, et al. Hypoxemia in the absence of blood loss upregulates iNOS expression and activity in macrophages. Am J Physiol. 1999;276:C285–90. doi: 10.1152/ajpcell.1999.276.2.C285. [DOI] [PubMed] [Google Scholar]
- 26.Meffert M, Hanninen EL, Menzel T, et al. In vivo time and dose dependency of interleukin-6 secretion in response to lowdose subcutaneous recombinant interleukin-2. Cancer Biother. 1994;9:307–16. doi: 10.1089/cbr.1994.9.307. [DOI] [PubMed] [Google Scholar]
- 27.Waage A. Production and clearance of tumor necrosis factor in rats exposed to endotoxin and dexamethasone. Clin Immunol Immunopathol. 1987;45:348–55. doi: 10.1016/0090-1229(87)90087-0. [DOI] [PubMed] [Google Scholar]
- 28.Castell JV, Geiger T, Gross V, et al. Plasma clearance, organ distribution and target cells of interleukin-6/hepatocyte-stimulating factor in the rat. Eur J Biochem. 1988;177:357–61. doi: 10.1111/j.1432-1033.1988.tb14384.x. [DOI] [PubMed] [Google Scholar]
- 29.Ertel W, Morrison MH, Ayala A, Chaudry IH. Hypoxemia in the absence of blood loss or significant hypotension causes inflammatory cytokine release. Am J Physiol. 1995;269:R160–6. doi: 10.1152/ajpregu.1995.269.1.R160. [DOI] [PubMed] [Google Scholar]
- 30.Keel M, Schregenberger N, Steckholzer U, et al. Endotoxin tolerance after severe injury and its regulatory mechanisms. J Trauma. 1996;41:430–7. doi: 10.1097/00005373-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Shalaby MR, Waage A, Aarden L, Espevik T. Endotoxin, tumor necrosis factor-alpha and interleukin 1 induce interleukin 6 production in vivo. Clin Immunol Immunopathol. 1989;53:488–98. doi: 10.1016/0090-1229(89)90010-x. [DOI] [PubMed] [Google Scholar]
- 32.Fletcher EC. Effect of episodic hypoxia on sympathetic activity and blood pressure. Respir Physiol. 2000;119:189–97. doi: 10.1016/s0034-5687(99)00114-0. [DOI] [PubMed] [Google Scholar]
- 33.Fletcher EC. Sympathetic over activity in the etiology of hypertension of obstructive sleep apnea. Sleep. 2003;26:15–9. doi: 10.1093/sleep/26.1.15. [DOI] [PubMed] [Google Scholar]
- 34.Ng DK, Chan C, Chow AS, Chow P, Kwok K. Childhood sleep-disordered breathing and its implications for cardiac and vascular diseases. J Paediatr Child Health. 2005;41:640–6. doi: 10.1111/j.1440-1754.2005.00751.x. [DOI] [PubMed] [Google Scholar]
- 35.Hu J, Chen Z, Gorczynski CP, et al. Sleep-deprived mice show altered cytokine production manifest by perturbations in serum IL-1ra, TNFa, and IL-6 levels. Brain Behav Immun. 2003;17:498–504. doi: 10.1016/j.bbi.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Maes M. Psychological stress and the inflammatory response system. Clin Sci (Lond) 2001;101:193–4. [PubMed] [Google Scholar]
- 37.Welsh CJ, Bustamante L, Nayak M, et al. The effects of restraint stress on the neuropathogenesis of Theiler's virus infection II: NK cell function and cytokine levels in acute disease. Brain Behav Immunol. 2004;18:166–74. doi: 10.1016/S0889-1591(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 38.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–3. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 39.Vigushin DM, Pepys MB, Hawkins PN. Metabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and disease. J Clin Invest. 1993;91:1351–7. doi: 10.1172/JCI116336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ehl S, Gehring B, Pohlandt F. A detailed analysis of changes in serum C-reactive protein levels in neonates treated for bacterial infection. Eur J Pediatr. 1999;158:238–42. doi: 10.1007/s004310051058. [DOI] [PubMed] [Google Scholar]
- 41.Kemna E, Pickkers P, Nemeth E, van der HH, Swinkels D. Timecourse analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood. 2005;106:1864–6. doi: 10.1182/blood-2005-03-1159. [DOI] [PubMed] [Google Scholar]
- 42.Jansky L, Reymanova P, Kopecky J. Dynamics of cytokine production in human peripheral blood mononuclear cells stimulated by LPS or infected by Borrelia. Physiol Res. 2003;52:593–8. [PubMed] [Google Scholar]
- 43.Zangerle PF, De Groote D, Lopez M, et al. Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood: II. Application to rheumatoid arthritis and osteoarthritis. Cytokine. 1992;4:568–75. doi: 10.1016/1043-4666(92)90021-i. [DOI] [PubMed] [Google Scholar]
- 44.Karkela J, Vakkuri O, Kaukinen S, Huang WQ, Pasanen M. The influence of anaesthesia and surgery on the circadian rhythm of melatonin. Acta Anaesthesiol Scand. 2002;46:30–6. doi: 10.1034/j.1399-6576.2002.460106.x. [DOI] [PubMed] [Google Scholar]
- 45.Nishimura S, Fujino Y, Shimaoka M, Hagihira S, Taenaka N, Yoshiya I. Circadian secretion patterns of melatonin after major surgery. J Pineal Res. 1998;25:73–7. doi: 10.1111/j.1600-079x.1998.tb00542.x. [DOI] [PubMed] [Google Scholar]
- 46.Reyes L, Tinworth KD, Li KM, Yau DF, Waters KA. Observerblinded comparison of two nonopioid analgesics for postoperative pain in piglets. Pharmacol Biochem Behav. 2002;73:521–8. doi: 10.1016/s0091-3057(02)00820-1. [DOI] [PubMed] [Google Scholar]
- 47.Dimofte G, Alexander A, Carlson G, Little R, Irving M. TNF alpha and IL-6 involvement in surgical trauma. II. In vitro cytokine production. Rev Med Chir Soc Med Nat Iasi. 2001;105:493–8. [PubMed] [Google Scholar]