Abstract
Study Objectives:
To evaluate the effect of bariatric surgery on sleep apnea symptoms and obesity-associated morbidity in patients with severe obesity.
Design:
Prospective study.
Setting:
University hospitals and community centers in Sweden.
Intervention:
We investigated the influence of weight loss surgery (n=1729) on sleep apnea symptoms and obesity-related morbidity using a conservatively treated group (n=1748) as a control.
Measurements and Results:
Baseline BMI in surgical group (42.2±4.4 kg/m2) and control group (40.1±4.6 kg/m2) changed −9.7±5 kg/m2 and 0±3 kg/m2, respectively, at 2-year follow-up. In the surgery group, there was a marked improvement in all obstructive sleep apnea (OSA) symptoms compared with the control group (P <0.001). Persistence of snoring (21.6 vs 65.5%, adjusted OR 0.14, 95% CI 0.10–0.19) and apnea (27.9 vs 71.3%, adjusted OR 0.16, 95% CI 0.10–0.23) were much less in the surgery group compared with controls. Compared with subjects with no observed apnea at follow-up (n=2453), subjects who continued to have or developed observed apnea (n=404) had a higher incidence of diabetes (adjusted OR 2.03, 95% CI 1.19–3.47) and hypertriglyceridemia (adjusted OR 1.86, 95% CI 1.07–3.25) but not hypertension (adjusted OR 1.09, 95% CI 0.65–1.83) or hypercholesterolemia (adjusted OR 0.91, 95% CI 0.53–1.58).
Conclusion:
Bariatric surgery results in a marked improvement in sleep apnea symptoms at 2 years. Despite adjustment for weight change and baseline central obesity, subjects reporting loss of OSA symptoms had a lower 2-year incidence of diabetes and hypertriglyceridemia. Improvement in OSA in patients losing weight may provide health benefits in addition to weight loss alone.
Citation:
Grunstein RR; Stenlöf KS; Hedner JA et al. Two year reduction in sleep apnea symptoms and associated diabetes incidence after weight loss in severe obesity. SLEEP 2007;30(6):703-710.
Keywords: Sleep, snoring, sleep apnea, obesity, diabetes, bariatric surgery
OBESITY IS A MAJOR WORLDWIDE PUBLIC HEALTH PROBLEM, AND RECENT STUDIES HAVE HIGHLIGHTED THE HEALTH BENEFITS OF WEIGHT REDUCTION IN obese subjects.1 Obstructive sleep apnea (OSA) is a common and major complication of obesity.2 An obese male is 5–18 times as likely to have OSA as males in the normal weight range.3 It is estimated that over 50% of obese males have OSA.4 Although the health consequences of obesity, particularly central obesity, are well established, there is increasing evidence that the presence of OSA independent of obesity contributes to the cardiovascular disease and metabolic abnormalities seen in these patients.5,6
A number of small, uncontrolled studies have demonstrated a reduction in OSA severity after weight loss by caloric restriction.7 However, dietary weight loss programs alone have variable success in weight reduction. Adjunctive pharmacotherapy for obesity in addition to dietary strategies appears to be a promising treatment, and there is emerging evidence of benefit in reduction of sleep disordered breathing.8 Weight loss surgery has previously been shown to reduce OSA severity in small uncontrolled cohort studies.9
Therapies such as nasal continuous positive airway pressure (CPAP), mandibular advancement splints, and upper airway surgery are frequently used in OSA but have variable compliance or efficacy.10 Treatment efficacy may be particularly impaired in patients with OSA and obesity.11,12 Therefore sustained weight loss may have definite advantages in overall management of OSA, by reducing apnea severity and reducing other morbidity in obesity such as diabetes, hypertension, and hyperlipidemia. Given the potential independent contribution of OSA to morbidity in severe obesity, it is of interest to determine the effect of change in sleep apnea status on comorbidities of obesity in subjects undergoing weight loss.
The Swedish Obese Subjects (SOS) cohort13 is an ongoing, long-term, prospective investigation of the effect of surgical weight loss on morbidity, mortality, and social impairment. We have previously used baseline data from this study to establish an important role for OSA in the comorbidities of obesity.4,14 The purpose of the present study was to prospectively investigate the influence of sustained weight loss on symptoms of sleep apnea and morbidity typically associated with obesity.
METHODS
General Design
SOS (Swedish Obese Subjects) is an ongoing nationwide project designed to determine whether the morbidity and mortality rates among obese people who lose weight by surgical means differ from those in a matched obese reference group.13,15 SOS consists of a registry study and an intervention study.13 The criteria for inclusion in the intervention study are age between 37 and 60 years, BMI ≥38 kg/m2 for women, and BMI ≥34 kg/m2 for men. Severe illness, abuse of alcohol or drugs, and previous bariatric surgery were reasons for exclusion, whereas diabetes, hypertension, and previously experienced (not within the last 6 months) myocardial infarction were not.
The study was not randomized since the ethics committees in Sweden did not approve randomization into surgical or control groups. Instead, patients willing to undergo surgical therapy were computer matched to those preferring conventional treatment with respect to sex and 18 other clinical variables. These were age, height, weight, waist and hip circumferences, waist/hip ratio, systolic blood pressure, cholesterol, triglycerides, smoking, diabetes, menopause, and six parameters evaluating psychological status: perceived health, psychasthenia, monotony avoidance, available social interaction, availability of attachment, and stressful life events.
Surgical procedures used for weight reduction included gastric bypass, vertical banded gastroplasty, and gastric banding.15 Surgery and follow-up was conducted at 25 different surgical departments in Sweden. Control subjects received routine obesity management offered at 480 primary health care centres located throughout the country. These treatments included dietary advice, physical training, low calorie diets, and behavior modification. No anti-obesity drugs were registered in Sweden during the study period.
Treatments and Study Groups
We examined data at baseline and at 2-year follow-up for subjects included in the study prior to September 30, 1998. Table 1 shows the clinical characteristics of the surgery and control groups who completed 2-year follow-up. At baseline, the surgical and control groups were similar with respect to gender and living arrangements. The average interval between registry examination and baseline examination in the intervention study was 9 months. During this initial period between matching and inclusion, the group awaiting surgery gained weight while the control group lost weight, resulting in a difference in body weight. Patients in the surgical group were slightly younger than those in the control group, had a higher prevalence of hypertension and diabetes, and were more frequently smokers.
Table 1.
Baseline Characteristics Among Subjects Completing 2-year Follow-up (+/−SD).
| Surgery | Control | P-value§ | |||
|---|---|---|---|---|---|
| Theoretically possible for follow-up (n) | 1729 | 1748 | |||
| Actually followed up (n) | 1592 | 1431 | |||
| Dropout rate (%) | 7.9 | 18.1 | |||
| Age (years) | 46.9 | (5.9) | 48.1 | (6.2) | <0.001 |
| Sex (% male) | 29.8 | 29.9 | 0.937 | ||
| Smoker (%) | 24.2 | 20.9 | 0.033 | ||
| Weight (kg) | 120.4 | (16.2) | 114.9 | (16.6) | <0.001 |
| Height (m) | 1.69 | (0.09) | 1.69 | (0.09) | 0.210 |
| BMI (kg/m2) | 42.2 | (4.4) | 40.1 | (4.6) | <0.001 |
| Waist (cm) | 125.5 | (10.8) | 120.2 | (11.1) | <0.001 |
| Waist Hip Ratio | 0.992 | (0.078) | 0.978 | (0.073) | <0.001 |
| Neck circumference (cm) | 43.7 | (4.2) | 42.9 | (4.3) | <0.001 |
| Systolic blood pressure (mmHg) | 145.8 | (18.7) | 138.2 | (17.8) | <0.001 |
| Diastolic blood pressure (mmHg) | 90.6 | (11.1) | 85.3 | (10.8) | <0.001 |
| Glucose (mmol/L) | 5.4 | (2.1) | 5.2 | (1.9) | 0.003 |
| Insulin (mmol/L) | 21.5 | (12.7) | 18.3 | (11.3) | <0.001 |
| Triglycerides (mmol/L) | 2.26 | (1.56) | 2.06 | (1.41) | <0.001 |
| High density lipoprotein (mmol/L) | 1.19 | (0.28) | 1.18 | (0.29) | 0.336 |
| Total cholesterol (mmol/L) | 5.91 | (1.12) | 5.65 | (1.07) | <0.001 |
| Diabetes (%) | 18.7 | 15.9 | 0.048 | ||
| Hypertension (%) | 78.4 | 61.2 | <0.001 | ||
| Hypertriglyceridemia (%) | 21.5 | 16.8 | 0.001 | ||
| Hypercholesterolemia (%) | 74.2 | 64.9 | <0.001 |
P-value: test of equality between groups. T-test for continuous variables, Fisher's exact test for proportions.
Sleep Apnea Questions
All subjects completed an 8-item sleep questionnaire at baseline and at 2-year follow-up using questions identical to those used in previous cross-sectional and longitudinal surveys in Sweden and validated against polysomnography in a subsample.16,17 Subjects were asked if they had a current regular home partner. The survey included the following questions directly related to sleep apnea—two questions utilizing a 5-point scale (never, rarely, sometimes, often, very often), one related to presence of loud and disruptive snoring, and another related to frequent daytime sleepiness. Subjects reporting “often” or “very often” were considered to be frequent snorers or to have frequent daytime sleepiness, respectively. In addition to these questions, subjects were asked whether a partner or another family member had observed frequent pauses in breathing during sleep (yes/no).4,14
Measurements
Anthropomorphic measurements (body weight, height, neck, waist and hip circumference) were measured as previously described. Systolic and diastolic (phase V) blood pressures were measured in the right arm using a mercury sphygmomanometer after 15 minutes of supine rest (single reading). Cuff width and upper arm circumference were recorded in each individual case. The blood pressures were adjusted for any incongruities in these measurements before analysis.18
Laboratory Data
Blood samples were obtained in the morning after 10–12 hours of fasting. Serum insulin was measured radioimmunochemically. Blood glucose and the remaining serum tests were analysed by enzymatic techniques.
Criteria for Health and Disease
The diagnosis of diabetes was based on the presence of fasting glucose ≥6.1 mmol/L and/or self-reported use of anti-diabetic medication. Cut-off values used for definitions of hypertriglyceridemia and hypercholesterolemia were serum triglycerides ≥ 2.8 mmol/L and total serum cholesterol ≥5.2 mmol/L, respectively. The diagnosis of hypertension required a systolic blood pressure ≥140 mm Hg or a diastolic blood pressure ≥90 mm Hg and/or medication prescribed specifically against hypertension. The incidence calculations are based on the diagnoses of developed diabetes, hypertension, hypertriglyceridemia, or hypercholesterolemia among individuals who were not affected by these conditions at the start of the intervention. Persistence of disease was calculated based on continuation of diagnoses at 2-year follow-up. For the purpose of analysing data on incidence or persistence of comorbidity, the presence of sleep apnea was determined by a positive response to the question regarding frequent observed breathing pauses during sleep and is denoted as observed apnea in the text. Analysis using observed apneas plus snoring and sleepiness resulted in a restricted population not suitable for multivariate analyses.
Statistical Methods
Fisher's exact test was used to compare incidence and persistence proportions of sleep apnea symptoms between the 2 treatment groups. Further, to control for baseline differences between the 2 treatment groups, a logistic regression model was used, adjusting for sex, age, BMI, smoking, diabetes, alcohol, and neck circumference. We report odds ratios (OR) with 95% confidence intervals (CI). The control group was always used as the reference group. Data were analyzed according to intention to treat, so that subjects in the control group who may have had surgery outside of the study are included in the control group.
After pooling the 2 treatment groups, the incidence and persistence proportions were examined in relation to degree of change in body weight. Changes in body weight were calculated as percent of the initial value and grouped in quartiles. Logistic regression models adjusting for baseline differences were used to compared the 4 weight change groups. The quartile with least weight change was used as the reference, and odds ratios and 95% CIs are reported.
Similarly logistic regression models were used to study incidence and persistence of diabetes, hypertension, and hyperlip idemia in relation to sleep apnea status. All the analyses were performed using the Stata statistics package (Stata Statistical Software: Release 7.0, Stata Corporation, College Station, TX, USA).
RESULTS
Baseline characteristics of subjects completing the 2-year follow-up are shown in Table 1. Among the 1592 surgically treated patients available at 2 years, 1143 (71.8%) underwent vertical banded gastroplasty (VBG), 325 (20.4%) gastric banding, and 124 (7.8%) gastric bypass. These operations were performed at 25 surgical departments located throughout Sweden. Calculations performed in February 2000 showed a perioperative mortality rate of 0.21%, and the incidence of other surgical complications (bleeding, embolus and/or thrombosis, wound complications, deep infections, pulmonary, and other complications) was 13%.
There was substantial weight reduction in the surgery group at 2 years. The mean weight loss in surgically treated patients was 27.8 kg, corresponding to 23% of baseline weight. Mean BMI decreased from 42.2 to 32.5 kg/m2 compared with baseline weight. The average weight in the control group remained essentially unchanged. Greater than 10% weight loss was achieved by approximately 50% of the entire SOS study group. Average relative weight loss in the surgery group was 21.9% and 23.3% in men and women, respectively (P = 0.015). This could in part be explained by different inclusion criteria for men and women in the study.
Weight Change and Sleep Apnea Symptoms
To evaluate appearance of new sleep apnea-related symptoms, we analysed data in subjects without symptoms at baseline. The development of new symptoms of apneas and snoring was approximately 4 to 5 times lower in the surgery group than the control group (Table 2). The incidence of sleepiness was also lower in the surgery group (Table 2). Of the subjects in the surgery group with frequent complaints at baseline, only 20%–30% had persisting symptoms at follow-up (Table 2). In contrast, approximately 50%–70% of control subjects reported persistent symptoms.
Table 2.
Sleep Apnea Symptoms in Surgery and Control Groups
| Surgery | Control | P-value | OR (95% CI) | |
|---|---|---|---|---|
| Number of subjects | 1592 | 1431 | ||
| Freq. apneas | ||||
| Baseline, % | 24.0 | 21.8 | <0.001 | |
| Follow-up, % | 8.3 | 20.8 | <0.001 | |
| Incidence, % | 2.3 | 6.7 | <0.001 | 0.28 (0.16 to 0.49) |
| Persistence, % | 27.9 | 71.3 | <0.001 | 0.16 (0.10 to 0.23) |
| Freq. snoring | ||||
| Baseline, % | 44.5 | 35.6 | <0.001 | |
| Follow-up, % | 10.8 | 29.8 | <0.001 | |
| Incidence, % | 2.1 | 10.0 | <0.001 | 0.18 (0.10 to 0.32) |
| Persistence, % | 21.6 | 65.5 | <0.001 | 0.14 (0.10 to 0.19) |
| Freq. daytime sleepiness | ||||
| Baseline, % | 25.8 | 20.4 | <0.001 | |
| Follow-up, % | 12.7 | 17.8 | <0.001 | |
| Incidence, % | 5.9 | 8.4 | 0.018 | 0.66 (0.45 to 0.96) |
| Persistence, % | 32.6 | 54.6 | <0.001 | 0.44 (0.30 to 0.63) |
Incidence: proportion of subjects with symptoms at 2-year among those without reported symptoms at baseline
Persistence: proportion of subjects with symptoms at 2-year among those with reported symptoms at baseline
Proportions are unadjusted values. P-value: Fischer's exact test, unadjusted
OR (95% CI): odds-ratio (95% confidence interval), adjusted for age, sex, BMI, smoking, diabetes, alcohol, and neck circumference at baseline
The improvement in symptoms of apneas and daytime sleepiness was similar in surgically treated men and women. In contrast, reduction in the incidence of snoring was larger in surgically treated women as compared to men. The ORs for comparison of treatment groups were 0.36 and 0.13 in men and women, respectively (P = 0.024 for sex-treatment interaction).
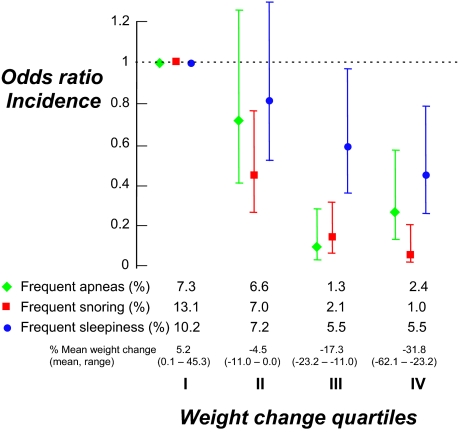
To investigate the relationship between degree of weight loss and change in sleep apnea symptoms, we stratified the entire study sample (surgery and control groups) into quartiles, according to change in weight from baseline. There was a dose-response effect of weight loss on sleep apnea symptoms (Figures 1 and 2). Subjects in the quartile with the greatest weight loss were 2–13 times less likely to report the development of new sleep apnea symptoms (Figure 1) and 2.5–7 times less likely to report continuing sleep apnea symptoms than subjects in the quartile with the least weight change (Figure 2). Even in the second quartile, corresponding to a weight reduction of 0.0% to 11.0% of baseline body weight, the odds ratio for a report of frequent apneas, snoring, and daytime sleepiness was reduced by approximately 50%.
Figure 1.
Subjects divided in the quartile according to weight change. Subjects with the greatest weight loss were 2–13 times less likely to report the development of new sleep apnea symptoms. Odds ratios and confidence intervals expressed relative to the quartile with the least weight loss (OR 1.0)
Figure 2.
Subjects divided in the quartile according to weight change. Subjects with the greatest weight loss were 2.5–7 times less likely to report continuing sleep apnea symptoms than subjects in the quartile with the least weight change. Odds ratios and confidence intervals expressed relative to the quartile with the least weight loss (OR 1.0)
In general, the effect of weight loss on symptoms of apneas, snoring, and daytime sleepiness was similar in men and women. For snoring and daytime sleepiness, however, there was a statistically significant interaction between sex and weight loss, so that men with greatest weight loss had relatively larger improvement in these symptoms compared with women with greatest weight loss (sex-weight loss interaction, P = 0.003 for snoring, P = 0.029 for daytime sleepiness).
Obesity-Associated Morbidity
The impact of sleep apnea (defined as the presence of frequent witnessed apnea, i.e., “observed apnea”) on the development of obesity-associated morbidity, the 2-year incidence of diabetes and hypertension was calculated in the entire group. The 2-year incidence of diabetes, adjusted for weight change and other variables, was more than 2 times higher in subjects who developed or continued to have observed apnea as those who did not (Table 3). However, the adjusted incidence of hypertension over the 2-year period was similar in subjects with and without this symptom at follow-up. Similarly, the adjusted incidence and persistence of hypercholesterolemia was unrelated to observed apnea status, while hypertriglyceridemia was 1.9 times more likely in subjects developing or maintaining observed apnea compared with those who did not (Table 3).
Table 3.
Incidence/persistence of Diabetes, Hypertension, and Hyperlipidemia by Apnea Status.
| Sleep apnea status, baseline/follow-up | |||
|---|---|---|---|
| no/no | yes/no | no/yes or yes/yes | |
| n: | 2116 | 337 | 404 |
| Diabetes | |||
| Incidence, % | 3.1 | 3.0 | 9.2 |
| OR (95% CI) | 1 | 0.95 (0.38 to 2.35) | 2.03 (1.19 to 3.47) |
| Persistence, % | 55.5 | 39.1 | 62.0 |
| OR (95% CI) | 1 | 0.67 (0.33 to 1.38) | 0.77 (0.38 to 1.57) |
| Hypertension | |||
| Incidence, % | 25.5 | 26.7 | 36.8 |
| OR (95% CI) | 1 | 0.83 (0.41 to 1.65) | 1.09 (0.65 to 1.83) |
| Persistence, % | 73.0 | 68.7 | 78.1 |
| OR (95% CI) | 1 | 1.14 (0.81 to 1.61) | 0.97 (0.67 to 1.40) |
| Hypercholesterolemia | |||
| Incidence, % | 26.7 | 27.3 | 24.6 |
| OR (95% CI) | 1 | 1.13 (0.64 to 2.00) | 0.91 (0.53 to 1.58) |
| Persistence, % | 81.7 | 77.4 | 81.1 |
| OR (95% CI) | 1 | 1.05 (0.71 to 1.54) | 0.85 (0.58 to 1.26) |
| Hypertriglyceridemia | |||
| Incidence, % | 3.6 | 3.2 | 10.2 |
| OR (95% CI) | 1 | 1.09 (0.46 to 2.54) | 1.86 (1.07 to 3.25) |
| Persistence, % | 38.5 | 29.4 | 51.4 |
| OR (95% CI) | 1 | 0.80 (0.41 to 1.56) | 0.97 (0.56 to 1.68) |
Diabetes: ≥6.1 mmol/L/self-reported use of antidiabetic medication
Hypertension: ≥140/90 mmHg/self-reported use of antihypertensive medication
Hypertriglyceridemia: triglycerides ≥2.8 mmol/L
Hypercholesterolemia: total cholesterol ≥5.2 mmol/L
Proportions are unadjusted values.
OR (95% CI): odds-ratio (95% confidence interval), adjusted for age, sex, BMI, waist circumference, relative weight change, smoking, diabetes, alcohol, and neck circumference at baseline.
DISCUSSION
This study has demonstrated that symptoms of sleep apnea such as snoring, breathing pauses during sleep, and sleepiness are markedly igmp1roved following surgical weight loss in a dose-dependent fashion. These findings have important implications for the management of sleep disordered breathing in patients with severe obesity, supporting an important therapeutic role for bariatric surgery in such patients. These data also add to previous reports from the SOS cohort demonstrating that a 2-year weight loss leads to a dramatic reduction in obesity-related comorbidity such as diabetes, hypertension, and hyperlipidemia.15 Moreover, the observation that there was a doubling of the odds ratio for incidence of either diabetes or hypertriglyceridemia with development or maintenance of observed apnea suggests a contributory role for OSA in some obesity related morbidity. These metabolic findings persisted even after adjustment for weight change and a range of other confounding variables, including neck size, as a marker of central obesity. In contrast, observed apneas in this cohort were not associated with the development of hypertension or hypercholesterolemia during the follow-up period.
The SOS study offers a unique opportunity to investigate OSA symptom change prospectively in a large cohort of obese subjects. It provides the first controlled study addressing the impact of weight loss on these symptoms and associated morbidity. There are several key observations in this analysis of 2-year follow-up data. Firstly, sustained weight loss leads to a marked reduction in symptoms of sleep apnea. Secondly, symptom reduction was proportional to the degree of weight loss. Subjects in the quartile with the greatest weight loss were 2.5–7 times less likely to report OSA symptoms such as frequent snoring, witnessed apnea, and excessive sleepiness than subjects in the quartile with the least weight change. At 2-year follow-up, among symptomatic subjects in the quartile with the greatest weight loss, only 17.0% still reported frequent apneas, 9.6% reported frequent snoring, and 24.8% reported frequent daytime sleepiness.
Symptoms suggestive of sleep apnea were extremely common in the SOS cohort at baseline.4,14 Frequent snoring was reported in 44.5% of subjects who subsequently underwent bariatric surgery. In these subjects at 2-year follow-up, 10.8% still reported frequent snoring. This level of snoring prevalence is similar to that (14.7%) found in a previously published random sample of 2,668 Swedish non-obese males (mean BMI 27) with an almost identical mean age using identical survey questions.17 Therefore, the surgical intervention in the SOS study had a major impact on snoring prevalence reducing the frequency of this symptom to that found randomly in the population. In fact, there appeared to be an almost linear association between the degree of weight loss in the whole SOS cohort and reduction in symptoms of sleep disordered breathing.
Weight loss is frequently mentioned as a treatment alternative for OSA. Small and uncontrolled studies of bariatric surgery have reported a dramatic reduction of OSA in the short term,7,9,19 though the amount of weight loss achieved by surgical intervention may not be correlated with improvement in OSA.19 Long-term controlled studies examining the effect of weight loss on OSA or OSA-related symptoms have not previously been performed. Observations in the Wisconsin Sleep Cohort20 have demonstrated that subjects who lose weight reduce the severity of sleep disordered breathing. However, this study involved a population with a mean BMI of 29 at baseline, and the median number of OSA events was low in contrast to our population that was more obese and therefore more likely to have worse OSA. Our data supports a dose-response effect for weight loss on sleep apnea symptoms and further shows that the impact of weight loss on OSA is sustained long-term.
Weight loss may reduce OSA severity by a number of mechanisms. Fat deposition adjacent to the upper airway promotes mass loading and obstruction of the upper airway during sleep and is reduced by weight loss.21 In addition, abdominal obesity reduces lung volumes and may, by means of altered air flow dynamics, mechanically reduce pharyngeal cross-sectional area, and increase pharyngeal resistance.22 Weight loss leads to an increase in lung volume and reduction in upper airway collapsibility.23
We also observed, after adjustment for weight loss, fat distribution, and other variables, that the incidence of diabetes was more than doubled in subjects with newly developed or continuing observed apnea. A number of studies suggest that there is biological plausibility for the role of sleep apnea in the development of obesity-associated comorbidities.24 An obesity-independent association between OSA and increased insulin levels has previously been observed in the SOS cohort4 and in other cross-sectional studies.25 Some studies suggest an improvement in insulin sensitivity with CPAP treatment,26,27 although this observation was more pronounced in non-obese patients.27 The 2-year weight loss adjusted incidence of diabetes in the present study was more than 3 times higher in subjects who developed or maintained observed apnea than those who did not. Similar findings were observed for hypertriglyceridemia. The increased likelihood of comorbid diabetes and hypertriglyceridemia in these patients may be explained by a number of mechanisms. Recent studies in patients with OSA have found evidence of endothelial dysfunction,28 increased concentrations of leptin, tumour necrosis factor-alpha and free fatty acids,29 and elevated muscle sympathetic nerve activity.36 All these abnormalities have been identified as contributors to the pathogenesis of diabetes in severe obesity.30 It is possible that the diabetes in these SOS study subjects may be influenced by coexistent sleep disordered breathing. This situation may be different in less obese subjects, in whom there does not seem to be an obesity-independent effect of OSA in the development of diabetes.31
Previous work in the general population has observed that the likelihood of newly developed hypertension appears to be nearly three times higher in subjects with sleep apnea independent of other known hypertension associated risk factors, including central obesity.5 Treatment of sleep apnea with CPAP has also been associated with a fall in blood pressure.32 However in this study, in contrast to the finding with diabetes, hypertension incidence or prevalence over the 2-year period (persistence) was unaffected by change in observed apnea. It is possible that hypertension in the severely obese with OSA may involve mechanisms different from those occurring in the general OSA population or that elimination of sleep disordered breathing following weight reduction may be less complete than that generally seen after CPAP treatment of sleep apnea. Moreover, others have recently questioned the independent impact of sleep apnea on hypertension and the efficacy of CPAP in reducing blood pressure.33,34
The strengths of this study include the large size of the cohort, the availability of a control group for surgical intervention, and the use of identical survey instruments and objective testing at 2-year follow-up. One obvious limitation of such a study is the use of survey questions as indices of sleep disordered breathing instead of full polysomnography. Polysomnography was not readily available when the SOS study was conceived in the mid 1980s; instead, survey questions that had been validated against polysomnography in a subgroup were utilized.35 We used frequent breathing pauses to define sleep apnea for the purposes of investigating OSA influences of obesity-associated morbidity. Although using such a symptom will produce some misclassification relative to polysomnography, it is a specific symptom, and those reporting frequent breathing pauses during sleep are likely to have OSA.36,37
Single blood pressure readings were used as is common in large scale studies of this type, but it is possible that this may lead to misclassification of hypertension compared with serial readings, such as 24-hour blood pressure. Another limitation is that the subjects were not randomised to surgical or control groups, as this was refused by ethics committees at the time the study was initiated. Therefore SOS subjects self-selected therapy, and there may be unrecognised biases with potential effects on measured outcomes. In addition, the BMI of the SOS subjects was at the higher end of weight ranges seen in sleep clinics, and these data may not be able to be generalized to overweight or less obese individuals.
The effectiveness of intentional weight loss in reducing symptoms of sleep disordered breathing has important implications for management of sleep apnea in the obese patient. Nasal CPAP has high efficacy in OSA, but effectiveness is limited. Recent data suggest that less than 30% of patients in Sweden prescribed CPAP comply adequately with this treatment at 1-year follow-up.38 Patients with obesity also respond poorly to mandibular advancement splints and upper airway surgery.11,12 Therefore sustained weight loss has advantages in the overall management of OSA in addition to therapeutic benefits for other morbidity in obesity. For patients with milder degrees of obesity, nonsurgical weight loss strategies39 may also have a role in the management of OSA, though this will need to be verified by randomized controlled trials (RCT) for the endpoint of OSA. Emerging data from open “proof of concept” studies suggest the potential of this approach.8 For more severe obesity, bariatric surgery in the SOS study had low morbidity and mortality15 with newer, simpler surgical techniques becoming available.40 When data from randomized studies demonstrating longer term benefits in mortality and morbidity become available, weight loss surgery should be considered as an important treatment alternative in patients with severe obesity and sleep apnea.
ACKNOWLEDGEMENTS
The Swedish Obese Subjects (SOS) study is supported by the Swedish Medical Research Council (Grant 19X-05239), Hoffman-La Roche, and the Volvo Research Foundation. Ronald Grunstein was supported by an NHMRC of Australia Practitioner Fellowship
The SOS Study involves the assistance of 380 primary health care centres in Sweden. Members of the SOS Steering and Safety Committee are B. Larsson, S. Lindstedt, L. Lissner, L. Olbe, M. Sullivan, L. Sjöström (chairman), Sahlgrenska Hospital, Gothenburg, Sweden; C. Bengtsson, Institute of Primary Care, Gothenburg, Sweden; I. Näslund, Örebrö Hospital, Örebro, Sweden; S. Dahlgren, Umeå Hospital, Umeå, Sweden; E. Jonsson, Karolinska Hospital, Stockholm; L. Backman, Danderyd's Hospital, Stockholm, Sweden; H. Wedel, Nordic Health University, Gothenburg, Sweden; C. Bouchard, Laval University, Quebec. We would also thank to Dr. David Wang for assistance in preparation of this manuscript.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Grunstein has received research support from GlaxoSmithKline, Neurocrine, Sanofi-Aventis, and Cephalon; has participated in a speaking engagement for Roche Pharmaceuticals; and has received travel assistance form Respironics. Drs. Stenlöf, Hedner, Peltonen, Karason, and Sjöström have indicated no financial conflicts of interest.
REFERENCES
- 1.Caterson ID, Hubbard V, Bray GA, et al. American Heart Association. Circulation; Prevention Conference VII: Obesity, a worldwide epidemic related to heart disease and stroke: Group III: worldwide co-morbidities of obesity; 2004. pp. e476–83. [DOI] [PubMed] [Google Scholar]
- 2.Grunstein RR, Wilcox I, Yang TS, Gould Y, Hedner JA. Snoring and sleep apnoea in men: association with central obesity and hypertension. Int J Obesity. 1993;17:533–40. [PubMed] [Google Scholar]
- 3.Bearpark H, Elliott L, Grunstein R, et al. Snoring and sleep apnea. A population study in Australian men. Am J Respir Crit Care Med. 1995;151:1459–65. doi: 10.1164/ajrccm.151.5.7735600. [DOI] [PubMed] [Google Scholar]
- 4.Grunstein RR, Stenlöf K, Hedner JA, Sjoström L. Impact of sleep apnea and sleepiness on metabolic and cardiovascular risk factors in the Swedish Obese Subjects (SOS) Study. Int J Obes. 1995;19:410–8. [PubMed] [Google Scholar]
- 5.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disodered breathing and hypertension. N Eng J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 6.Gami AS, Caples SM, Somers VK. Obesity and obstructive sleep apnea. Endocrinol Metab Clin North Am. 2003;32:869–94. doi: 10.1016/s0889-8529(03)00069-0. [DOI] [PubMed] [Google Scholar]
- 7.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–9. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 8.Yee BJ, Phillips CL, Banerjee D, Caterson I, Hedner JA, Grunstein RR. The effect of sibutramine-assisted weight loss in men with obstructive sleep apnoea. Int J Obes. 2007;31:161–8. doi: 10.1038/sj.ijo.0803363. [DOI] [PubMed] [Google Scholar]
- 9.Dixon JB, Schachter LM, O'Brien PE. Polysomnography before and after weight loss in obese patients with severe sleep apnea. Int J Obes. 2005;29:1048–54. doi: 10.1038/sj.ijo.0802960. [DOI] [PubMed] [Google Scholar]
- 10.Grunstein RR, Hedner J, Grote L. Treatment options for sleep apnoea. Drugs. 2001;61:237–51. doi: 10.2165/00003495-200161020-00007. [DOI] [PubMed] [Google Scholar]
- 11.Marklund M, Persson M, Franklin KA. Treatment success with a mandibular advancement device is related to supine-dependent sleep apnea. Chest. 1998;114:1630–5. doi: 10.1378/chest.114.6.1630. [DOI] [PubMed] [Google Scholar]
- 12.Schechtman KB, Sher AE, Piccirillo JF. Methodological and statistical problems in sleep apnea research: the literature on uvulopalatopharyngoplasty. Sleep. 1995;18:659–66. doi: 10.1093/sleep/18.8.659. [DOI] [PubMed] [Google Scholar]
- 13.Sjöström L, Larsson B, Backman L, et al. Swedish Obese Subjects (SOS). Recruitment for an intervention study and a selected description of the obese state. Int J Obesity. 1992;16:465–79. [PubMed] [Google Scholar]
- 14.Grunstein RR, Stenlöf K, Hedner JA, Sjoström L. Impact of self reported sleep-breathing disturbances on psycho-social performance in the Swedish Obese Subjects (SOS) Study. Sleep. 1995;18:635–43. doi: 10.1093/sleep/18.8.635. [DOI] [PubMed] [Google Scholar]
- 15.Sjostrom CD, Peltonen M, Wedel H, Sjostrom L. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension. 2000;36:20–5. doi: 10.1161/01.hyp.36.1.20. [DOI] [PubMed] [Google Scholar]
- 16.Gislason T, Aberg H, Taube A. Snoring and systemic hypertension- -an epidemiological study. Acta Med Scand. 1987;222:415–21. doi: 10.1111/j.0954-6820.1987.tb10958.x. [DOI] [PubMed] [Google Scholar]
- 17.Gislason T, Almqvist M, Eriksson G, Taube A, Boman G. Prevalence of sleep apnea syndrome among Swedish men--an epidemiological study. J Clin Epidemiol. 1988;41:571–6. doi: 10.1016/0895-4356(88)90061-3. [DOI] [PubMed] [Google Scholar]
- 18.Maxwell MH, Waks AU, Schroth PC, Karam M, Dornfeld LP. Error in blood-pressure measurement due to incorrect cuff size in obese patients. Lancet. 1982;3:33–36. doi: 10.1016/s0140-6736(82)91163-1. [DOI] [PubMed] [Google Scholar]
- 19.Rasheid S, Banasiak M, Gallagher SF, et al. Gastric bypass is an effective treatment for obstructive sleep apnea inpatients with clinically significant obesity. Obes Surg. 2003;13:58–61. doi: 10.1381/096089203321136593. [DOI] [PubMed] [Google Scholar]
- 20.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000 Dec 20;284:3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 21.Welch KC, Foster GD, Ritter CT, et al. A novel volumetric magnetic resonance imaging paradigm to study upper airway anatomy. Sleep. 2002;25:532–42. [PubMed] [Google Scholar]
- 22.Series F, Cormier Y, Lampron N. Influence of passive changes of lung volume on upper airways. J Appl Physiol. 1990;68:2159–64. doi: 10.1152/jappl.1990.68.5.2159. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz AR, Gold AR, Schubert N, et al. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1991;144:494–8. doi: 10.1164/ajrccm/144.3_Pt_1.494. [DOI] [PubMed] [Google Scholar]
- 24.Polotsky VY, Li J, Punjabi NM, et al. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol. 2003;552:253–64. doi: 10.1113/jphysiol.2003.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strohl KP, Novak RD, Singer W, et al. Insulin levels, blood pressure and sleep apnea. Sleep. 1994;17:614–8. doi: 10.1093/sleep/17.7.614. [DOI] [PubMed] [Google Scholar]
- 26.Brooks B, Cistulli PA, Borkman M, et al. Obstructive sleep apnea in obese noninsulin-dependent diabetic patients: effect of continuous positive airway pressure treatment on insulin responsiveness. J Clin Endocrinol Metab. 1994;79:1681–5. doi: 10.1210/jcem.79.6.7989475. [DOI] [PubMed] [Google Scholar]
- 27.Harsch IA, Schahin SP, Radespiel-Troger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156–62. doi: 10.1164/rccm.200302-206OC. 15. [DOI] [PubMed] [Google Scholar]
- 28.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169:348–53. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 29.Vgontzas AN, Bixler EO, Chrousos GP. Metabolic disturbances in obesity versus sleep apnoea: the importance of visceral obesity and insulin resistance. J Intern Med. 2003;254:32–44. doi: 10.1046/j.1365-2796.2003.01177.x. [DOI] [PubMed] [Google Scholar]
- 30.Hedner J, Ejnell H, Sellgren J, Hedner T, Wallin G. Is high and fluctuating muscle nerve sympathetic activity in the sleep apnoea syndrome of pathogenetic importance for the development of hypertension? J Hypertens Suppl. 1988;6:S529–31. doi: 10.1097/00004872-198812040-00166. [DOI] [PubMed] [Google Scholar]
- 31.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–5. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359(9302):204–10. doi: 10.1016/S0140-6736(02)07445-7. 19. [DOI] [PubMed] [Google Scholar]
- 33.Stradling J. Con: Sleep apnea does not cause cardiovascular disease. Am J Respir Crit Care Med. 2004;169:148–9. doi: 10.1164/rccm.2310012. [DOI] [PubMed] [Google Scholar]
- 34.Robinson GV, Smith DM, Langford BA, Davies RJO, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Resp J. doi: 10.1183/09031936.06.00062805. in press. [DOI] [PubMed] [Google Scholar]
- 35.Lindberg E, Elmasry A, Gislason T, et al. Evolution of sleep apnea syndrome in sleepy snorers: a population-based prospective study. Am J Respir Crit Care Med. 1999;159:2024–7. doi: 10.1164/ajrccm.159.6.9805070. [DOI] [PubMed] [Google Scholar]
- 36.Teculescu DB, Hannhart B, Benamghar L, Michaely JP. Witnessed breathing pauses during sleep: a study in middle-aged French males. Respir Med. 2005;99:1268–74. doi: 10.1016/j.rmed.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 37.Kump K, Whalen C, Tishler PV, et al. Assessment of the validity and utility of a sleep-symptom questionnaire. Am J Respir Crit Care Med. 1994;150:735–41. doi: 10.1164/ajrccm.150.3.8087345. [DOI] [PubMed] [Google Scholar]
- 38.Grote L, Hedner J, Grunstein R, Kraiczi H. Therapy with nCPAP: incomplete elimination of sleep related breathing disorder. Eur Respir J. 2000;16:921–7. doi: 10.1183/09031936.00.16592100. [DOI] [PubMed] [Google Scholar]
- 39.Wadden TA, Berkowitz RI, Womble LG, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353:2111–20. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 40.O'Brien PE, Dixon JB, Laurie C, et al. Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: a randomized trial. Ann Intern Med. 2006;144:625–33. doi: 10.7326/0003-4819-144-9-200605020-00005. [DOI] [PubMed] [Google Scholar]