Abstract
Study Objectives:
To implement a chronic rat model of recurrent airway obstructions to study the obstructive sleep apnea (OSA) syndrome.
Design:
Prospective controlled animal study.
Setting:
University laboratory.
Patients or Participants:
24 male Sprague-Dawley rats (250–300 g).
Interventions:
The rats were placed in a setup consisting of a body chamber and a head chamber separated by a neck collar specially designed to apply recurrent airway obstructions with OSA patterns. Rats in the Obstruction group (n=8) were subjected to 5-s obstructions at a rate of 60 per hour, 6 h/day during 4 weeks. Sham rats (n=8) were placed in the setup but no obstructions were applied. Naive rats (n=8) were subjected to no intervention.
Measurements and Results:
Breathing flow, pressure, CO2 air concentration, and SpO2 showed that the model mimicked OSA respiratory events (obstructive apneas, increased respiratory efforts, and oxygen saturation dips). Animal stress, assessed by body weight and plasma corticosterone, was not significantly different across Obstruction and Sham groups. This supports the concept that this novel model does not introduce a significant burden of stress in the rat after acclimatization to the chamber. Thromboxane-B2/6-keto-Prostaglandin-F1α ratio in plasma, which is an index of vasoconstriction, was significantly increased in the rats subjected to obstructions.
Conclusions:
The designed animal model of chronic recurrent airway obstructions is feasible and potentially useful to study the mechanisms involved in the cardiovascular consequences of OSA.
Citation:
Farré R; Nácher M; Serrano-Mollar A et al. Rat model of chronic recurrent airway obstructions to study the sleep apnea syndrome. SLEEP 2007;30(7):930-933.
Keywords: Obstructive sleep apnea, animal model, airway obstruction
INTRODUCTION
THE OBSTRUCTIVE SLEEP APNEA (OSA) SYNDROME IS A VERY PREVALENT RESPIRATORY SLEEP DISTURBANCE THAT INDUCES EXCESSIVE SLEEPINESS LEADING TO an increased risk of accidents and deterioration in quality of life. OSA is also an important risk factor for cardiovascular diseases,1 although the exact mechanisms involved are not well known despite the considerable effort made in recent years. Given the concurrence of a number of confounding variables, such as those characterizing the metabolic syndrome, it is difficult to draw precise and definitive conclusions from patient studies. Patient data also make it difficult to investigate the detailed mechanisms causing the consequences of OSA. Specifically, the relative roles played in the pathophysiology of sleep apnea by fluctuations in oxygen, carbon dioxide, behavioral state, respiratory drive, ventilatory effort, and upper airway intraluminal pressures are not known. To advance our understanding of which patients with sleep apnea are at greater risk for morbidities and to target treatment to relevant perturbances, it is essential that we understand the interactions of physiological variables.
Accordingly, animal models, which avoid comorbidities and allow invasive measurements under well-controlled experimental conditions, are a useful tool to investigate the pathophysiological mechanisms determining the consequences of OSA. Although a number of animal models have been proposed to this end,2,3 no model has been so far described to noninvasively apply a chronic pattern of controlled airway obstructions mimicking the ones experienced by OSA patients. The aim of this work was, therefore, to develop and test the feasibility of such a model for OSA research.
METHODS
Experimental Setup
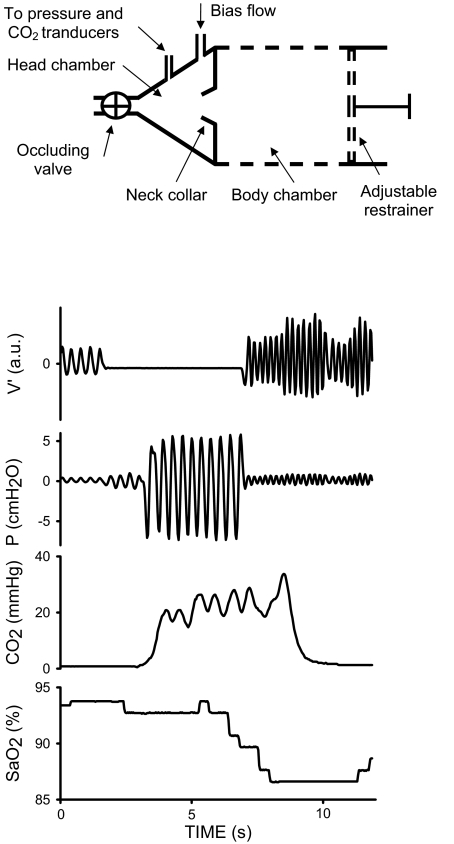
The rat model proposed to apply recurrent airway obstructions is based on a setup consisting of 2 chambers separated by a latex neck collar (Figure 1, top). The body chamber was a transparent plastic cylinder (6.5 cm in diameter) with a dense distribution of holes (1 cm in diameter) in the walls to allow air circulation and maintenance of temperature and humidity. The position of the rear base of the cylinder (which incorporates a hole for the rat tail) was adjustable to restrain the rat movement. The neck collar was selected to almost eliminate air leaks between the body and head chambers while ensuring that no excessive pressure was applied on the neck. The transparent head chamber was conical (Figure 1, top), with dimensions designed to reduce its air volume when the rat was in place.
Figure 1.
Top: Diagram of the experimental setup to noninvasively apply recurrent airway obstructions in the rat. See text for explanation. Bottom: Example of the signals recorded during one of the recurrent obstructions. During the obstruction, flow (V') was nil owing to valve closure, pressure swings in the head chamber (P) were markedly increased as a result of the breathing efforts, partial pressure of carbon dioxide in the air of the head chamber (CO2) increased and the rat's arterial oxygen saturation (SpO2) exhibited a transient decrease.
The rat breathed room air through an orifice at the vertex of the conical head chamber. A valve to allow closure of the orifice was placed at the entrance of the head chamber (Figure 1, top). The valve, which was described in detail elsewhere,4 allowed the application of a controlled pattern of obstruction and incorporated a built-in pneumotachograph to monitor breathing flow. A bias airflow source electronically synchronized with the valve was connected to the head chamber to rinse it with room air. A flow of 2 ml/s entered the rear part of the head chamber during the time that the valve was open. When the valve was closed the bias flow was interrupted, keeping the head chamber closed and imposing a virtual airway obstruction to the rat breathing.
A transducer (Honeywell 176 PC; Microswitch, Boston, MA, USA) measuring pressure in the head chamber was used to assess breathing effort. The CO2 concentration in the air of the head chamber was intermittently monitored (Tonometrics, Datex Engstrom, Helsinki, Finland). The arterial oxygen saturation (SpO2) was periodically measured by pulse oximetry at the rat tail (504; Criticare Systems, Inc, Waukesha, WI). Signals were sampled at 100 Hz and stored in a conventional computer-based data acquisition system.
Model Evaluation
The study, which was approved by the Animal Research Ethical Committee of the University of Barcelona, was carried out on 24 Sprague-Dawley male rats (250–300 g). The animals were randomized into 3 groups (n=8 each): Obstruction, Sham, and Naive.
Rats in the Obstruction and Sham groups were progressively adapted to the experimental setting during the week prior to the start of the experiment. To this end, the time that rats were placed in the experimental device (without airway obstructions) was increased each day up to 6 h on the last day. At the end of the adaptation period, the rats in the Obstruction group were subjected to 5-s obstructions at a rate of 60 per hour, 6 h/day for 4 weeks. A 5-s duration of obstructions was chosen because these resulted in transient SpO2 reductions to 85%±1% (10-s and 15-s obstructions transiently decreased SpO2 to 83%±2% and 75%±2%, respectively). Sham rats were subjected to the same experimental procedure, but no airway obstructions were applied. Naive rats were subjected to no intervention.
To assess the level of stress induced in the animals, the body weight and the plasma concentration of corticosterone, which is a conventional index of stress,5 were measured every 3 days for the 4 weeks of the study in all the rats. Plasma corticosterone was measured by ELISA (Cayman Chemical Company, Ann Arbor, MI, USA) from 200 μl of blood obtained from the saphena. At the end of the study, and before the animal was sacrificed, a blood sample was obtained from the aorta to measure the plasma levels of Thromboxane-B2 (TxB2) and 6-keto-Prostaglandin-F1α (6kPGF1α) by ELISA (Cayman Chemical Company, Ann Arbor, MI, USA). The ratio TxB2/6kPGF1α was used as an index of vasoconstriction.6
Data were expressed as mean±SEM. Statistical analysis was carried out by ANOVA. Two-way ANOVA tests were used to ascertain whether body weight and plasma corticosterone varied with time and/or group (Obstruction, Sham, and Naive). When the differences were significant, a Bonferroni post-test (GraphPad Software Inc, San Diego, CA, USA) was performed. A one-way ANOVA test was employed to assess the differences between groups in TxB2/6kPGF1α. When the differences were significant, a Newman-Keuls post-test was performed. The differences were considered significant when P <0.05.
RESULTS
Figure 1, bottom, shows an example of the signals recorded during one of the airway obstructions applied to the rats. During the 5-s period of obstruction, flow through the valve was nil owing to its closure. As a result of the breathing efforts of the rat against the closed valve, the swings in air pressure markedly increased in the head chamber. The CO2 concentration of the air in the head chamber consistently increased as a result of the occlusion. As expected, after some delay from the start of the occlusion, SpO2 exhibited a transient dip.
Along the 4 weeks of the study, Naive rats showed significantly greater weight gain (46%±5%) than rats in the Obstruction and Sham groups (27%±3% and 28%±4%, respectively); there was no significant difference between Obstructive and Sham rats. Plasma corticosterone was significantly higher in the Obstructive and Sham rats than in the Naive group, but there was no significant difference between the Obstruction and the Sham groups. In these 2 groups of instrumented rats, corticosterone levels recovered the baseline values at the end of the 4-week period: 29.7±8.1 μg/ml at day 1 and 30.2±8.5 μg/ml at day 28 for the Obstruction group, and 35.4±8.9 μg/ml at day 1 and 32.7±7.2 μg/ml at day 28 for the Sham group. TxB2 was found to increase and 6kPGF1α remained unchanged in the instrumented rats. The ratio TxB2/6kPGF1α was significantly greater in the Obstruction group (3.13±0.42) than in the Sham (1.16±0.39) and Naive (0.42±0.07) groups; the difference between the Sham and Naive groups was not significant.
DISCUSSION
The novel model designed in this work allows the rat to be chronically subjected to a pattern of respiratory events realistically mimicking the ones that characterize OSA. Indeed, the animal experienced intermittent airway obstructions resulting in increased breathing efforts and oxygen desaturations.
A number of animal models have been employed to date to investigate the mid- and long-term effects of OSA. A dog model based on the implant of an obstruction valve at the tracheal level was used to study systemic hypertension and breathing control in OSA.2 However, the relatively low availability of gene expression assays for this species in comparison with rodents have enhanced the interest in murine models. The most widely used model to investigate the effects of the respiratory disturbances in OSA consists in subjecting rodents to a hypoxia/normoxia pattern by breathing air with variable O2 concentration.3 This approach has been successfully employed to study the changes in systemic arterial pressure and rat behavior and the presence of metabolic abnormalities and cardiovascular consequences.7,8 Nevertheless, the model based on changing the air concentration does not allow us to study the potential consequences of strenuous breathing against an obstructed airway. This mechanical stimulus actually experienced by OSA patients acts as an immune challenge9 resulting in inflammatory and metabolic responses that could play a significant role in determining the consequences of OSA. However, the only study that investigated the systemic effects of recurrent airway obstructions mimicking OSA in rats required an invasive setting, with the result that only short term effects could be studied.10
To avoid manipulation of the airway of the animal, i.e., to apply a noninvasive procedure, the described model imposes airway obstruction indirectly. Virtual airway occlusion is achieved by occluding the head chamber, which was designed to reduce air volume and to provide air sealing at the neck collar. Because the airway of the animal is not actually occluded, the rat is able to breathe the very small airflow associated with the pressure changes induced by muscle effort exerted on the low compliance of the gas in the chamber. This low amplitude breathing flow accounts for the increase in CO2 concentration in the head chamber during valve obstruction (Figure 1, top). The restriction of movement imposed on the rat during the occlusions is a potential source of stress.5 Although the rats were familiarized with the setting before the application of the obstructive challenge, some stress remained as evidenced by the differences observed in weight gain and plasma corticosterone. Nevertheless, the fact that the level of corticosterone at the end of the experiment resembled that of the baseline and the observation that the animals exhibited no discomfort in the setting, suggest that the stress induced by restraint was moderate.
The use of a Sham group, which takes into account the restraining effect as well as the whole experimental procedure, allows us to analyze the effects induced by the airway obstructions exclusively. The fact that the ratio TxB2/6kPGF1α was significantly greater in the Obstruction than in both the Sham and Naive rats, together with the absence of significance in the difference between Obstruction and Sham suggests that the vasoconstriction response observed was specifically triggered by the stimulus of recurrent airway obstructions. This result should not be interpreted simply on the basis of a vasoconstriction/vasodilation imbalance. The fact that TxB2 is mainly produced by platelets suggests that, in addition to the vasoconstriction pattern, there is an involvement of platelet activation that could be related to common cardiovascular complications in patients with OSA.
The chronic model described can be readily applied to the investigation of the consequences of the respiratory events in OSA. Indeed, dose-response studies can be carried out by modifying the frequency and/or the duration of the obstructions, the number of hours of challenge per day and the total duration of the study. Implementation of this novel research tool in a series of animal studies is expected to provide new data regarding the interactions of each physiological variable across obstructive apneic events. For example, this system would allow the independent study of the modification of one physiological perturbance within or across an apnea and its resolution without significantly altering the other physiological parameters. In conclusion, the described animal model provides a useful tool for investigating the mechanisms involved in the mid- and long-term consequences of OSA.
ACKNOWLEDGEMENTS
This work was supported in part by Ministerio de Ciencia y Tecnologia (SAF2005-00110, SAF2004-00684), Ministerio de Sanidad y Consumo (FIS-PI040929), SEPAR, Carburos Metalicos, FUCAP and Ciberes (ISCiii-CB06/06/0025 and ISCiii-CB06/06/0026).
The authors wish to thank Dr. David Gozal for his helpful suggestions and Mr. Miguel A. Rodriguez for his technical assistance.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have reported no financial conflicts of interest.
REFERENCES
- 1.Caples SM, Garcia-Touchard A, Somers VK. Sleep disordered breathing and cardiovascular risk. Sleep. 2007;30:291–304. doi: 10.1093/sleep/30.3.291. [DOI] [PubMed] [Google Scholar]
- 2.Kimoff RJ, Brooks D, Horner RL, et al. Ventilatory and arousal responses to hypoxia and hypercapnia in a canine model of obstructive sleep apnea. Am J Respir Crit Care Med. 1997;156:886–94. doi: 10.1164/ajrccm.156.3.9610060. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher EC. Invited review: physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol. 2001;90:1600–5. doi: 10.1152/jappl.2001.90.4.1600. [DOI] [PubMed] [Google Scholar]
- 4.Farre R, Rotger M, Montserrat JM, Calero G, Navajas D. Collapsible upper airway segment to study the obstructive sleep apnea syndrome in rats. Respir Physiol Neurobiol. 2003;36:199–209. doi: 10.1016/s1569-9048(03)00082-x. [DOI] [PubMed] [Google Scholar]
- 5.Marin MT, Cruz FC, Planeta CS. Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiol Behav. 2007;90:29–35. doi: 10.1016/j.physbeh.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 6.Bolla M, You D, Loufrani L, et al. Cyclooxygenase involvement in thromboxane-dependent contraction in rat mesenteric resistance arteries. Hypertension. 2004;43:1264–9. doi: 10.1161/01.HYP.0000127438.39744.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gozal D, Reeves SR, Row BW, Neville JJ, Guo SZ, Lipton AJ. Respiratory effects of gestational intermittent hypoxia in the developing rat. Am J Respir Crit Care Med. 2003;167:1540–7. doi: 10.1164/rccm.200208-963OC. [DOI] [PubMed] [Google Scholar]
- 8.Campen MJ, Shimoda LA, O'Donnell CP. Acute and chronic cardiovascular effects of intermittent hypoxia in C57BL/6J mice. J Appl Physiol. 2005;99:2028–35. doi: 10.1152/japplphysiol.00411.2005. [DOI] [PubMed] [Google Scholar]
- 9.Vassilakopoulos T, Roussos C, Zakynthinos S. The immune response to resistive breathing. Eur Respir J. 2004;24:1033–43. doi: 10.1183/09031936.04.00067904. [DOI] [PubMed] [Google Scholar]
- 10.Nacher M, Serrano-Mollar A, Farre R, Panes J, Segui J, Montserrat JM. Recurrent obstructive apneas trigger early systemic inflammation in a rat model of sleep apnea Respir Physiol Neurobiol. 2007;155:93–6. doi: 10.1016/j.resp.2006.06.004. [DOI] [PubMed] [Google Scholar]