Abstract
Study Objective:
To study prospectively the relations of insomnia to the development of anxiety disorders and depression in a population-based sample.
Design:
Cohort study based on data from 2 general health surveys of the adult population.
Setting:
Two general health surveys in the adult population in Nord-Trøndelag County of Norway, HUNT-1 performed in 1984–6 and HUNT-2 in 1995–7
Participants:
Participants without significant anxiety and depression in HUNT-1 were categorized according to the presence and absence of insomnia in the 2 surveys (N=25,130).
Measurements and Results:
Anxiety disorders and depression in HUNT-2 were assessed by the Hospital Anxiety and Depression Scale and analyzed using multivariate logistic regression analysis adjusted for age, gender, education, comorbid depression/anxiety, and history of insomnia. Anxiety disorders in HUNT-2 were significantly associated with the group with insomnia in HUNT-1 only (OR 1.6; 95% CI, 1.1–2.3), the group with insomnia in HUNT-2 only (OR 3.4; 95% CI, 3.1–3.8), as well as with the group with insomnia in both surveys (OR 4.9; 95% CI, 3.8–6.4). Depression in HUNT-2 was significantly associated with the group with insomnia in HUNT-2 only (OR 1.8; 95% CI, 1.6–2.0), but not with the groups with insomnia in HUNT-1 only or with insomnia in both surveys.
Conclusions:
Only a state-like association between insomnia and depression was found. In addition to being a state marker, insomnia may be a trait marker for individuals at risk for developing anxiety disorders. Results are consistent with insomnia being a risk factor for the development of anxiety disorders.
Citation:
Neckelmann D; Mykletun A; Dahl AA. Chronic insomnia as a risk factor for developing anxiety and depression. SLEEP 2007;30(7):873–880.
Keywords: Insomnia, anxiety disorders, depression, cohort study, risk factors
INTRODUCTION
SLEEP DISTURBANCES ARE COMMON IN MOST FORMS OF PSYCHOPATHOLOGY.1 THERE ARE SEVERAL INDICATIONS THAT THE RELATIONSHIP BETWEEN AFFECTIVE disorders and sleep disturbances goes beyond mere co-occurrence. Most somatic treatments for depression have a specific inhibitory effect on REM sleep, and acute sleep deprivation has antidepressant effects.2 A disturbed sleep pattern predicts poor response to psychotherapy better than does the severity of depression.3 This close relation between the neurobiology of sleep and of affective disorders has been used to formulate hypotheses of the neurobiology of depression (original publications include2,4–6). There has been a particular focus on the distinct patterns of monoaminergic, serotonergic, and cholinergic neuromodulation associated with each of the different sleep/wake states.7 Sleep is also contingent upon reduced thalamic input8 of which a major source is cortex.9–11 Psychopathological states can be assumed to affect descending corticothalamic activity and thus both the initiation and maintenance of sleep by impairing the thalamic dampening of sensory information that characterizes sleep.8,12
Insomnia has been suggested to cause depression and/or anxiety disorders.13,14 However, most studies on the consequences of insomnia have either been cross-sectional or have used a follow-up interval of only one to two years. Since the prodromal period of a first episode of major depression or anxiety disorder may be up to 8–10 years,15,16 studies with shorter follow-up intervals do not cover a sufficient time span to distinguish between prodromal symptoms and putative risk factors.
Insomnia is also common in anxiety disorders13,15,17–19 which are highly comorbid with depression.20–22 This comorbidity must be addressed to avoid a confounding effect of anxiety when the effects of insomnia on the risk of developing major depression are studied,14,23 as insomnia has been reported to predict the later occurrence of anxiety disorder.13,24–26
In the current study we aimed to test the following 3 hypotheses about the associations between insomnia and anxiety disorder/depression: 1) Insomnia is a trait marker of a general vulnerability to anxiety disorder and depression, present whether or not these disorders are present; 2) Insomnia is a state marker of current anxiety disorder and depression, present when these disorders are present; and 3) Insomnia is associated with an exposure-related increased risk of developing anxiety disorder and depression.
METHODS
Design
This cohort study uses data from 2 general health surveys of the adult population in the Norwegian county of Nord-Trøndelag, performed in 1984–6 (HUNT-1) and 1995–7 (HUNT-2). Both surveys were carried out in the same 2-stage sequence. Over a period of 2 years, all inhabitants aged ≥20 years received a personal invitation to participate in the survey. A general health questionnaire accompanying the invitation was returned at the appointed examination. Anthropometric measures and blood pressure were recorded. A blood sample was drawn only in HUNT-2. Participants were given a supplementary questionnaire to be returned by mail within 2 weeks. Non-responders received one reminder.27 HUNT-2 was approved by the Norwegian Data Inspectorate and the Regional Committee for Ethics in Medical Research, while these official organs were not established at the time of HUNT-1 in 1984. All participants gave written informed consent.
Selection of Sample
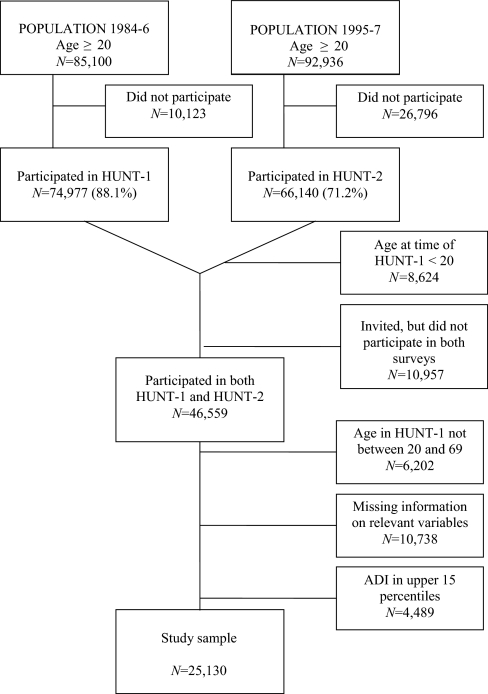
A flow diagram of the selection is presented in Figure 1. The population analyzed in this paper consists of the individuals that participated in both HUNT-1 and HUNT-2 and were aged 20–69 years at the time of HUNT-1. In a previous study, 10% of non-attenders in HUNT-1 were compared to attenders in an attempt to study why they did not take part in the survey.28 Reasons for non-attendance in HUNT-1 included being too busy (the dominating reason in the age range 20–55 years), being not interested in participating in a study, having moved, and health problems (the dominating reason in those aged ≥60 years). Non-attenders had a somewhat higher morbidity than attenders, but only 12% of the non-attenders reported health problems as a reason. Reasons for non-attendance in HUNT-2 were studied in a random sample (2%, N=685) of non-attenders.29 The most important reason for non-attending in age group 20–69 years was lack of time/moved away (54%), while in those aged ≥70 years, immobilizing disease (21%) and follow-up by their own physician (28%) were important reasons. Causes for non-attendance in HUNT-2 for HUNT-1 attenders include death, migration, and the general reasons for non-attendance as mentioned above. Reasons for HUNT-2 attendees not to have participated in HUNT-1 include age ≤20 years at the time of HUNT-1, not living in Nord-Trøndelag at the time of HUNT-1, as well as those of general non-attendance. The migration rate in Nord-Trøndelag in this time period was 0.3% per year.27 The age distribution of the sample in this study differed from the whole HUNT-2 sample in that there were fewer individuals in the age group 30–39 years. This may have been caused by the design; the HUNT-1 study was sampling from 1984–86, while the HUNT-2 sampling was from 1995–97. The sex distribution of the study sample was comparable to the whole HUNT-2 sample.
Figure 1.
Flow Diagram of Sample Selection
Since our goal was to identify factors associated with the later onset of anxiety and depression as reported in HUNT-2, we excluded individuals with significant anxiety and depression in HUNT-1 from the study sample using an anxiety and depression index (ADI). Twelve questions on anxiety and depression were included in HUNT-1.30 A one-dimensional anxiety and depression symptom index (the ADI-12 Index) based on these 12 items correlated strongly (r = 0.82) with the Hopkins Symptoms Checklist-25 (HSCL-25 anxiety and depression subscales) in a subsample re-examined for other purposes after baseline screening.30 The test-retest correlation for the ADI-12 Index was considered good (r = 0.66, with a 2-year time-lag).30 Five of the ADI-12 items correspond to ICD-10 criteria of F32 Depressive episode, and 3 items correspond to ICD-10 criteria of F41.1, generalized anxiety disorder. Two items concern feelings of being under pressure, one concerns use of tranquillizers or sleeping pills, and one concerns being more aware of responsibilities than other persons. We modified the ADI by removing the 2 items “Sleep disturbance” and “Use of sleeping pills” from the original 12-item list. A secondary analysis was performed with only the individuals with ADI scores in the lowest 20th percentile included to verify that the associations observed were not due to subclinical anxiety and/or depression in HUNT-1. Age and sex characteristics of the study sample are given in Table 1.
Table 1.
Age and Sex Characteristics at the Time of the HUNT-2 Survey
| Age groups | Female | Male | Total |
|---|---|---|---|
| 30–39 years | 1,738 | 2,072 | 3,810 |
| 40–49 years | 3,242 | 3,678 | 6,920 |
| 50–59 years | 2,893 | 3,097 | 5,990 |
| 60–69 years | 2,284 | 2,339 | 4,623 |
| 70–79 years | 1,935 | 1,852 | 3,787 |
| Total | 12,092 | 13,038 | 25,130 |
Dependent Variables
In HUNT-2 the Hospital Anxiety and Depression Scale (HADS) was used to self-rate symptoms of anxiety and depression.31 The HADS does not include somatic symptoms of anxiety and depression (such as fatigue and insomnia). It is a widely used questionnaire for anxiety disorders and depression in population studies, primary care, and in hospital settings.32 The HADS consists of 7 items for depression (HADS-D) and 7 for anxiety (HADS-A). Each item is scored from 0 (not present) to 3 (maximally present). HADS-D is mainly focused on anhedonia, which by many is seen as the core feature of depression,31 while HADS-A mainly contains items related to generalized anxiety disorder, particularly as conceptualized by Rickels.33 The psychometric properties of HADS in HUNT-2 have been published elsewhere.34 In this paper the term “anxiety disorders” refers to a score of 8 or more on the anxiety subscale (HADS-A), and “depression” to a score of 8 or more on the depression (HADS-D) subscale. A cut-off score of 8 for disorders is in accordance with other published studies, resulting in a sensitivity and specificity of 0.80 with respect to DSM-IV defined anxiety disorders and depression.32 In HUNT-2 HADS was completed by 62,491 individuals (94.5% of the participants). The prevalence of anxiety disorders and depression by age and sex are given in Table 2.
Table 2.
Prevalence in Percent (CI) of HADS Defined Depression, Anxiety Disorders and DIMS by Age and Gender at the Time of the HUNT-2 Survey
| Age groups | Depression |
Anxiety |
DIMS |
|||
|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | |
| 30–39 years | 5.4 (4.4–6.4) | 5.1 (4.1–6.2) | 14.0 (12.5–15.5) | 9.7 (8.3–11.1) | 6.4 (5.4–7.5) | 5.4 (4.3–6.5) |
| 40–49 years | 6.3 (5.5–7.1) | 7.8 (6.9–8.7) | 13.8 (12.7–14.9) | 10.6 (9.6–11.7) | 10.0 (9.1–11.0) | 8.6 (7.6–9.6) |
| 50–59 years | 8.1 (7.1–9.1) | 10.6 (9.5–11.7) | 13.2 (12.0–14.4) | 9.3 (8.2–10.3) | 17.2 (15.9–18.6) | 10.4 (9.3–11.5) |
| 60–69 years | 9.6 (8.4–10.8) | 10.1 (8.8–11.3) | 12.4 (11.0–13.7) | 5.8 (4.9–6.8) | 19.0 (17.4–20.6) | 13.0 (11.7–14.4) |
| 70–79 years | 11.9 (10.4–13.4) | 13.7 (12.2–15.2) | 11.1 (9.6–12.5) | 5.2 (4.2–6.2) | 23.2 (21.3–25.1) | 11.2 (9.8–12.6) |
Independent Variables
Insomnia
Insomnia is the subjective feeling of having difficulties initiating or maintaining sleep (DIS and DMS respectively, jointly referred to as DIMS) or of non-restorative sleep. Insomnia is considered to be chronic if present most nights for at least a month (DSM-IV). The questionnaires in HUNT included questions about frequency of DIS and DMS last month with 4 possible responses (Never/Occasionally/Often/Almost every night). DIS and DMS were dichotomized into ‘Present’ (Often or Almost every night) and ‘Absent’ (Never or Occasionally). Chronic insomnia (DIMS) was coded as present if DIS and/or DMS were present. In HUNT-2, questions about DIMS were answered by 54,374 individuals (82.2% of the participants). The prevalence of DIMS by age and sex is given in Table 2.
We divided the study sample into 4 groups based on the presence and absence of DIMS in HUNT-1 and HUNT-2 as follows: The first group consisted of individuals that did not report DIMS in either survey, referred to as (group −/−). The second group of individuals that reported DIMS in HUNT-1, but not in HUNT-2, referred to as (group H1/−). The third group individuals that reported DIMS in HUNT 2, but not in HUNT-1 (group −/H2). The fourth group consisted of individuals that reported DIMS in both surveys (group H1/H2). Group membership was coded by the variable History of DIMS (see Table 3).
Table 3.
Sex, Age, Education, Depression, and Anxiety Disorders by History of DIMS
| History of DIMS | No DIMS (−/−) | HUNT-1 only (H1/−) | HUNT-2 only (−/H2) | HUNT-1&2 (H1/H2) | Total | |
|---|---|---|---|---|---|---|
| Sex | χ2=163, df=3, P <0.001 | |||||
| (%) | Female | 10,930 (50.3) | 198 (62.3) | 1,674 (60.7) | 236 (69.6) | 13,038 (51.9) |
| Male | 10,785 (49.7) | 120 (37.7) | 1,084 (39.3) | 103 (30.4) | 12,092 (48.1) | |
| Total | 21,715 | 318 | 2,758 | 339 | 25,103 | |
| Age (95% CI) | χ2=24.8, df=3, P<0.001 | 53.0 (52.8–53.2) | 57.2 (55.75–8.7) | 57.0 (56.6–57.5) | 62.2 (60.9–63.5) | 53.6 (53.5–53.8) |
| Education | χ2=249, df=6, P <0.001 | |||||
| (%) | < 9 years | 8,632 (39.8) | 167 (52.5) | 1,435 (52.0) | 217 (64.0) | 10,451 (41.6) |
| 9 to 12 years | 9,128 (42.0) | 115 (36.2) | 963 (34.9) | 95 (28.0) | 10,301 (41.0) | |
| > 12 years | 3,955 (18.2) | 36 (11.3) | 360 (13.1) | 27 (8.0) | 4,378 (17.4) | |
| Depression | χ2=446, df=3, P <0.001 | |||||
| HADS-D ≥ 8 | Case | 1,571 (7.2) | 34 (10.7) | 521 (18.9) | 55 (16.2) | 2,181 (8.7) |
| (%) | Non-case | 20,144 (92.8) | 284 (89.3) | 2,237 (81.1) | 284 (83.8) | 22,949 (91.3) |
| Anxiety | χ2=978, df=3, P<0.001 | |||||
| HADS-A ≥8 | - Case | 1,836 (8.5) | 42 (13.2) | 737 (26.7) | 101 (29.8) | 2,716 (10.8) |
| (%) | Non-case | 19,879 (91.5) | 276 (86.8) | 2,021 (73.3) | 238 (70.2) | 22,414 (89.2) |
In HUNT-2 individuals aged 20–69 years were asked about the frequency of sleeplessness the year prior to their HUNT-2 visit and whether their sleeplessness had affected their work performance. The responses to these questions were used to assess whether the groups defined by the variable History of DIMS differed with respect to prevalence of sleep disturbance in the period between the 2 surveys (see Results).
Other Putative Prodromal Markers
To assess to what extent DIMS was unique among putative prodromal symptoms in predicting anxiety disorders or depression, we used the response “Discontent” to the question “How do you feel about your life?” in the HUNT-1 survey as a putative prodromal symptom of these conditions.
Sociodemographic Variables
Age was analyzed in decades. Education was divided into 3 levels, based on duration of education (< 10 years, 10–12 years, >12 years).
Statistical Analysis
Distributions of nominal and ordinal characteristics were tested with the Pearson χ2 test. Somers' d was used as a directional measure of ordinal associations. Differences in HADS scores and age were tested with the median test.
The effects of History of DIMS on the presence of anxiety disorders and depression at the time of HUNT-2 were analyzed by linear, multi-nominal, and logistic regression models. All 3 types of regression analyses yielded similar results, and therefore we present results from the logistic regression analyses only (Table 4).
Table 4.
Adjusted OR (95% CI) from Multivariate Logistic Regression Analyses of HUNT-2 Depression, Anxiety, and Comorbid Anxiety and Depression. In the Analysis of Comorbid Anxiety and Depression, Individuals with Either Anxiety Disorders or Depression only were Excluded (See Text).
| Independent variables | N | Characteristics | Depression OR (95%CI) | Anxiety OR (95% CI) | N | Anxiety & Depression OR (95% CI) |
|---|---|---|---|---|---|---|
| History of DIMS | 21,715 | −/− | Reference | Reference | 19,458 | Reference |
| 318 | H1/− | 1.2 (0.8–1.8) | 1.6 (1.1–2.3) | 262 | 1.4 (0.7–2.6) | |
| 2,758 | −/H2 | 1.8 (1.6–2.0) | 3.4 (3.1–3.8) | 2,134 | 5.8 (5.0–6.6) | |
| 339 | H1/H2 | 1.1 (0.8–1.6) | 4.9 (3.8–6.4) | 249 | 5.4 (3.7–7.9) | |
| Sex | 12,092 | Male | Reference | Reference | 10,731 | Reference |
| 13,038 | Female | 0.6 (0.6–0.7) | 1.7 (1.5–1.8) | 11,372 | 1.1 (1.0–1.3) | |
| Age group | 3,810 | 30–39 years | Reference | Reference | 3,387 | Reference |
| 6,920 | 40–49 years | 1.3 (1.1–1.6) | 0.9 (0.8–1.0) | 6,128 | 1.2 (0.9–1.5) | |
| 5,990 | 50–59 years | 1.8 (1.5–2.1) | 0.6 (0.6–0.7) | 5,261 | 1.1 (0.9–1.2) | |
| 4,623 | 60–69 years | 1.9 (1.6–2.3) | 0.4 (0.4–0.5) | 4,077 | 0.9 (0.7–1.1) | |
| 3,787 | 70–79 years | 2.7 (2.3–3.3) | 0.3 (0.3–0.4) | 3,250 | 0.8 (0.6–1.1) | |
| Education | 10,451 | < 9 years | Reference | Reference | 8,995 | Reference |
| 10,301 | 9–12 years | 0.8 (0.7–0.9) | 0.9 (0.8–1.0) | 9,132 | 0.7 (0.6–0.8) | |
| 4,378 | 12 + years | 0.6 (0.6–0.7) | 0.8 (0.7–0.9) | 3,976 | 0.5 (0.4–0.6) | |
| Comorbidity | No | Reference | Reference | |||
| N=22,414 | N=22,949 | |||||
| Yes | N=2,716 | N=2,181 | ||||
| 9.4 (8.5–10.5) | 9.3 (8.4–10.4) | |||||
| N in the analysis | 25,130 | 25,130 | 22,103 |
The α level was set at P = 0.05, and reported P values are the results of 2-sided tests. All confidence intervals (CI) reported are at the 95% level.
All statistical analyses were made with SPSS 13.0 (SPSS Inc, Chicago, IL).
RESULTS
Sample
The selection of the sample is shown in Figure 1. The age and sex characteristics of the sample are given in Table 1.
Prevalence of DIMS, Anxiety Disorders, and Depression in HUNT-2
The point prevalence of DIMS, anxiety disorders, and depression by age and gender at the time of HUNT-2 are given in Table 2. The prevalence of DIMS increased with age (χ2.= 350.9, df = 4, P <0.001, Somers' d = 0.20, t = 18.9, P <0.001). Females tended to report more DIMS than males (χ2̣= 135.6, df = 1, P <0.001). Detailed analyses of the prevalence of depression have been published elsewhere.35 Logistic regression analyses yielded crude odds ratios (CI) of HUNT-2 DIMS for HADS defined anxiety disorders of 4.0 (3.6–4.4, Wald=860.6, df=1, P <0.001), and for depression of 2.9 (2.6–3.2, Wald = 406.3, df = 1, P <0.001). Odds ratios (CI) of HUNT-2 DIMS adjusted for age, sex, and education for HADS defined anxiety disorders were 4.2 (3.8–4.6, Wald = 859.4, df=1, P <0.001), and depression 2.7 (2.5–3.0, Wald = 343.8, df=1, P <0.001).
Characteristics of Groups Defined by History of DIMS
Age, gender, education level, anxiety disorders and depression grouped by the variable History of DIMS are given in Table 3. Significant gradients (−/− < H1/− < −/H2 < H1/H2) were evident with respect to both symptom burden (Data not shown; HAD-D score: Somers' d = 0.26, t = 24.5, P <0.001; HAD-A score: Somers' d = 0.33, t = 28.7, P <0.001) and prevalence of disorder (HADS defined depression: Somers' d = 0.159, t = 15.4, P <0.001;
HADS defined anxiety disorders: Somers' d = 0.214, t = 21.6, P <0.001).
Sleep disturbances the year prior to the HUNT-2 survey (frequency of sleeplessness and impaired work performance attributed to sleep problems) showed gradients related to History of DIMS. Significant gradients (−/− < H1/− < −/H2 < H1/H2) were observed for frequency of sleeplessness (Somers' d = 0.66, t = 47.2, P <0.001) as well as for impaired work performance (Somers' d = 0.30, t = 28.4, P <0.001).
Associations of History of DIMS to Anxiety Disorders and/or Depression in HUNT-2
The adjusted ORs for HUNT-2 depression and anxiety disorders from multivariate logistic regression models that included the variables History of DIMS, age, gender, educational level, and comorbidity (coexistence of depression when analyzing for anxiety disorder, and vice versa) are given in Table 4. Chronic insomnia (operationalized as DIMS) was associated with the development of anxiety disorders (History of DIMS: Wald = 583, df = 3, P <0.001) as well as depression (History of DIMS: Wald=81.1, df = 3, P <0.001). Support for chronic insomnia being a trait marker for vulnerability to develop the disorder (OR associated with the History of DIMS group H1/− significantly larger than that of the reference group [−/−]) was found for anxiety disorders, but not for depression. Support for chronic insomnia as a state marker (OR associated with the group −/H2 significantly larger than that of [−/−]) was found for both anxiety disorders and depression. Support for chronic insomnia being associated with an exposure-related increase in the risk of developing the disorder (The OR associated with the group H1/H2 significantly larger than that of the group [−/H2]) was found for anxiety disorders, but not for depression.
There were no significant interactions between History of DIMS and gender for either depression (χ2= 6.99, df = 3, P = 0.07) or anxiety disorders (χ2= 0.70, df = 3, P = 0.87).
Reflecting the comorbidity of HADS-defined anxiety disorders and depression in HUNT-2, the variable most strongly associated with depression was having anxiety disorders (Wald = 1718, df = 1, P <0.001), and for anxiety disorders it was having depression (Wald = 1702, df = 1, P <0.001).
Female gender was associated with increased risk for anxiety disorders (Wald = 121.3, df = 1, P <0.001) and decreased risk for depression (Wald = 88.1, df=l, P <0.001). There were effects of age on the risk of both anxiety disorders (age group: Wald = 229, df = 4, P <0.001) and depression (age group: Wald = 136, df = 4, P <0.001). Educational level was associated with the risk of both anxiety disorders (Wald = 12.4, df = 2, P <0.01) and depression (Wald = 61.5, df = 2, P <0.001).
Since including the comorbid condition as an independent variable in the model may lead to overadjusting, a separate analysis was performed to study the ORs of developing a comorbid condition, after excluding individuals with either anxiety disorders or depression only (Table 4, model Anxiety & Depression). The comorbid group had a higher HADS-A score than the group with anxiety disorders only (10.7 vs. 9.42 χ2=183, df = 1, P <0.001), as well as a higher HADS-D score than the group with depression only (9.9 vs. 9.22 χ2= 92.4, df = 1, P <0.001).
We repeated the analyses of the multivariate logistic regression models in Table 4 using as a sample only the participants with HUNT-1 ADI scores within the lower 20th percentile (n = 5,499) to reduce the probability that the observed associations were caused by individuals in HUNT-1 with subsyndromal anxiety disorder or depression. As expected, this resulted in wide confidence intervals due to small group sizes (group H1/− [n = 17] and group H1/H2 [n = 18]). Numerically the ORs of the association between DIMS and anxiety disorder increased compared to the study sample (History of DIMS: Wald = 95.3, df = 3, P <0.001; ORs H1/−: 1.3 [0.2–11.4], −/H2: 5.0 [3.5–7.1], H1/H2: 17.9 [5.2–61.5]), while the ORs of the associations between DIMS and depression were stable (History of DIMS: Wald=9.1, df=3, P <0.05; ORs H1/−: 1.5 [0.1–15.5], −/H2: 1.9 [1.3–3.0], H1/H2: 1.0 [0.2–5.5]).
In a supplementary analysis to assess whether DIMS in HUNT-1 was unique as a prodromal marker for anxiety disorders or depression in HUNT-2 we included the HUNT-1 variable “Contentment with life” in the multivariate logistic regression models presented in Table 4. Being discontent with life in the HUNT-1 survey, putatively a negative affective symptom, was associated with depression (Wald 4.0, df=1; P<0.05; OR 2.2 [CI, 1.0–4.8]) but not anxiety disorders (Wald 0.9, df=1, P = 0.36; OR 0.6 [CI, 0.2–1.7]) in the HUNT-2 survey. The associations of History of DIMS to HADS-defined anxiety disorders and depression did not change.
It has been suggested that use of hypnotics or tranquilizers may increase the incidence of depression or anxiety.36,37 The participants in HUNT-1 were asked how frequently they had used hypnotic or tranquilizing drugs in the last month. The available hypnotics in Norway at the time of HUNT-1 were all benzodiazepines (BZD). At the time of HUNT-2, zopiclone was the most prescribed hypnotic drug. In the study sample, the association between frequency of BZD use in HUNT-1 (“Never” N=23,059, “Less than weekly” N=1,143, “Weekly but not every day” N=417 and “Daily” N=266) and depression and anxiety disorders in HUNT-2 were analyzed. The crude associations of this variable were significant both with respect to depression (Wald = 96.6, df = 3, P <0.001) and anxiety disorders (Wald = 191.5, df = 3, P <0.001). Among BZD users, there was no dose-response association between frequency of use and outcome.
To reduce the probability that the observed associations were caused by individuals in HUNT-1 with subsyndromal anxiety disorders or depression, repeat-analyses were performed, using as a sample only the HUNT-1 participants with very low levels of anxiety and depressive symptoms (ADI scores within the lower 20th percentile [N=5,113]). In this sample the crude associations between frequency of use (“Never” N=5,000, “Less than weekly” N=86, “Weekly but not every day” N=15, and “Daily” N=12) and HUNT-2 depression (Wald=4.70, df=3, N.S, Reference category “Never”; OR “Less than weekly”= 0.92, “Weekly but not daily”=0.00, “Daily”=0.96) and anxiety (Wald=2.83, df=3, N.S. Reference category “Never”; OR “Less than weekly”= 0.34, “Weekly but not daily”=1.28, “Daily”=1.32) were all non-significant.
DISCUSSION
Main Results
In this population-based cohort study based on data from 2 general health surveys performed 11 years apart, we found significant relations between the longitudinal course of chronic insomnia (DIMS) and the development of anxiety disorders. Compared to the group of participants without chronic insomnia in both HUNT-1 and HUNT-2 (History of DIMS group −/−), the groups with DIMS in HUNT-1 only (H1/−), in HUNT-2 only (−/H2) and the group with chronic insomnia in both HUNT-1 and HUNT-2 (H1/H2) all had increased associations to having developed anxiety disorders. The association to developing an anxiety disorder was significantly stronger for the group H1/H2 than for the group −/H2. This suggests that chronic insomnia may be a trait marker for individuals at risk for developing anxiety disorders; a state marker of anxiety disorders. Furthermore the results were consistent with the hypothesis that chronic insomnia may confer a risk related to the accumulated exposure for the development of anxiety disorders.
Compared to individuals without chronic insomnia in both HUNT-1 and HUNT-2 (−/−), only the group −/H2 had an increased association to having developed depression. This suggests that chronic insomnia is a state marker of depression. We did not find evidence to suggest that chronic insomnia was either a trait marker for increased risk of developing depression, or that it conferred an exposure-related increase in the risk of developing depression.
Strengths and Limitations
We believe there are several important methodological advantages of the current study in addition to the prospective design with a large sample that is representative with respect to demographic characteristics.
First, the analyses were adjusted for comorbidity of anxiety disorder and depression. An extensive comorbidity of anxiety disorder and depression has been found in the general population.20–22,38 To have either anxiety disorder or depression at the time of HUNT-2 was the major risk factor for the other disorder (ORs >9 in our multivariate models). Sleep disturbances are known to be associated with both anxiety disorder and depression. Our results substantiated the concern expressed by others that comorbid anxiety disorders might have confounded observed associations between the symptoms of disturbed sleep and depression.14,23
Second, the 11-year interval between index and follow-up survey can be expected to ensure that few individuals in the first survey are in a prodromal phase of an episode of depression and anxiety disorder observed in the second survey. The prodromal period for a first onset of a major depressive episode or an anxiety disorder is long,16 and associations observed in studies with a follow-up time shorter than the prodromal period may be caused by insomnia being a symptom in the prodromal phase of the disorder rather than a risk factor. In support of this argument are the results from several studies that have analyzed sleep disturbances and other affective symptoms at baseline with respect to the subsequent development of major depression. These studies have reported that several other affective symptoms or symptom domains were associated more strongly with the development of depression than was insomnia.15,23,25 Consistent with this, we found that reporting discontentment with life in HUNT-1 was significantly associated with depression in HUNT-2, whereas DIMS in HUNT-1 (History of DIMS group H1/-) was not. There was no significant association between reporting discontentment with life in HUNT-1 and anxiety disorders in HUNT-2. We are aware of only 2 studies that have used a follow-up time of sufficient duration to address this problem. A cohort study of 1,053 male medical school students at the Johns Hopkins University39 with a median follow-up period of 34 years (range 1–45) found the relative risk of clinical depression to be increased in those who reported insomnia in medical school (relative risk [RR] 2.0) and in those with difficulty sleeping under stress in medical school (RR 1.8) compared with those who did not report sleep difficulties. Insomnia was also related to midlife psychiatric distress as assessed with the General Health Questionnaire. Adjustments for possible confounding anxiety disorders were not made. To what extent results from this cohort of young males of high sociodemographic status can be generalized to the general population has been questioned.
The other study examined the associations between persistent childhood sleep problems and adulthood.24 Parent-reported sleep problems in children that persisted from ages 5–7 years to 9 years of age were found to predict adulthood anxiety disorders (OR=1.60), but not depressive disorders (OR=0.99) at age 21 and/or 26. The authors conclude that persistent sleep problems in childhood may be an early risk indicator of anxiety in adulthood.
Third, the dependent variables were measured using a validated diagnostic instrument that does not contain sleep disturbances as a symptom (the HADS).31 Others have reported inflated associations between the mental disorders and sleep disturbances as a consequence of the latter being a diagnostic criterion for depression and anxiety disorders. These authors found an attenuation of 30%-40% in the observed ORs between insomnia and depression when sleep disturbances were omitted from the diagnostic criteria of depression.23,25
Fourth, HADS in being a dimensional instrument enabled us to use multiple thresholds to differentiate between disorder and no-disorder as well as linear regressions to assess the stability of the reported relations. Increasing the threshold for disorder elevated the observed ORs but predictably widened the confidence intervals due to a decrease in the number of cases. The results reported here were calculated using the accepted thresholds for defining disorder.32 While having depression or anxiety disorder according to the HADS does not overlap fully with any DSM-IV diagnoses, the documented sensitivity and specificity of HADS-defined disorders are good, and the dimensions are valid expressions of anxiety and depression.34
We excluded from the sample individuals with a clinically significant symptom burden of anxiety and/or depression in HUNT-1 based on an earlier published index measure (ADI).30 The observed associations between DIMS and anxiety increased numerically, while the associations between DIMS and depression did not change when we analyzed the subsample selected for very low levels of anxiety or depressive symptoms in HUNT-1 as assessed by ADI. These results further reduce the probability that our findings are explained by subsyndromal anxiety or depression at the time of HUNT-1.
The validity of grouping the respondents according to the variable History of DIMS depends on the assumption that the 4 groups differed with respect to the exposure to DIMS in the study period. Our data support that assumption. The chronic course of DIMS in this sample is evident from Table 3; of the 657 individuals with DIMS in HUNT-1, more than half (N=339) also had DIMS in HUNT-2. The groups defined by History of DIMS differed with a statistically and clinically significant gradient (−/− < H1/− < −/H2 < H1/H2) with respect to sleep disturbances in the year prior to HUNT-2 (“Frequency of sleeplessness” and “Work impairment attributed to sleep problems”); e.g., the prevalence of reporting sleeplessness “once a week” or “more than once a week” in the different groups was (−/−: 6.5%) < (H1/−: 26.5%) < (−/H2: 62.1%) < (HI/H2: 84.6%). These results suggest differential exposure to DIMS in the study period for the History of DIMS defined groups and thus the validity of our approach.
Our data on sleep disturbances are limited to DIMS. We have no information on the relations of nonrestorative sleep or hypersomnia to anxiety disorder and depression.
Due to our design based on the 2 health surveys 11 years apart, the absence or presence of periods of anxiety disorders or depression in the interval between the 2 surveys is unknown. The study population was selected for having less anxiety and depression than the normal population by excluding individuals with a high ADI in HUNT-1 (the upper 15th percentile). As expected, low prevalences of anxiety disorders and depression were thus observed in the study sample at the time of the HUNT-2 survey compared to the full sample from HUNT-2 (data not shown). The consequence of an underestimation of accumulated morbidity would be an underestimation of the effect size of risk factors. The finding that an affective symptom like “discontent with life” in HUNT-1 was significantly associated with depression in HUNT-2 supports the validity of our design, and further suggests that even putatively modest risk factors could be identified with this design. The use of hypnotics or tranquilizers in HUNT-1 was associated with having developed depression or anxiety disorders in HUNT-2. This association did not increase as a function of frequency of use, in either crude or multivariate adjusted models. Among the participants with very low levels of anxiety and depressive symptoms in HUNT-1 (sample defined by ADI scores within the lower 20th percentile [n=5,499]) the use of hypnotics or tranquilizers in HUNT-1 was not associated with having developed depression or anxiety disorders in HUNT-2. This loss of significance and reduced ORs (not shown) in this latter population contrasts with the stable significance and increased ORs of the association between History of DIMS and HUNT-2 anxiety in the same subsample. This may suggest that observed association between the use of hypnotics or tranquilizers in HUNT-1 and depression or anxiety disorders in HUNT-2 could be a result of subsyndromal anxiety or depression at the time of HUNT-1.
Since separate data for use of hypnotic and tranquilizers were not available, to what extent the use of these drugs represents hypnotic use or treatment of a psychiatric condition is not known.
Studies of clinical populations with DIMS have generally shown surprisingly little difference in the amount of sleep compared to good sleepers as assessed by polysomnographic evaluation.40 Some studies of sleep EEG in subjects with self-reported DIMS have demonstrated distinct changes in frequency bands that are compatible with increased levels of arousal.41 It is not possible through an epidemiological study to study the neurobiological mechanisms underlying the associations between prolonged DIMS and the development of anxiety disorders. There may be common risk factors of possibly neuromodulatory origin for both conditions that may be aggravated by the development of DIMS. Alternatively, altered corticothalamic activity associated with insomnia may predispose to the development of anxiety through other pathophysiological mechanisms, possibly related to hyper-arousal and lack of coping mechanisms. One possible theoretical framework for the observed results is given by the cognitive activation theory of stress.42
In conclusion, these results suggest that chronic insomnia may be a trait marker for individuals at risk for developing anxiety disorders. Chronic insomnia is a state marker of both anxiety disorder and depression. The results were consistent with the notion that chronic insomnia may confer a risk related to the accumulated exposure for the development of anxiety. We did not find evidence suggesting that chronic insomnia was either a trait marker for risk of developing depression or that it conferred an exposure related increase in the risk of developing depression. A study with more detailed information about the cumulative exposure to chronic insomnia and accumulated morbidity would be needed to exclude similar relations to depression.
From a clinical point of view, these results imply that individuals reporting DIMS, in addition to receiving adequate treatment for their sleep disturbance should be carefully examined for the presence of anxiety disorder as well as depression. Focus on DIMS as a symptom of both anxiety and depression may facilitate the early detection of a mental disorder as well as the detection of comorbidity. Though not demonstrated, alleviating DIMS may reduce the risk of developing anxiety disorders.
ACKNOWLEDGEMENT
The Nord-Trøndelag Health Study (the HUNT study) is a collaboration between HUNT Research Centre, Faculty of Medicine, Norwegian University of Science and Technology (Verdal), Norwegian Institute of Public Health, and Nord-Trøndelag County Council.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have reported no financial conflicts of interest.
REFERENCES
- 1.Benca KM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. 1992;49:651–68. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- 2.Wu JC, Bunney WE. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry. 1990;147:14–21. doi: 10.1176/ajp.147.1.14. [DOI] [PubMed] [Google Scholar]
- 3.Thase ME, Buysse DJ, Frank E, et al. Which depressed patients will respond to interpersonal psychotherapy? The role of abnormal EEG sleep profiles. Am J Psychiatry. 1997;154:502–9. doi: 10.1176/ajp.154.4.502. [DOI] [PubMed] [Google Scholar]
- 4.Weitzman ED, Kripke DF, Goldmacher D, McGregor P, Nogeire C. Acute reversal of the sleep-waking cycle in man. Effect on sleep stage patterns. Arch Neurol. 1970;22:483–9. doi: 10.1001/archneur.1970.00480240003001. [DOI] [PubMed] [Google Scholar]
- 5.Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2:632–5. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- 6.Borbely AA, Wirz-Justice A. Sleep, sleep deprivation and depression. A hypothesis derived from a model of sleep regulation. Hum Neurobiol. 1982;1:205–10. [PubMed] [Google Scholar]
- 7.McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 8.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–85. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 9.Steriade M. The GABAergic reticular nucleus: a preferential target of corticothalamic projections. Proceedings of the National Academy of Sciences of the United States of America; 2001. pp. 3625–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steriade M. Impact of network activities on neuronal properties in corticothalamic systems. J Neurophysiol. 2001;86:1–39. doi: 10.1152/jn.2001.86.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 12.Neckelmann D, Ursin R. Sleep stages and EEG power spectrum in relation to acoustical stimulus arousal threshold in the rat. Sleep. 1993;16:467–77. [PubMed] [Google Scholar]
- 13.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 14.Gillin JC. Are sleep disturbances risk factors for anxiety, depressive and addictive disorders? Acta Psychiatr Scand Suppl. 1998;393:39–43. doi: 10.1111/j.1600-0447.1998.tb05965.x. [DOI] [PubMed] [Google Scholar]
- 15.Dryman A. Eaton WW Affective symptoms associated with the onset of major depression in the community: findings from the US National Institute of Mental Health Epidemiologic Catchment Area Program. Acta Psychiatr Scand. 1991;84:1–5. doi: 10.1111/j.1600-0447.1991.tb01410.x. [DOI] [PubMed] [Google Scholar]
- 16.Eaton WW, Badawi M, Melton B. Prodromes and precursors: epidemiologic data for primary prevention of disorders with slow onset. Am J Psychiatry. 1995;152:967–72. doi: 10.1176/ajp.152.7.967. [DOI] [PubMed] [Google Scholar]
- 17.Culpepper L. Generalized anxiety disorder in primary care: emerging issues in management and treatment. J Clin Psychiatry. 2002;63(Suppl 8):35–42. [PubMed] [Google Scholar]
- 18.Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37:9–15. doi: 10.1016/s0022-3956(02)00052-3. [DOI] [PubMed] [Google Scholar]
- 19.Vollrath M, Wicki W, Angst J. The Zurich study. VIII. Insomnia: association with depression, anxiety, somatic syndromes, and course of insomnia. Eur Arch Psychiatry Neurol Sci. 1989;239:113–24. doi: 10.1007/BF01759584. [DOI] [PubMed] [Google Scholar]
- 20.Kessler RC, Nelson CB, McGonagle KA, Liu J, Swartz M, Blazer DG. Comorbidity of DSM-III-R major depressive disorder in the general population results from the US National Comorbidity Survey. Br J Psychiatry Suppl. 1996:17–30. [PubMed] [Google Scholar]
- 21.Preisig M, Merikangas KR, Angst J. Clinical significance and comorbidity of subthreshold depression and anxiety in the community. Acta Psychiatr Scand. 2001;104:96–103. doi: 10.1034/j.1600-0447.2001.00284.x. [DOI] [PubMed] [Google Scholar]
- 22.Sartorius N, Ustun TB, Lecrubier Y, Wittchen HU. Depression comorbid with anxiety results from the WHO study on psychological disorders in primary health care. Br J Psychiatry Suppl. 1996:38–43. [PubMed] [Google Scholar]
- 23.Roberts RE, Shema SJ, Kaplan GA, Strawbridge WJ. Sleep complaints and depression in an aging cohort: Aprospective perspective. Am J Psychiatry. 2000;157:81–8. doi: 10.1176/ajp.157.1.81. [DOI] [PubMed] [Google Scholar]
- 24.Gregory AM, Caspi A, Eley TC, Moffitt TE, Oconnor TG, Poulton R. Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. J Abnorm Child Psychol. 2005;33:157–63. doi: 10.1007/s10802-005-1824-0. [DOI] [PubMed] [Google Scholar]
- 25.Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–8. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 26.Weissman MM, Greenwald S, Nino-Murcia G, Dement WC. The morbidity of insomnia uncomplicated by psychiatric disorders. Gen Hosp Psychiatry. 1997;19:245–50. doi: 10.1016/s0163-8343(97)00056-x. [DOI] [PubMed] [Google Scholar]
- 27.Holmen J, Midthjell K, Kruger O, Langhammer A, Holmen TL, Bratberg GH. The Nord-Trøndelag Health Study 1995–97 (HUNT 2): Objectives, contents, methods and participation. Norsk Epidemiologi. 2003;13:19–32. [Google Scholar]
- 28.Holmen J, Midthjell K, Bjartveit K. Verdal: National Institute of Public Health, Community Medicine Research Unit; 1990. The Nord-Trøndelag Health Survey 1984–1986. [Google Scholar]
- 29.Langhammer A, Johnsen R, Holmen J, Gulsvik A, Bjermer L. Cigarette smoking gives more respiratory symptoms among women than among men. The Nord-Trondelag Health Study (HUNT) J Epidemiol Community Health. 2000;54:917–22. doi: 10.1136/jech.54.12.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tambs K, Harris JR, Magnus P. Genetic and environmental contributions to the correlation between alcohol consumption and symptoms of anxiety and depression. Results from a bivariate analysis of Norwegian twin data. Behav Genet. 1997;27:241–50. doi: 10.1023/a:1025662114352. [DOI] [PubMed] [Google Scholar]
- 31.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 32.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 33.Rickels K, Rynn MA. What is generalized anxiety disorder? J Clin Psychiatry. 2001;62(Suppl 11):4–12. discussion 13–4.:4–12. [PubMed] [Google Scholar]
- 34.Mykletun A, Stordal E, Dahl AA. Hospital Anxiety and Depression (HAD) scale: factor structure, item analyses and internal consistency in a large population. Br J Psychiatry. 2001;179:540–4. doi: 10.1192/bjp.179.6.540. [DOI] [PubMed] [Google Scholar]
- 35.Stordal E, Bjartveit KM, Dahl NH, Kruger O, Mykletun A, Dahl AA. Depression in relation to age and gender in the general population: the Nord-Trondelag Health Study (HUNT) Acta Psychiatr Scand. 2001;104:210–6. doi: 10.1034/j.1600-0447.2001.00130.x. [DOI] [PubMed] [Google Scholar]
- 36.Commitee on Halcion IoM. Halcion: an independent assessment of safety and efficacy data. Washington DC: National Academy Press; 1997. [PubMed] [Google Scholar]
- 37.Kripke DF. Chronic hypnotic use: deadly risks, doubtful benefit. Sleep Med Rev. 2000;4:5–20. doi: 10.1053/smrv.1999.0076. [DOI] [PubMed] [Google Scholar]
- 38.Lenze EJ, Mulsant BH, Shear MK, Alexopoulos GS, Frank E, Reynolds CF., III Comorbidity of depression and anxiety disorders in later life. Depress Anxiety. 2001;14:86–93. doi: 10.1002/da.1050. [DOI] [PubMed] [Google Scholar]
- 39.Chang PP, Ford DE, Mead LA, Cooper-Patrick L, Klag MJ. Insomnia in young men and subsequent depression. The Johns Hopkins Precursors Study. Am J Epidemiol. 1997;146:105–14. doi: 10.1093/oxfordjournals.aje.a009241. [DOI] [PubMed] [Google Scholar]
- 40.Rosa RR, Bonnet MH. Reported chronic insomnia is independent of poor sleep as measured by electroencephalography. Psychosom Med. 2000;62:474–82. doi: 10.1097/00006842-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Merica H, Blois R, Gaillard JM. Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998;10:1826–34. doi: 10.1046/j.1460-9568.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- 42.Ursin H, Eriksen HR. The cognitive activation theory of stress. Psychoneuroendocrinology. 2004;29:567–92. doi: 10.1016/S0306-4530(03)00091-X. [DOI] [PubMed] [Google Scholar]