Abstract
Study objectives:
To establish a normal range of data in 3-month-old infants in relation to changes in cardiovascular measurements, with particular reference to pulse transit time (PTT), following subcortical arousals and awakenings from sleep.
Design:
Prospective study.
Setting:
Sleep laboratory, Dunedin Hospital
Participants:
Twenty healthy infants aged 9–12 weeks.
Methods:
Nap studies were performed using a standard polysomnographic setup with the addition of a Portapres blood pressure (BP) cuff (wrist application) and a piezoelectric sensor on the foot. PTT was measured from the ECG-R waveform to the arrival of the pulse peripherally. Infants were exposed to white noise from 50 to 100 dB at 10 dB intervals within REM and NREM sleep.
Results:
Awakening thresholds were higher (P = 0.01) in NREM (>90 dB) than REM sleep (mean ± SD; 74.3 ± 9.4dB). Subcortical thresholds were always 10 dB below waking thresholds. Following awakening, there was an immediate increase in HR, SBP, and DBP of 21%, 14%, and 17%, respectively, and a 13% decrease in PTT returning to baseline within 25–30 seconds. PTT at baseline measured 140 ± 11 and 139 ± 9 msec in NREM and REM sleep, respectively, and decreased approximately 20 msec with waking. PTT changes were negatively correlated with heart rate (HR) but not BP, although a trend was evident.
Conclusions:
At 3 months of age, infants provoked to arouse from sleep showed PTT changes that inversely mimicked BP trends, suggesting that PTT could be useful in infant studies as a marker for autonomic perturbations that occur during sleep in both clinical and research settings.
Citation:
Galland BC; Tan E; Taylor BJ. Pulse transit time and blood pressure changes following auditory-evoked subcortical arousal and waking of infants. SLEEP 2007;30(7):891-897.
Keywords: Arousal, auditory stimulation, autonomic function, blood pressure, infant, microarousal, pulse transit time
INTRODUCTION
EXPERIMENTAL METHODS TO INVESTIGATE AUTONOMIC CARDIOVASCULAR CONTROL IN INFANTS DURING SLEEP REQUIRE NONINVASIVE TECHNIQUES. CHANGES in BP were difficult to measure noninvasively until the advent of measures of finger pulse beat-to-beat BP, developed for adult use and modified for wrist application in infants.1
Pulse transit time (PTT) is the time taken for the pulse to travel between 2 arterial sites, and may be a useful estimate of BP. The time at which a heartbeat causes the expulsion of blood from the left ventricle through the aortic valve can be deduced from the R wave on an electrocardiogram (ECG). The pulse pressure waveform then travels to the periphery where it is traditionally measured in adults and children on the finger.2,3 However there is a slight inaccuracy in this measurement because of a short electromechanical delay between the occurrence of the R wave on the ECG and the opening of the aortic valve, and thus PTT measured in this way is considered an “estimate.” “True PTT” will take into account this electromechanical delay (left ventricular isometric contraction time, or pre-ejection period), and provide a measure of pulse wave velocity. PTT is affected by both cardiac and vascular activity. With an increase in blood pressure there is an increase in arterial wall stiffness and a resultant increase in pulse wave velocity. Accordingly, for a fixed vessel distance, as blood pressure increases, PTT decreases; conversely as blood pressure falls, the speed of the pulse waveform decreases and PTT increases.4
PTT can also be used as an autonomic marker of arousal during sleep.5 In response to a minor alerting stimulus, the arousal process from sleep begins at the brainstem level and can be identified by reflex increases in sympathetic activity; heart rate, respiratory rate, blood pressure, and skin vasoconstriction. It can also be detected by a dip in PTT. These arousals are termed subcortical arousals, microarousals, transient arousals, autonomic arousals, or blood pressure arousals. In response to more significant alerting stimuli, the arousal process radiates upwards to the cortex. This results in cortical arousal associated with behavioral awakening, characterized in infants by eyes opening, vigorous movements often accompanied by crying,6 and more profound changes in sympathetic activity.
Electroencephalography (EEG) recordings are the most comprehensive method of measuring arousals and wakenings but carry a limitation, in that the technical requirements of running polysomnographic studies often confine the studies to a set-up in an attended sleep laboratory.
Subcortical arousals can contribute to sleep fragmentation, and may result in daytime sleepiness in adults.7 In that sense, they may be as important to measure as awakenings. However, those arousals do not always result in obvious changes in EEG rhythms. The application of PTT in pediatrics has mainly been in respiratory-related sleep studies, aiding the identification of respiratory events that do not terminate in waking. In infants and children, airway obstructions terminate without visible EEG arousals about 50% of the time.8
PTT has rarely been used as a measure of autonomic arousal in infants, although one study reports significant changes in PTT with sleep position, albeit without any actual data being detailed.9 The main aims of this study were to establish a normal range of PTT data in 3-month-old infants and to profile beat-to-beat BP changes with simultaneous PTT in response to auditory stimulation, which provokes both subcortical arousal and waking.
METHODS
Participants
Twenty healthy infants were included in the study. A summary of infant group characteristics is given in Table 1. The infants were born >37 weeks gestation at a birth weight >2500 g. They had no congenital defects; there was no history of maternal smoking and no evidence of maternal complications during pregnancy or at birth. Infants were aged 9 to 12 weeks at the time of the study. The Otago Ethics Committee gave ethical approval for the study. Written informed consent for each study was obtained from at least one parent/guardian.
Table 1.
Infant Group Characteristics
| Number of infants | 20 |
|---|---|
| Birthweight (g) | 3613.0 (460) |
| Gestation (wk) | 39.5 (1) |
| Age at study (days) | 76.0 (5.5) |
| Weight at study (g) | 6142.0 (974) |
| Crown-heel length at study (cm) | 59.0 (3.3) |
| Male sex (n) | 12.0 (60%) |
| Exclusively/predominantly breastfed (n) | 15.0 (75%) |
Values in brackets without units represent SD
Signal Acquisition
Babies were brought into the sleep laboratory in the morning, approximately an hour before the baby would normally settle for a nap. The polysomnography recordings included two EEG leads (C4/A1 and C3/A2) according to the 10–20 placement system), two electro-oculograms (EOG) leads, chin electromyogram (EMG), respiratory pattern from thoracic and abdominal inductance plethysmography (Respitrace model 150, Respitrace Co, NY, USA) and pulse oximetry for on-line HR and oxygen saturation (Nellcor N-200, Nellcor, Pleasanton, CA, USA). ECG electrodes were placed in a modified lead II position on the chest wall and the signal sampled at a frequency of 500 Hz. A peripheral pulse waveform was recorded from a piezoelectric pulse transducer (ADInstruments Pty Ltd, Bella Vista, NSW, Australia) placed on the plantar aspect of the foot. Systolic and diastolic pressures were measured using the Portapres finger blood pressure recording system (Portapres-Model 2, Finapres Medical Systems, Amsterdam, the Netherlands). The finger cuffs were modified for attachment around the baby's wrist. Prerecording measurements were taken to ensure stable and accurate measurements. Continuous recordings were made for a maximum of 5 min. This continuous measure of blood pressure utilized the volume clamp method,10 in which the diameter of the artery under the cuff is kept constant by an inflatable cuff clamped so that the intra-arterial pressure and cuff pressure are the same (i.e., transmural pressure is zero). A servocontroller system detects this using the signal from an infrared plethysmograph. The cuff pressure was measured with an electronic pressure transducer and the resultant signal displayed as arterial pressure. All signals were relayed through the integrated hardware/software system of the PowerLab (ADInstruments Pty Ltd, Bella Vista, NSW, Australia). Each baby was also videotaped throughout the study.
Auditory Stimulation
The baby was placed supine in a pram that was modified at the head to enclose a speaker unit. This modification ensured integrity of white noise delivered over a range of 50–100 dB, calibrated by a sound level meter. After the baby had fallen asleep, and a sleep state was firmly established within no less than 5 minutes, the blood pressure cuff was turned on. Once a stable and accurate measurement was recorded, an auditory stimulus was administered, beginning at 50 dB, and successively and incrementally (10 dB) administered every 2 min until the baby exhibited a full awakening, or until a maximum 100 dB intensity was reached. Stimulus duration was 3 seconds. The BP could only be recorded continuously for 5 minutes so as to avoid venous congestion. Therefore the machine was turned on and off briefly after 5 minutes (in between auditory test runs) and turned off at the completion of each run. Infants rarely responded to the switching on and off of the cuff during sleep, as noted by others,11 but the test was abandoned if this did happen. Auditory stimuli in the 50–100 dB range of intensities were administered twice in NREM and REM sleep states; the order varied, depending on the state shifts of the infant. If the infant switched sleep state during a test, the test was abandoned and started again once the new sleep state was established. The duration of recordings for data collection, from time of sleep onset to the end of the study, ranged from 20–150 min (median 80 min). The end of the study was determined when the data set was complete, or if the infant would not resettle after having been woken from a test.
Subcortical Arousal and Awakening Criteria
The criteria for subcortical and awakening thresholds used at the time of experimentation were based primarily on behavioral responses. Awakenings were scored if the infant opened his/her eyes together with gross movement, with or without crying. A subcortical arousal was scored if the infant did not open his/her eyes, but responded to the auditory stimulus with a startle.12 The startle is a sudden abrupt movement with a body jerk and sometimes extension of the arms. The EEG data and video data were later reviewed to confirm that the thresholds had been reached. Awakenings were then confirmed as an abrupt shift in EEG frequency according to the criteria of the ASDA13 and meeting the subsequently published criteria for the consensus of scoring of awakenings in infants aged 1 to 6 months.14 A subcortical arousal for the study was thus defined as a startle or gross body movement without an EEG arousal, together with the HR changes (on review) meeting the criteria of the consensus.14
PTT
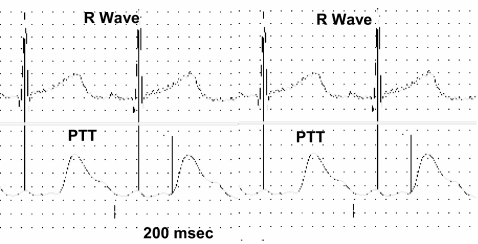
PTT was measured using the difference in recorded time between the R wave on the ECG waveform and the arrival of the pulse waveform at the periphery recorded by the piezoelectric transducer (Figure 1). Calculations were performed off-line using macro files created within Chart 5.1 incorporating signal peak detection. Artifact-contaminated sections were corrected manually. Since PTT values are semiquantitative,4,15 relative changes from baseline are given together with the relative changes in other cardiovascular measures.
Figure 1.
Pulse transit time is the time delay difference between 2 arterial sites, in this case the time from onset of ventricular ejection, estimated as the R wave on the ECG waveform (top channel) to the arrival of the pulse peripherally represented at the beginning of the pulse waveform as it reaches the foot (bottom channel).
Statistical Analyses
As the data from these babies involved repeated measures, linear mixed models (modeling outcome on sleep state and time) were fitted using SAS version 9.1.2. A random subject effect was included in all models, taking into account the correlation between measures (within-subject). Hazard ratios were calculated using a discrete time version of the proportional hazards model, with sandwich variance estimated to adjust for repeat observations. P values less than 0.05 were considered statistically significant.
RESULTS
Oxygen saturation was continuously monitored throughout the study and was always within the normal range. There were no pathological respiratory events recorded in any of the infants under study. Twenty-eight auditory test recordings were obtained from NREM sleep, and 31 from REM sleep, out of a total possible of 40 in each sleep state. Tests were limited to NREM sleep in one infant and in REM sleep in 2 infants. REM/NREM trials were performed in the same sleep episodes in 12 infants and successive sleep episodes in 5 infants.
Subcortical Arousal and Waking Thresholds
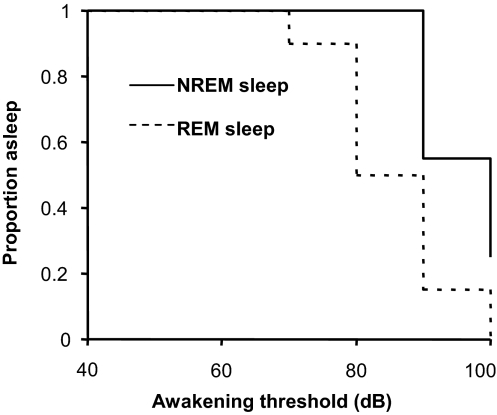
Data for the number of tests and arousal thresholds classified into Subcortical arousal and waking responses are summarized in Table 2. Higher mean waking thresholds (P = 0.01) were recorded in NREM (>90 dB) compared to REM sleep (mean ± SD; 74.3 ± 9.4dB). Kaplan-Meier curves in relation to waking, and comparing sleep state responses, are shown in Figure 2. Seven tests in NREM sleep, conducted in 4 infants, failed to arouse infants with the maximum auditory stimulus of 100 dB. All tests in REM sleep resulted in waking, so that there was a much higher likelihood that the infant would wake from REM than NREM sleep, i.e., within this 50–100 dB range (hazard ratio 9.46; 95 % CI, 1.15 to 77.6). Waking thresholds were very reproducible within sleep state and within individual babies. Repeat test data were obtained from 10 individuals in NREM sleep and 15 individuals in REM sleep. Waking thresholds were identical in 8/10 of these NREM repeat tests, and 10/15 of the REM repeat tests. All differences in thresholds were of the 10 dB magnitude, but inconsistent as to whether the first or second presentation produced the higher or lower threshold.
Table 2.
Number of Tests Resulting in Subcortical Arousal or Waking in Each Sleep State by Intensity of Auditory Stimulus.
| Subcortical Arousal |
Waking |
|||
|---|---|---|---|---|
| NREM | REM | NREM | REM | |
| Total # | 13 | 8 | 28 | 31 |
| 50 dB | 0 | 0 | 0 | 0 |
| 60 dB | 0 | 3 | 0 | 3 |
| 70 dB | 2 | 2 | 0 | 13 |
| 80 dB | 5 | 3 | 13 | 11 |
| 90 dB | 1 | 0 | 7 | 3 |
| 100 dB | 5 | 0 | 1 | 1 |
| No waking at 100 dB | - | - | 7 | 0 |
Figure 2.
Kaplan-Meier curves showing the proportion of tests in which the children were still asleep for state REM and state NREM against the decibel level inducing waking.
Subcortical arousals were evident in 46% of NREM and 26% of REM tests (Table 2). In all cases these occurred 10 dB below the waking threshold.
Cardiovascular Measurements at Baseline
Baseline measurements for HR, DBP, SBP, and PTT were obtained from the 30 s of data prior the stimulus that woke the infant. Mean ± SD values in NREM sleep were: HR, 121 ± 6.2 bpm; SBP, 99.4 ± 13.4 mm Hg; DBP, 59.0 ± 16.9 mm Hg; PTT, 142 ± 11 msec. In REM sleep, baseline values were: HR, 126 ± 7.1 bpm; SBP, 101 ± 13.7 mm Hg; DBP, 62.6 ± 15.5 mm Hg; PTT, 139 ± 9 msec. HR was significantly higher in REM than NREM sleep (P = 0.0104), but no significant difference was found according to sleep state for other measurements.
Cardiovascular Measurements in Response to Waking
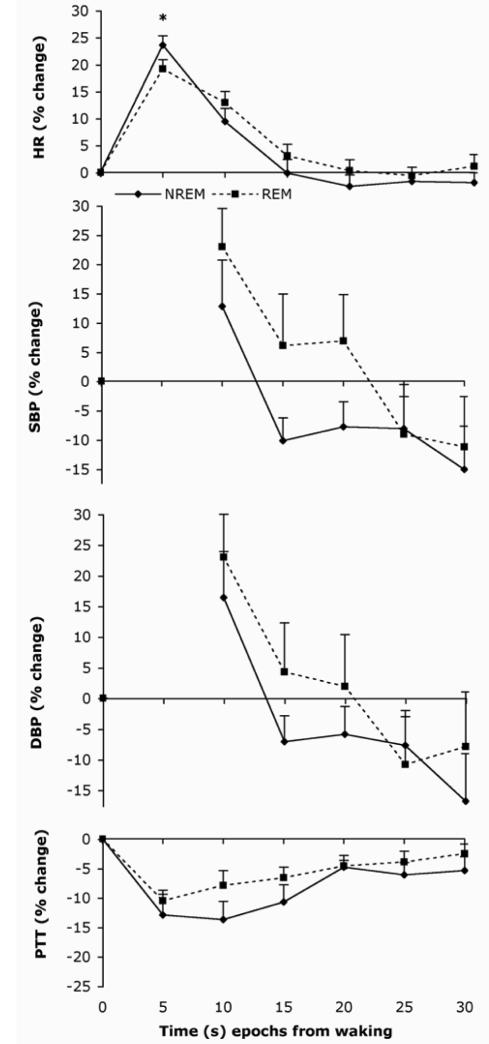
The auditory stimulus at waking induced a characteristic cardiovascular response with maximal mean changes in HR, SBP, and DBP of 21%, 14%, and 17%, respectively (combining the 2 sleep states). It induced a minimal decrease in PTT of approximately 13% occurring within 5–10 seconds. HR returned to baseline within 20 seconds of the arousal stimulus as shown in Figure 3. Movement artifact prevented the 0–5-second recordings of BP. Three infants responded with a sustained increase in HR when woken from REM sleep, but none responded with a sustained decrease. BP changes show a characteristic rebound overshoot to below baseline levels. A comparison of cardiovascular patterns following waking showed that sleep state differences were only significant for HR at time epoch 5 seconds (P = 0.006), revealing a higher percentage increase in HR when waking from NREM sleep than REM sleep. HR was positively correlated with SBP (P <0.0001) and DBP (P = 0.0005) measures and negatively correlated with PTT (P = 0.0004). PTT showed a trend towards a negative correlation with SBP and DBP, but this was not significant. There was no correlation between the strength of arousal stimulus at awakening threshold, and the subsequent change in HR, SBP, DBP, or PTT.
Figure 3.
HR, BP, and PTT changes following the auditory test stimulation in NREM and REM sleep. Values are means ± s.e.m. averaged over each 5-sec time epoch from stimulus presentation at time zero. * P < 0.05
Cardiovascular Measurements in Response to Subcortical Arousal
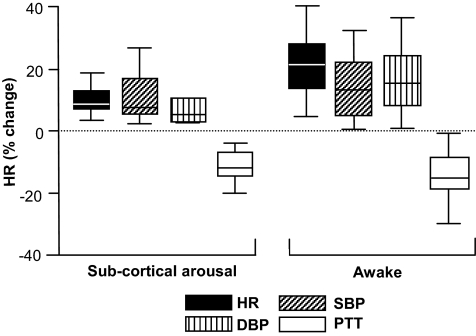
Because fewer tests induced subcortical arousal, it was not possible to complete any meaningful statistical analyses. As a result, the data are presented descriptively with the maximum changes in HR, SBP, and DBP, and minimum changes in PTT plotted in terms of the range and variability (Figure 4). These data illustrate reduced autonomic responses associated with subcortical arousal compared to waking. Accordingly the mean HR increased 10% in response to subcortical arousal compared with 21% in response to waking, 11% vs 14% for SBP, 8% vs 17% for DBP, and −11% vs −13% for PTT.
Figure 4.
Maximal values of cardiovascular parameters recorded following subcortical arousal and waking responses. The box and whisker plots represent the median, interquartile range and maximum and minimum (as defined by an equation that takes the spread of the interquartile range into account) for a range of variables. Maxima and minima are represented as the whiskers attached to the box.
DISCUSSION
This study presents normative data in regard to PTT and simultaneous heart rate and beat-to-beat blood pressure in healthy 9–12 week old sleeping infants, and includes changes in these variables in response to auditory-induced subcortical arousal and waking. As far as we are aware, the PTT data is novel for this age range, chosen to reflect the peak age of SIDS occurrence.16 SIDS mechanism/s are unknown, although there are many reports suggesting that depressed autonomic activity involving cardio-respiratory control, and arousal from sleep, may contribute to sudden death.17–19 The usefulness of this data was therefore targeted for studies involving physiological investigations into the mechanism/s of SIDS. Clinical utility for this mode of investigating autonomic function in infancy has yet to be established.
Abrupt changes in HR, BP, and PTT followed subcortical and waking responses. The BP responses following waking were mostly biphasic, with an immediate increase followed by a paradoxical decrease and a return to baseline. The immediate response is the sympathetic pressor response to arousal stimuli. The second reflects activation of baroreceptor-mediated reflex responses acting to restrain the pressor response via central mechanisms. This causes vagal activation and sympathetic withdrawal. The decelerated HR and increased PTT mimicked the immediate BP response with a slower return to baseline but without paradoxical overshoot. This suggests the PTT value is less influenced by reflex activity than blood pressure is. Biphasic HR responses to an acoustic stimulus of sudden noise have been reported previously in studies of normal healthy infants11,20,21 and adults.22 The less common response of a sustained tachycardia was noted here. Others have noted a sustained bradycardia in response to acoustic stimuli in infants.11
Following subcortical arousal, the pressor responses were less profound, but because there were far fewer subcortical arousals, we could not apply any statistical analyses to compare with the waking responses. Only 10 of the 20 infants exhibited subcortical arousals. The reduced but obvious changes in cardiovascular variables observed in association with subcortical arousals suggest these data could serve as markers of sleep disruption events in infants, even though full arousals, conventionally detected as changes in cortical EEG activity, are more obvious. For example, in studies of children and adults with obstructive sleep apnea, PTT improves the detection of microarousals.3,5 It has been suggested that these microarousals may serve to terminate respiratory events during sleep through subcortical polysynaptic reflexes,3 perhaps serving to protect the immediate sleep state.
In response to waking, a trend for the profile of SBP and DBP to be negatively correlated with PTT was noteworthy but did not reach statistical significance. Perhaps larger cardiovascular changes, and/or precisely controlled stepwise conditions, would be more useful in establishing this relationship. Others report a near linear correlation between PTT and BP changes23 suggesting that PTT may be a useful surrogate marker of blood pressure. A change of 1 msec in PTT in adults is considered equivalent to a 1 mm Hg change in blood pressure.15,24 Payne et al25 suggest that PTT is not a reliable marker for SBP but may be useful for assessing BP variability and in detecting rapid changes in BP. They state that PTT cannot be used as a marker for DBP or mean pressure without correcting for the pre-ejection period. This is because the pre-ejection period cannot be assumed to remain constant, accounting for a substantial and variable proportion of PTT ranging from 12%–35%.
For the measure of PTT, the ECG-R wave is always used as the proximal time point because it is simple to detect and tolerates motion artifact. For the distal time point, a peripheral pulse waveform is used, traditionally accessed from a raw photoplethysmograph signal directly from a pulse oximeter output.15,26 We chose to use a much simpler signal to time the arrival of the pulse waveform — a pressure signal to the piezoelectric sensor on the foot. The distal point being the foot rather than the finger (as is conventional in children and adults) would create a longer PTT being further from the heart. Although most studies using fully automated analyses take the time of the arrival of the pulse waveform distally as 25% or 50% of the height of the maximal point of the waveform, we chose to use the exact time point of arrival of the pulse. This was easily identified for manual correction in the absence of fully automated analyses. PTT length varies in relation to pulse site detection and in the timing point of the arrival of the pulse waveform. Therefore the PTT values documented here reflect the conditions of our particular set-up and analyses, but no other data are available from infants for comparison. The method was suitable for our brief period of data collection, but we acknowledge it would be unsuitable for analyzing continuous long recordings. It is too labor intensive and relies heavily on subjective judgment during manual correction as to the exact location of the distal time point.
The device we used for measuring blood pressure was the Portapres (the portable equivalent of the Finapres device; TNO Biomedical Instrumentation, Amsterdam, the Netherlands) designed for use in adults for finger arterial pressure. We modified the device for use in infants for measuring radial artery pressure as described by others.11,27 One limitation is that the device is very sensitive to movement artifact; hence we had difficulties in obtaining reliable measures around the point of arousal. The equipment is cumbersome, and because of the risk of venous congestion with prolonged application, the method is only suitable for intermittent data collection and not for use in unattended domiciliary studies involving infants. However the technique offers enormous advantages in providing a continuous beat-to-beat blood pressure record that would otherwise be impossible in normal healthy infants without an arterial line. Accuracy of the estimate in neonates has been examined by comparing radial blood pressure readings with intra-arterial measurements in the umbilical artery. The studies show good agreement between the 2 measures on the order of 5–10 mm Hg,11,27 remaining constant with changing BP values.11
More heightened alertness in REM than NREM sleep was evident in the lower arousal thresholds required to induce waking, supporting findings from others using auditory,28 chemosensory,29 and tactile/chemosensory30 stimuli in infants. These sleep state differences in arousal thresholds appear very clear in healthy infants but less so in healthy adults, where higher arousal thresholds in REM sleep are reported.31 Others report lower thresholds,32,33 and some report similar thresholds between REM and NREM sleep.34,35 Unlike adults, infants always enter REM sleep at the beginning of the sleep cycle. NREM sleep, with higher arousal thresholds, thus follows REM sleep. These higher arousal thresholds perhaps fit with the theoretical concept of sleep architecture serving as a protective field where arousal thresholds increase with the duration of undisturbed sleep,36 i.e., in this case, a protective field within a single sleep cycle where the infant slowly entrusts him/herself to the safety of the sleeping environment.
A further limitation of this study was that not all infants aroused at the maximal 100 dB, making it impossible to assign an exact arousal threshold in NREM sleep. The failure of some infants to arouse at 100 dB has also been reported in children.32,37 The noise level is equivalent to the noise from a jet aircraft taking off from 300 metres away, or a chainsaw in use.
The auditory stimulation test was chosen because some comparative data is available in the literature for this infant age, and the test was brief to administer. Also, the test targets only one sensory system, resulting in reproducible and predictable arousal thresholds. Kahn and coworkers have used the stimulus method extensively in infants during REM sleep,38–40 but no data exist on arousal thresholds elicited in NREM sleep. Less data were produced from NREM because only 75% of tests evoked waking, as opposed to 100% of tests in REM sleep. Nevertheless it is important to include these data, because the infant, at this age, spends at least half of his/her sleep time in NREM. The relative proportion of NREM over REM sleep increases with maturity. Others preserve the NREM state from testing to avoid sleep fragmentation40,41 and because habituation to an arousal stimulus can occur.42 Habituation to the arousal test was not evident here, probably because there were only a small number of tests (maximum of 4), and these were applied at relatively longer intervals than those known to induce habituation.42
In summary, acoustic stimulation in 9- to 12-week-old infants resulted in abrupt changes in cardiovascular variables, including PTT, which followed the classic biphasic profile. The stimulation evoked seemingly smaller responses from subcortical arousal than from waking. The main new aspect of this study was in providing the first normative data in relation to PTT and changes following arousal. Further investigations are required from larger cross-sectional studies to establish the use of PTT as a surrogate marker for blood pressure in infants and its utility and application in autonomic development studies and investigation cardiovascular and autonomic pathologies.
ACKNOWLEDGMENTS
The authors thank Marie Goulden for research assistance and Andrew Gray for his statistical assistance. We are grateful to the parents whose babies participated in the study.
ABBREVIATIONS:
- DBP
Diastolic blood pressure
- NREM
Non rapid eye movement sleep
- PTT
Pulse transit time
- SBP
Systolic blood pressure
- REM
Rapid eye movement sleep
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have reported no financial conflicts of interest.
REFERENCES
- 1.Drouin E, Gournay V, Calamel J, Mouzard A, Roze JC. Assessment of spontaneous baroreflex sensitivity in neonates. Arch Dis Child. 1997;76:F108–12. doi: 10.1136/fn.76.2.f108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pitson D, Sandell A, van den Hout R, Stradling J. Use of pulse transit time as a measure of inspiratory effort in patients with obstructive sleep apnoea. Eur Respir J. 1995;8:1669–74. doi: 10.1183/09031936.95.08101669. [DOI] [PubMed] [Google Scholar]
- 3.Pepin JL, Delavie N, Pin I, et al. Pulse transit time improves detection of sleep respiratory events and microarousals in children. Chest. 2005;127:722–30. doi: 10.1378/chest.127.3.722. [DOI] [PubMed] [Google Scholar]
- 4.Smith RP, Argod J, Pepin JL, Levy PA. Pulse transit time: an appraisal of potential clinical applications. Thorax. 1999;54:452–7. doi: 10.1136/thx.54.5.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitson DJ, Stradling JR. Autonomic markers of arousal during sleep in patients undergoing investigation for obstructive sleep apnoea, their relationship to EEG arousals, respiratory events and subjective sleepiness. J Sleep Res. 1998;7:53–9. doi: 10.1046/j.1365-2869.1998.00092.x. [DOI] [PubMed] [Google Scholar]
- 6.Guilleminault C, Souquet M. Sleep states and related pathology. In: Korobkin R, Guilleminault C, editors. Advances in perinatal neurology. New York: Spectrum Publications Inc; 1979. pp. 225–47. [Google Scholar]
- 7.Martin SE, Wraith PK, Deary IJ, Douglas NJ. The effect of nonvisible sleep fragmentation on daytime function. Am J Respir Crit Care Med. 1997;155:1596–601. doi: 10.1164/ajrccm.155.5.9154863. [DOI] [PubMed] [Google Scholar]
- 8.McNamara F, Issa FG, Sullivan CE. Arousal pattern following central and obstructive breathing abnormalities in infants and children. J Appl Physiol. 1996;81:2651–7. doi: 10.1152/jappl.1996.81.6.2651. [DOI] [PubMed] [Google Scholar]
- 9.Franco P, Van de Borne P, Chabanski S, et al. Physiological relationship between autonomic reactions and arousals in infancy. Sleep Med. 2002;3:S49–52. doi: 10.1016/s1389-9457(02)00165-x. [DOI] [PubMed] [Google Scholar]
- 10.Penaz J. Criteria for set point estimation in the volume clamp method of blood pressure measurement. Physiol Res. 1992;41:5–10. [PubMed] [Google Scholar]
- 11.Harrington C, Kirjavainen T, Teng A, Sullivan CE. Cardiovascular responses to three simple, provocative tests of autonomic activity in sleeping infants. J Appl Physiol. 2001;91:561–8. doi: 10.1152/jappl.2001.91.2.561. [DOI] [PubMed] [Google Scholar]
- 12.McGraw MB. The moro reflex. Am J Dis Child. 1937;51:240–51. [Google Scholar]
- 13.Atlas Task Force; American Sleep Disorders Association. EEG arousals: scoring rules and examples. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 14.The International Paediatric Work Group on Arousals. The scoring of arousals in healthy term infants (between the ages of 1 and 6 months) J Sleep Res. 2005;14:37–41. doi: 10.1111/j.1365-2869.2004.00426.x. [DOI] [PubMed] [Google Scholar]
- 15.Pitson D, Chhina N, Knijn S, van Herwaaden M, Stradling J. Changes in pulse transit time and pulse rate as markers of arousal from sleep in normal subjects. Clin Sci. 1994;87:269–73. doi: 10.1042/cs0870269. [DOI] [PubMed] [Google Scholar]
- 16.Little RE, Peterson DR. Sudden infant death syndrome epidemiology: a review and update. Epidemiol Rev. 1990;12:241–6. doi: 10.1093/oxfordjournals.epirev.a036057. [DOI] [PubMed] [Google Scholar]
- 17.Schechtman VL, Harper RM, Kluge KA, Wilson AJ, Hoffman HJ, Southall DP. Cardiac and respiratory patterns in normal infants and victims of the sudden infant death syndrome. Sleep. 1988;11:413–24. doi: 10.1093/sleep/11.5.413. [DOI] [PubMed] [Google Scholar]
- 18.Franco P, Szliwowski H, Dramaix M, Kahn A. Polysomnographic study of the autonomic nervous system in potential victims of sudden infant death syndrome. Clin Auton Res. 1998;8:243–9. doi: 10.1007/BF02277969. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz PJ, Stramba-Badiale M, Segantini A, et al. Prolongation of the QT interval and the sudden infant death syndrome. New Engl J Med. 1998;338:1709–14. doi: 10.1056/NEJM199806113382401. [DOI] [PubMed] [Google Scholar]
- 20.Anderssen SH, Nicolaisen RB, Gabrielsen GW. Autonomic response to auditory stimulation. Acta Paediatr. 1993;82:913–8. doi: 10.1111/j.1651-2227.1993.tb12598.x. [DOI] [PubMed] [Google Scholar]
- 21.Lagercrantz H, Edwards D, Henderson-Smart D, Hertzberg T, Jeffery H. Autonomic reflexes in preterm infants. Acta Paed Scand. 1990;79:721–8. doi: 10.1111/j.1651-2227.1990.tb11546.x. [DOI] [PubMed] [Google Scholar]
- 22.Catcheside PG, Chiong SC, Mercer J, Saunders NA, McEvoy RD. Noninvasive cardiovascular markers of acoustically induced arousal from non-rapid-eye-movement sleep. Sleep. 2002;25:797–804. doi: 10.1093/sleep/25.7.797. [DOI] [PubMed] [Google Scholar]
- 23.Leas S, Ali N, Goldman L, Loh J, Fleetham J, Strandling J. Sleep. 1990. Systolic blood pressure reflect inspiratory effort during simulated obstructive sleep apnea; pp. 178–81. [Google Scholar]
- 24.Pitson DJ, Stradling JR. Value of beat-to-beat blood pressure changes, detected by pulse transit time, in the management of the obstructive sleep apnoea/hypopnoea syndrome. Eur Respir J. 1998;12:685–92. doi: 10.1183/09031936.98.12030685. [DOI] [PubMed] [Google Scholar]
- 25.Payne RA, Symeonides CN, Webb DJ, Maxwell SR. Pulse transit time measured from the ECG: an unreliable marker of beat-to-beat blood pressure. J Appl Physiol. 2006;100:136–41. doi: 10.1152/japplphysiol.00657.2005. [DOI] [PubMed] [Google Scholar]
- 26.Katz ES, Lutz J, Black C, Marcus CL. Pulse transit time as a measure of arousal and respiratory effort in children with sleep-disordered breathing. Pediatr Res. 2003;53:580–8. doi: 10.1203/01.PDR.0000057206.14698.47. [DOI] [PubMed] [Google Scholar]
- 27.Drouin E, Gournay V, Calamel J, Mouzard A, Roze JC. Feasibility of using finger arterial pressure in neonates. Arch Dis Child. 1997;77:F139–40. doi: 10.1136/fn.77.2.f139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franco P, Scaillet S, Valente F, Chabanski S, Groswasser J, Kahn A. Ambient temperature is associated with changes in infants' arousability from sleep. Sleep. 2001;24:325–9. doi: 10.1093/sleep/24.3.325. [DOI] [PubMed] [Google Scholar]
- 29.Campbell AJ, Bolton DPG, Taylor BJ, Sayers RM. Responses to an increasing asphyxia in infants: effects of age and sleep state. Respir Physiol. 1998;112:51–8. doi: 10.1016/s0034-5687(98)00008-5. [DOI] [PubMed] [Google Scholar]
- 30.Read PA, Home RS, Cranage SM, Walker AM, Walker DW, Adamson TM. Dynamic changes in arousal threshold during sleep in the human infant. Pediatr Res. 1998;43:697–703. doi: 10.1203/00006450-199805000-00020. [DOI] [PubMed] [Google Scholar]
- 31.Williams H, Khammack J, Daly R, Dement W, Lubin A. Responses to auditory stimulation, sleep loss and the EEG stages of sleep. Electroenceph Clin Neurophysiol. 1964;16:269–79. doi: 10.1016/0013-4694(64)90109-9. [DOI] [PubMed] [Google Scholar]
- 32.Busby KA, Mercier L, Pivik RT. Ontogenetic variations in auditory arousal threshold during sleep. Psychophysiology. 1994;31:182–8. doi: 10.1111/j.1469-8986.1994.tb01038.x. [DOI] [PubMed] [Google Scholar]
- 33.Issa FG, Sullivan CE. Arousal and breathing responses to airway occlusion in healthy sleeping adults. J Appl Physiol. 1983;55:1113–9. doi: 10.1152/jappl.1983.55.4.1113. [DOI] [PubMed] [Google Scholar]
- 34.Bentley AJ, Newton S, Zio CD. Sensitivity of sleep stages to painful thermal stimuli. J Sleep Res. 2003;12:143–7. doi: 10.1046/j.1365-2869.2003.00352.x. [DOI] [PubMed] [Google Scholar]
- 35.Roehrs T, Merlotti L, Petiucelli N, Stepanski E, Roth T. Experimental sleep fragmentation. Sleep. 1994;17:438–43. doi: 10.1093/sleep/17.5.438. [DOI] [PubMed] [Google Scholar]
- 36.Voss U. Functions of sleep architecture and the concept of protective fields. Rev Neurosci. 2004;15:33–46. doi: 10.1515/revneuro.2004.15.1.33. [DOI] [PubMed] [Google Scholar]
- 37.Moreira GA, Tufik S, Nery LE, et al. Acoustic arousal responses in children with obstructive sleep apnea. Pediatr Pulmonol. 2005;40:300–5. doi: 10.1002/ppul.20219. [DOI] [PubMed] [Google Scholar]
- 38.Kahn A, Picard E, Blum D. Auditory arousal thresholds of normal and near-miss SIDS infants. Dev Med Child Neurol. 1986;28:299–302. doi: 10.1111/j.1469-8749.1986.tb03876.x. [DOI] [PubMed] [Google Scholar]
- 39.Franco P, Pardou A, Hassid S, Lurquin P, Groswasser J, Kahn A. Auditory arousal thresholds are higher when infants sleep in the prone position. J Pediatr. 1998;132:240–3. doi: 10.1016/s0022-3476(98)70438-x. [DOI] [PubMed] [Google Scholar]
- 40.Franco P, Scaillet S, Groswasser J, Kahn A. Increased cardiac autonomic responses to auditory challenges in swaddled infants. Sleep. 2004;27:1527–32. doi: 10.1093/sleep/27.8.1527. [DOI] [PubMed] [Google Scholar]
- 41.Franco P, Pardou A, Hassid S, Kahn A. Decreased cardiac responses to auditory stimulation during prone sleep. Pediatrics. 1996;97:174–8. [PubMed] [Google Scholar]
- 42.McNamara F, Wulbrand H, Thach BT. Habitation of the infant arousal response. Sleep. 1999;22:320–6. [PubMed] [Google Scholar]