Abstract
Introduction:
Adolescence is a time of rapid changes in sleep habits and rising prevalence of sleepiness. The importance of measuring sleep in this population is increasingly recognized. In adults, measurements of sleep by actigraphy correlate well with sleep data from EEG recordings. Since actigraphy is increasingly utilized in adolescent sleep studies, more information is needed about reliability in this age group. This analysis investigated which actigraphy data mode is optimal for data collection in adolescents and explored the level of agreement between actigraphy and polysomnography (PSG) in population subgroups.
Methods:
181 adolescents aged 12–16 years were concurrently monitored with PSG and wrist actigraphy (measured in 3 data modes: Time Above Threshold [TAT], Zero Crossing Mode [ZCM], and Proportional Integration Mode [PIM]) to measure total sleep time (TST).
Results:
The sample was 50% male, 55% African American, 9% with sleep disordered breathing (SDB; apnea-hypopnea index ≥ 5). Intraclass correlation coefficients (ICC) for TST between actigraphy and PSG were low to moderate and were highest for TAT (0.41) compared to ZCM (0.32) and PIM (0.34). Subgroup analyses revealed that ICCs were higher among those without SDB (0.55) than those with SDB (0.00), and for girls (0.66) compared with boys (0.31).
Conclusions:
Results suggest that actigraphy provides a reasonably good estimate of TST in adolescents without SDB. Recognition of the variation in sleep estimates among different data collection modes, among population subgroups, and across the age spectrum, may be of fundamental importance in the interpretation of actigraphy data for sleep duration estimation.
Citation:
Johnson NL; Kirchner HL; Rosen CL et al. Sleep estimation using wrist actigraphy in adolescents with and without sleep disordered breathing: a comparison of three data modes. SLEEP 2007;30(7):899-905.
Keywords: Actigraphy, sleep disordered breathing, pediatrics
INTRODUCTION
ADOLESCENCE IS A TIME OF RAPID CHANGES IN SLEEP PATTERNS AND OF RISING PREVALENCE OF SLEEPINESS, WITH ADOLESCENTS HAVING BEEN IDENTIFIED as being at high risk for problem sleepiness.1 Sleep deprivation among adolescents is a rising concern among parents, health professionals, and educators.2,3,4,5,6 In light of the concerns and growing awareness of the problems associated with adolescent sleep, the importance of measuring sleep duration in adolescents is increasingly recognized.
Wrist actigraphy is a noninvasive and relatively inexpensive means of estimating sleep based on recording and analyzing motion data. Although actigraphy has been used to estimate sleep patterns for over 25 years, the methodology by which actigraphy data should be accumulated, processed, and scored in order to optimize sleep/wake inference continue to be explored.7 Previous research has shown that sleep differs in adolescence as compared to earlier periods of childhood and to adulthood.8,9 Because the relationship between sleep and movement may vary over the age spectrum, researchers have identified the need to independently test the validity of actigraphy-based sleep-wake inference in an infant population.10 However, little has been done to validate current methods for actigraphy data processing in an adolescent population.
In healthy adults, actigraphy-based estimates of sleep correlate well with sleep data from EEG recordings obtained from polysomnography (PSG). However, estimates of sleep from actigraphy have been found to be less reliable as sleep becomes more disturbed.7,11,12,13 Others have shown that actigraphy appears to be limited in the accuracy of its estimation of sleep in the presence of the sleep fragmentation associated with sleep disordered breathing (SDB).12 Whether and to what extent these findings are generalizable to an adolescent population has yet to be shown.
This study compared sleep/wake inference from actigraphy data to that obtained from overnight PSG in 181 adolescents, comparing subgroups by gender and SDB, in order to identify the mode of data processing that best correlates with PSG-determined sleep estimates. This study (1) sought to determine the optimal data mode for processing actigraphy data in adolescents, and (2) investigated the following hypotheses: i) total sleep time (TST) from actigraphy has high agreement with TST from PSG in adolescents, and ii) the agreement between actigraphy and PSG will be stronger in adolescents without SDB than those with SDB. Due to previous findings that there are differences in sleep patterns and movement during sleep between adolescent girls and boys,14,15,16 possible gender differences in the agreement between actigraphy and PSG were also explored.
METHODS
Study Population
The study sample consisted of 181 adolescents who were participating in the Cleveland TeenZzz Study, an ongoing longitudinal cohort study designed to evaluate the role of sleep disturbances in a wide range of health outcomes. TeenZzz participants represent a sample of adolescents who initially participated in the Cleveland Children's Sleep and Health Study (CCSHS), an urban community-based cohort of 907 children assembled as a stratified random sample of full-term and preterm children born at Cleveland area hospitals between 1988 and 1993. This cohort was designed to overrepresent African American and former preterm children as described previously.17 For the TeenZzz Study, 250 of these children, including all snorers and children with SDB and a random sample of the remaining cohort were targeted for evaluation during adolescence. This study was based on 181 adolescents for whom actigraphy data were collected concurrently with polysomnography.
Study Protocol
In a standardized research protocol in a dedicated clinical research facility, each adolescent had overnight PSG and underwent measurement of height and weight, with calculation of BMI as the ratio of weight in kilograms to the square of height in meters. BMI percentile was generated based on population data from the National Health and Nutrition Examination Survey (NHANES) 1999–2000.18 Participants with a BMI percentile ≥95% were classified as overweight. For the week prior to the PSG (while at home) and during the overnight PSG study (in the clinical research center), the participant wore a wrist actigraph and completed daily sleep logs. Informed consent was obtained from the child's caregiver and written assent from the child. The study was approved by the governing institutional review boards.
Polysomnography and Sleep Data
Full-channel overnight PSG was collected (E-Series, Compumedics Ltd., Abbotsford, Victoria, Australia) recording brain wave activity (C3/A2 and C4/A1 electroencephalogram), eye movements (right and left electrooculogram), respiratory effort (thoracic and abdominal inductance belts), airflow (nasal/oral thermister and nasal pressure cannula), ECG, pulse oximetry, snoring (microphone), body position, and leg movements (piezoelectric leg paddles). The procedures for scoring PSG data have been described in detail previously.19 In brief, sleep stages were identified for each 30-second epoch according to Rechtshaffen and Kales criteria.20 Arousals were scored as characterized by the Academy of Sleep Medicine criteria.21 Obstructive apneas were identified if the amplitude of airflow was absent or nearly absent for at least 8 seconds and the duration of at least 2 breaths. Hypopneas were identified if the amplitude of respiratory signal (airflow, chest or abdominal effort) decreased below 70% of “baseline” breathing for at least 6 seconds and the duration of at least 2 breaths, and was associated with ≥3% drop in oxygen saturation. Central apneas were identified if no displacement was seen on the chest and abdominal effort channels. The apnea-hypopnea index (AHI) was calculated as the total number of obstructive apneas and hypopneas per hour of sleep. Total sleep time (TST) was defined as the total number of minutes scored as sleep. The AHI was dichotomized into those without SDB (AHI <5) and those with SDB (AHI ≥5), and further categorized into no SDB (AHI <5), mild SDB (5≤ AHI <15), and severe SDB (AHI ≥15).
Actigraphy Data and Scoring
Each adolescent wore a wrist actigraph for a period of 5 to 7 days. Due to scheduling and availability of equipment, actigraphy data was collected on the night of PSG for only 181 of the participants. Actigraphy data was collected using the Octagonal Sleep Watch 2.01 (AMI, Ambulatory Monitoring, Inc., Ardsley, NY) placed on the nondominant wrist, and configured for data collection using ACT-Millennium software (AMI). The actigraph uses a piezoelectric sensor that generates a signal based on movement. The analog signal is then digitized and for each 1-minute epoch an activity count is calculated and stored based on 1 of 3 data modes. There are currently 3 data modes available in wrist actigraphs: Proportional Integration Mode (PIM), also known as digital integration, calculates the area under the curve for each epoch; Time Above Threshold (TAT) counts the amount of time per epoch that the signal is above a set threshold; and Zero Crossing Mode (ZCM) counts the number of times per epoch that the signal crosses a threshold (set very close to zero).7 The equipment chosen for this study allowed simultaneous collection in all 3 data modes.
Actigraphy data were scored using Action-W analysis software (AMI). For this analysis, actigraphy data were edited to include only the night of the PSG, and the data analysis window was marked so as to match the PSG in start and end times. Timing was determined using lights out and lights on from PSG, which were set to occur no later than 23:00 and 08:00, respectively. The computers performing the PSG and setting up the actigraph were synchronized to a standard time. For actigraphy, sleep or wake is classified for each 1-minute epoch based on the activity score from that minute and applying an algorithm that weights the activity score from surrounding minutes.7,23 Our analyses used the UCSD algorithm, available within the analysis software, to calculate sleep or wake for each minute of data,22,23 and TST was calculated for each of the 3 data modes as the total number of minutes scored as sleep. Although other algorithms are available, our initial work showed that the agreement for TST estimation when comparing different algorithms was very high (i.e., intraclass correlation coefficients of 0.98 for UCSD vs. Cole-Kripke; 0.96 for UCSD vs. Sadeh; 0.98 for Cole-Kripke vs. Sadeh based on the sample of 181 records). Thus, to focus the analyses on the impact of different data modes on agreement relative to PSG-determined TST, actigraphic values were generated with the UCSD algorithm.
Statistical Analysis
Analyses were designed to (1) assess the agreement between actigraphy-based estimates of TST compared with PSG for each of the 3 data modes to determine which data mode is optimal for estimating TST in adolescents; (2) investigate changes in the level of this agreement in population subgroups, namely by SDB and gender. Analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC).
Sample characteristics were summarized by mean and standard deviation for normally distributed variables, median and interquartile range for skewed variables, and number and percent for categorical variables. Sample characteristics were also stratified by gender, and groups were compared using unpaired t-test for normally distributed variables, Wilcoxon rank-sum test for skewed variables, and Pearson chi-squared test or Fisher's exact test for categorical variables. Differences in TST between PSG and each data mode were calculated and were compared using paired t-tests. Bland-Altman plots24 and bar plots were generated as graphical tools for comparison of agreement, and groups were compared using Mantel-Haenszel chi-square test or Friedman's chi-square test. Intraclass correlation coefficients were used to compare the relative association of TST from PSG and actigraphy.
RESULTS
Sample characteristics are summarized in Table 1. The sample was 50% male, 55% African American, 28% overweight (mean BMI of 23.8 ± 6.8 kg/m2), and had a mean age of 13.7 ± 0.9 years. The median AHI for the sample was 0.47 (IQR: 0.16, 1.23). Seventeen adolescents (9%) had an AHI ≥5. Gender differences were seen in AHI, with more boys in the higher AHI categories.
Table 1.
Sample Characteristics Stratified by SDB and by Gendera
| All (n=181) | No SDB (n=164) | SDB (n=17) | Female (n=91) | Male (n=90) | |
|---|---|---|---|---|---|
| Participant characteristics | |||||
| Age (years) | 13.7 ± 0.9 (13.3) | 13.7 ± 0.9 (13.3) | 13.6 ± 0.7 (13.5) | 13.8 ± 0.9 (13.3) | 13.7 ± 0.9 (13.3) |
| African American (n;%) | 99 (54.7%) | 88 (53.7%) | 11 (64.7%) | 56 (61.5%) | 43 (47.8%) |
| BMI (kg/m2) | 23.8 ± 6.8 (21.7) | 23.2 ± 6.3 (21.4) | 29.6 ± 8.9 (30.6) | 23.5 ± 6.5 (21.5) | 24.1 ± 7.2 (22. 7) |
| Overweight b (n; %) | 50 (27.6%) | 39 (23.8%) | 30 (64.7%) | 20 (22.0%) | 30 (33.3%) |
| Sleep characteristics (from PSG) | |||||
| Total sleep time (minutes) | 477 ± 56 (486) | 475 ± 56 (483) | 485 ± 57 (491) | 483 ± 48 (495) | 470 ± 63 (476) |
| AHI (events/hour) | 0.47 (0.16,1.23) | 0.39 (0.13, 0.98) | 9.04 (6.72,19.01) | 0.38 (0.12, 0.92) | 0.64 (0.25, 2.76) |
| SDB (AHI ≥ 5) (n;%) | 17 (9.4%) | 0 (0.0%) | 17 (100%) | 4 (4.4%) | 13 (14.4%) |
| Severe SDB (AHI ≥ 15) (n; %) | 6 (3.3%) | 0 (0.0%) | 6 (35.3%) | 0 (0.0%) | 6 (6.7%) |
Mean ± SD (median) for normally distributed variables; Median (IQR) for skewed variables; N (%) for categorical variables.
Bold: P <0.05; Bold, Italic: P <0.01. P-values from unpaired Mest for normally distributed variables; Wilcoxon rank-sum test for skewed variables; Pearson chi-squared test or Fisher's exact test for categorical variables.
Overweight defined as BMI ≥ 95th age-gender adjusted percentile.
SDB = sleep disordered breathing; AHI = apnea-hypopnea index.
Comparison of PSG to Actigraphy by Data Mode
To assess the accuracy of measures of TST by each of the 3 actigraphy data modes, the mean TST and mean difference compared to concurrent measurement of sleep using PSG from each data mode are summarized in Table 2. On average, actigraphy tended to underestimate sleep duration compared to PSG, but of the 3 data modes, TAT provided estimates of sleep duration that were closest to measures made by PSG, with a mean difference from PSG of 11 minutes (95% CI: 0.8, 21.5; P = 0.035). This is contrasted with ZCM (33 minutes; 95% CI: 20.0, 32.7; P <0.001) and PIM (54 minutes; 95% CI: 41.7, 66.3; P <0.001). Subgroup analysis revealed that TAT provided estimates closer to PSG than ZCM and PIM in nearly all subgroups.
Table 2.
Comparison of TST from PSG and Actigraphy by Data Mode and Subgroupa
| Subgroup | N | PSG TST | PSG - TAT |
PSG - ZCM |
PSG - PIM |
|||
|---|---|---|---|---|---|---|---|---|
| Difference | P-value | Difference | P-value | Difference | P-value | |||
| Analytic sample | 181 | 477 ± 56 (486) | 11 ± 71 (−1) | 0.0354 | 33 ± 86 (12) | <0.0001 | 54 ± 84 (43) | <0.0001 |
| No SDB (AHI <5) | 164 | 476 ± 56 (483) | 2 ± 55 (−4) | 0.5645 | 23 ± 72 (8) | <0.0001 | 46 ± 72 (39) | <0.0001 |
| SDB (AHI ≥ 5) | 17 | 485 ± 57 (491) | 95 ± 132 (58) | 0.0093 | 130 ± 134 (91) | 0.0010 | 129 ± 140 (81) | 0.0017 |
| Girls | 91 | 483 ± 48 (495) | −9 ± 40 (−8) | 0.0378 | 6 ± 49 (1) | 0.2804 | 35 ± 54 (32) | <0.0001 |
| Boys | 90 | 470 ± 63 (476) | 31 ± 87 (14) | 0.0010 | 60 ± 104 (39) | <0.0001 | 73 ± 103 (58) | <0.0001 |
| Girls, No SDB | 87 | 483 ± 49 (497) | −11 ± 38 (−11) | 0.0067 | 1 ± 44 (0) | 0.7914 | 31 ± 49 (31) | <0.0001 |
| Girls, SDB | 4 | 481 ± 37 (487) | 48 ± 35 (61) | 0.0718 | 100 ± 68 (91) | 0.0622 | 120 ± 95 (105) | 0.0858 |
| Boys, No SDB | 77 | 467 ± 63 (470) | 18 ± 65 (9) | 0.0168 | 47 ± 89 (29) | <0.0001 | 63 ± 89 (55) | <0.0001 |
| Boys, SDB | 13 | 486 ± 63 (491) | 109 ± 148 (58) | 0.0211 | 140 ± 149 (91) | 0.0054 | 131 ± 155 (70) | 0.0099 |
PSG TST and differences presented in minutes as Mean ± SD (median); P-values from paired t-test comparing TST from PSG and actigraphy.
PSG = Polysomnography; TST = Total sleep time (minutes); TAT = Time Above Threshold; ZCM = Zero Crossing Mode; PIM = Proportional Integration Mode; AHI = apnea-hypopnea index
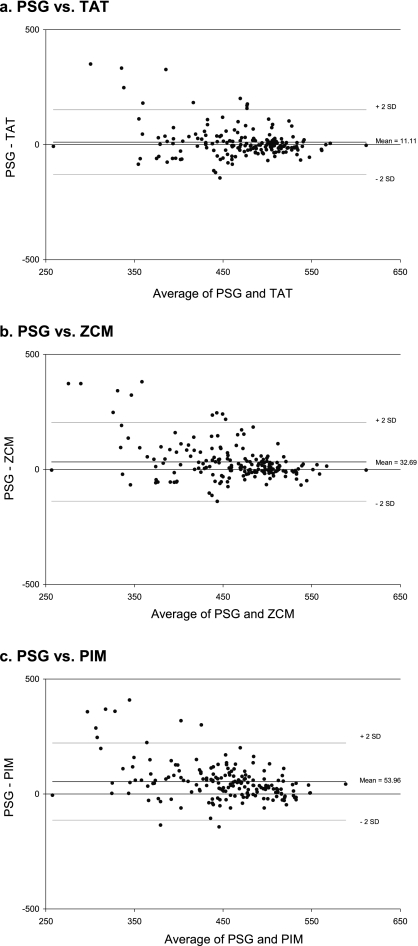
Bland-Altman plots were also employed to display the relative agreement of each data mode with PSG (Figure 1). The plots show that for all 3 data modes, actigraphy estimated TST more closely to that from PSG when the total sleep time was longer, and that the large differences tended to result from actigraphy underestimating TST. For TAT, the estimates of TST were clustered closer to those by PSG with fewer large differences in comparison with the other data modes.
Figure 1.
Bland-Altman Plots for TST (PSG) vs. TST (Actigraphy) by Data Mode
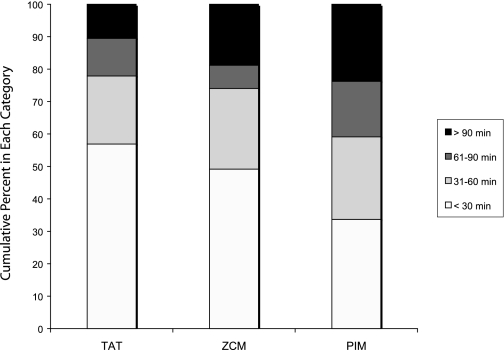
The absolute difference in TST between PSG and each actigraphy data mode was categorized into ≤30 minutes, 31–60 minutes, 61–90 minutes, and >90 minutes, and the number of measurements in each category were compared using Friedman's chi-square test. The percentage of measurements in each category are shown for each data mode in Figure 2. There was a significant difference between the 3 groups (P <0.001) and between TAT and ZCM (P = 0.001). Again, TAT had a larger percentage of measurements closer to PSG (57% within 30 minutes of PSG, compared to 49% for ZCM and 34% for PIM), with fewer large differences than ZCM and PIM (only 10% with differences >90 minutes, compared with 19% for ZCM and 24% for PIM).
Figure 2.
Categories of Absolute Difference in TST from PSG and Actigraphy by Data Mode
Finally, intraclass correlation coefficients (ICC) were calculated to measure the association between TST by PSG compared to that by each of the actigraphy data modes. The overall ICCs were low to moderate, but were highest for TAT compared to other data modes (Table 3). Subgroup analysis revealed that ICCs remained higher for TAT than for other data modes in nearly all subgroup categories.
Table 3.
Intraclass Correlation Coefficients (ICCs) for TST by Data Mode and Subgroup a
| N | PSG & TAT | PSG & ZCM | PSG & PIM | |
|---|---|---|---|---|
| Analytic sample | 181 | 0.41 (0.28–0.53) | 0.32 (0.19–0.45) | 0.34 (0.21–0.47) |
| No SDB (AHI <5) | 164 | 0.55 (0.43–0.65) | 0.42 (0.29–0.54) | 0.43 (0.30–0.55) |
| SDB (AHI ≥5) | 17 | 0.00 (0.00–0.42) | 0.00 (0.00–0.45) | 0.02 (0.00–0.48) |
| Girls | 91 | 0.66 (0.53–0.76) | 0.59 (0.44–0.71) | 0.56 (0.41–0.69) |
| Boys | 90 | 0.31 (0.11–0.49) | 0.21 (0.00–0.40) | 0.22 (0.02–0.41) |
| Girls, No SDB | 87 | 0.68 (0.55–0.78) | 0.64 (0.49–0.75) | 0.61 (0.46–0.73) |
| Girls, SDB | 4 | 0.71 (0.00–0.98) | 0.56 (0.00–0.96) | 0.44 (0.00–0.95) |
| Boys, No SDB | 77 | 0.49 (0.30–0.65) | 0.29 (0.08–0.48) | 0.29 (0.07–0.48) |
| Boys, SDB | 13 | 0.00 (0.00–0.47) | 0.00 (0.00–0.46) | 0.00 (0.00–0.49) |
ICC (95% CI)
AHI = Apnea-hypopnea index
Comparison of PSG to Actigraphy by Subgroup
Based on the above results, TAT was used as the data mode for the following subgroup analyses. Differences in TST from PSG and actigraphy by subgroup are also shown in Table 2. While measures of TST by actigraphy were close to those from PSG among those with no SDB, actigraphy underestimated sleep by over 90 minutes on average in the presence of SDB. Measures of TST by actigraphy were also closer to those by PSG for girls than boys, with TST among boys being underestimated by 31 minutes on average. Results showed that these gender differences were not fully explained by presence of SDB. ICCs were also calculated by subgroup (Table 3). ICCs were higher for those without SDB, while those with SDB had ICCs at or very near zero. ICCs were also higher for girls than boys, and this gender difference persisted when considering only girls and boys without SDB.
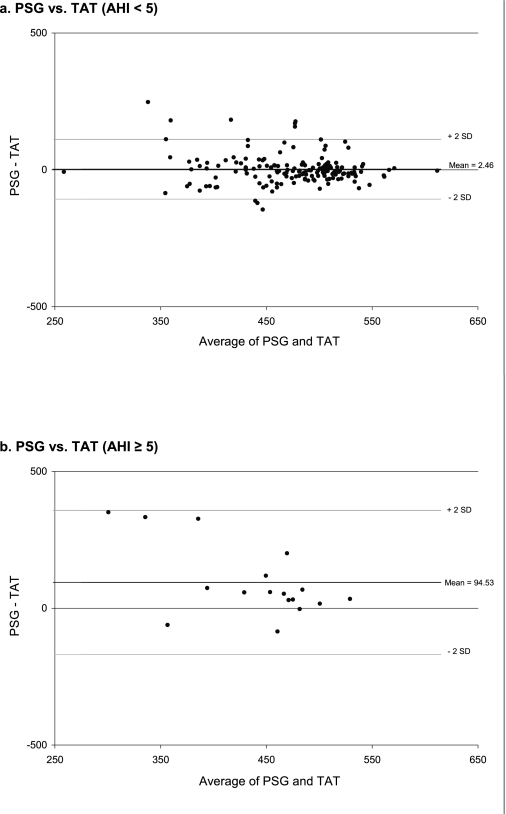
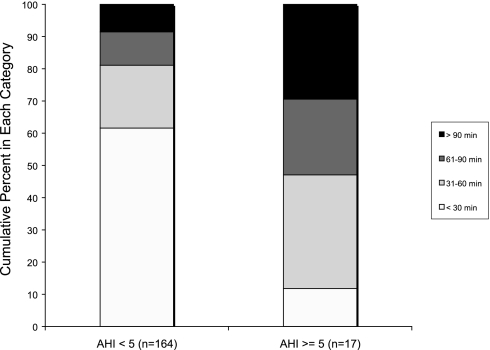
Differences in the level of agreement in TST from actigraphy among AHI subgroups were further explored graphically. BlandAltman plots are shown in Figure 3 for PSG vs. TAT stratified by AHI, showing that the mean difference between PSG and TAT was much closer to zero for those without SDB, and that fewer large differences were observed. This figure also shows that for those with SDB, actigraphy tended to underestimate sleep when compared with PSG. The number of measurements in each of the categories of absolute difference between PSG and actigraphy (using TAT) by categories of AHI were compared using the Mantel-Haenszel chi-square test, and a significant difference between the groups was observed (P <0.001). Figure 4 shows the percentage of measurements in each of these categories by categories of AHI. We see again from this figure that as AHI increases, larger differences are observed between actigraphy-based and PSG-based estimates of TST.
Figure 3.
Bland-Altman Plots for TST (PSG) vs. TST (TAT), Stratified by AHI
Figure 4.
Categories of Absolute Difference in TST from PSG and Actigraphy by AHI Category
DISCUSSION
Results showed that compared to PSG, Time Above Threshold provided estimates of TST that agreed more closely with PSG than either of the other data modes using the UCSD algorithm. Although it has been argued that PEVI most accurately identifies movement amplitude,7 estimates of TST based on this data mode compared poorly to PSG in this sample of adolescents. While some methodological discussion has been offered as to the relative benefits and limitations of each actigraphy data mode,7 little research has directly compared sleep-wake inference between data modes using clinical data. Zero Crossing Mode has typically been used for sleep-wake inference in adults, with little or no explanation for or justification of the choice of that mode.22,25 Our study questioned the assumption that this mode would be optimal in every population.
The use of wrist actigraphy as a method of estimating sleep time and sleep patterns is increasing as a measure of sleep that is simultaneously (1) more objective than sleep diaries and (2) less intrusive and expensive than polysomnography. A distinct benefit of actigraphy is the ability it provides researchers to collect data over multiple nights in the participant's normal sleep setting with minimal intrusion. This use is dependent upon actigraphy providing a valid objective measure of sleep duration compared to the “gold standard” of the PSG. Sleep-wake inference by actigraphy has been shown to be moderately accurate compared with PSG in adult and infant populations12,13,25,26 and in a mixed sample of healthy adults and adolescents.27 It has been unclear whether these findings may be generalized to a larger sample of adolescents with a range of sleep disordered breathing severity, who may have patterns of sleep and movement that are different from both younger and older individuals.4
This study compared estimates of TST from PSG and actigraphy using data collected concurrently on 181 adolescents. The nature of the study protocol allowed us to look at the agreement of actigraphy estimates of TST and the “gold-standard” of PSG, and also to explore this agreement for specific subgroups with different degrees of SDB identified by PSG. Our results showed that actigraphy provides good estimates of sleep time in adolescents without SDB but systematically underestimates TST in adolescents with SDB. This underestimation of sleep is consistent with results found in adults with severe SDB12 and may be explained by limitations in motion-based algorithms to accurately classify SDB-associated movement arousals. In particular, actigraphy data were collected in one-minute epochs, which may be classified as wake in the presence of movement that may not constitute the wake state when analyzed neurophysiologically in 30-second scoring epochs.
Actigraphy more accurately estimated sleep duration in girls than in boys. Girls tended to have a longer TST and lower prevalence of SDB than boys. However, exploratory analyses suggested that differences in SDB did not explain the gender differences observed in the level of agreement for actigraphy and PSG estimates of TST (Tables 2 and 3). It has been shown that the sleep patterns and movement during sleep vary between school-age girls and boys,14 between adolescent girls and boys,15,16 and between adolescents and younger children.28 This study gives additional objective justification for such conclusions by showing that female and male adolescents differ in sleep duration, and that the reliability of actigraphy as a measure of sleep differs in these groups. While the etiology of these gender differences in movement during sleep is not well understood, the differences observed are likely attributable to differences in the pattern of movement during sleep.
In the TeenZzz study, as in other epidemiological studies of sleep employing actigraphy,29,30 actigraphy was primarily utilized to estimate average sleep duration over multiple nights. In this context, there may be less of an imperative to expect actigraphy to provide complete agreement with PSG,31 but rather, provide estimates that are sufficient to distinguish subgroups with short, average, and long sleep durations. Thus, for our analysis we chose TST as a summary measure rather than pursuing an analysis of specificity and sensitivity of actigraphy sleep-wake inferences on a minute-by-minute basis.
The importance of estimating TST is underscored by recent studies showing clear associations of self-reported sleep duration with a variety of health outcomes, including diabetes, hypertension, coronary artery disease, and mortality.32,33,34 Actigraphy is often used to objectively estimate sleep where sleep duration is used as a predictor for functional impairment or health outcomes. The present analysis shows that actigraphy may systematically misclassify sleep duration in certain population subgroups. Our data suggest the need for caution in interpreting associations of actigraphy-based sleep duration and health outcomes in studies of heterogeneous populations, and to consider the potential impact of disturbed sleep on estimation of sleep duration.
The subgroup differences observed in this analysis also point to the need for increased sensitivity to how actigraphy data are processed. Using a particular data processing mode and specific scoring algorithm in a new population should incorporate an assessment of how that population may vary from the population that was used in validating that analytic approach. Further, it should not be assumed that if actigraphy is shown to be a valid measure of sleep in a population, it will be equally valid for all subgroups of the population. The changes in reliability of actigraphy among subgroups underscores the importance of considering the impact of underlying sleep patterns and pathology on the accuracy of movement-based approaches for estimating sleep duration. Future research would benefit from further exploration of the root of these systematic biases. The proprietary scoring software utilized in our study was not developed considering the impact of disturbed sleep on sleep-wake inference. In addition, while the correlations were very high between TST as calculated by other algorithms available in the software, a single algorithm was chosen for this analysis. Additional research is also necessary to develop and test algorithms that are optimized to be more sensitive to the sleep and movement patterns exhibited by adolescents or that could control for some easily measured indicators of SDB.
The strength of this study is the selection of a relatively large sample of participants in an age group for which little validation of actigraphy has been done. The study sample allowed assessment of the impact of mild to moderate levels of SDB on sleepwake inference. However, since the majority of subjects were recruited from a community setting, the study was limited by relatively small numbers of subjects, particularly females, with high AHI.
In summary, our data show that TAT is the optimal data collection mode for actigraphy in an adolescent population; that actigraphy correlates reasonably well with PSG in adolescents, but that the level of agreement varies by levels of respiratory disturbance and by gender. Recognition of the variation in estimates of sleep from actigraphy in different data collection modes, between population subgroups, and across the age spectrum, may be of fundamental importance in the interpretation of actigraphy data for sleep estimation.
ACKNOWLEDGEMENT
Support provided by RO1 HL709161, K23 HL04426, and M01 RR00080
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Rosen has received research support from Cephalon. Dr. Ancoli-Israel has been a consultant for or on the advisory board of Acadia, Cephalon, King Pharmaceuticals, Merck, Neurocrine Biosciences, Neurogen, Pfizer, Sanofi-Aventis, Sepracor, Somaxon, and Takeda and has participated in speaking engagements for Cephalon, King Pharmaceuticals, Neurocrine Biosciences, Pfizer, Sanofi-Aventis, Sepracor, and Takeda. Dr. Redline has been an advisor for Cypress Bioscience and Organon. Ms. Kibler was a study coordinator for clinical research study sponsored by Cephalon. Mr. Johnson, Dr. Kirchner, Ms. Storfer-Isser, Ms. Cartar, and Ms. Emancipator have reported no financial conflicts of interest.
REFERENCES
- 1.National Institutes of Health, National Center on Sleep Disorders Research and Office of Prevention, Education, and Control. Working group report on problem sleepiness. 1997. Aug,
- 2.National Institutes of Health, National Center on Sleep Disorders Research and Office of Prevention, Education, and Control. Problem sleepiness in your patient. 1997. Sep, NIH Publication No. 97-4073.
- 3.National Institutes of Health, National Center on Sleep Disorders Research and Office of Prevention, Education, and Control. Educating youth about sleep and drowsy driving. 1998. Sep,
- 4.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69:875–887. [PubMed] [Google Scholar]
- 5.Carskadon M. Adolescent sleepiness: increased risk in a high-risk population. Alcohol, Drugs and Driving. 1990;5/6:317–28. [Google Scholar]
- 6.Gupta NK, Mueller WH, Chan W, Meininger JC. Is obesity associated with poor sleep quality in adolescents? Am J Hum Biol. 2002;14:762–8. doi: 10.1002/ajhb.10093. [DOI] [PubMed] [Google Scholar]
- 7.Ancoli-Israel S, Cole R, Alessi C, et al. The role of actigraphy in the study of sleep and circadian rhythms. American Academy of Sleep Medicine Review Paper. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 8.Carskadon MA, Acebo C. Regulation of sleepiness in adolescents: update, insights, and speculation. Sleep. 2002;25:606–16. doi: 10.1093/sleep/25.6.606. [DOI] [PubMed] [Google Scholar]
- 9.Smith JR, Karacan I, Yang M. Automated measurement of alpha, beta, sigma, and theta burst characteristics. Sleep. 1979;1:435–43. [PubMed] [Google Scholar]
- 10.Sadeh A, Lavine P, Scher A, et al. Actigraphic home-monitoring sleep-disturbed and control infants and young children: a new method for pediatric assessment of sleep-wake patterns. Pediatrics. 1991;87:494–9. [PubMed] [Google Scholar]
- 11.Sadeh A, Hauri PJ, Kripke DF, Lavie P. The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18:288–302. doi: 10.1093/sleep/18.4.288. [DOI] [PubMed] [Google Scholar]
- 12.Hedner J, Pillar G, Pittman SD, et al. A novel adaptive wrist actigraphy algorithm for sleep-wake assessment in sleep apnea patients. Sleep. 2004;27:1560–6. doi: 10.1093/sleep/27.8.1560. [DOI] [PubMed] [Google Scholar]
- 13.Kushida CA, Chang A, Chirag G, et al. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2:389–96. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 14.Sadeh A, Raviv A, Gruber R. Sleep patterns and sleep disruptions in school-age children. Dev Psychol. 2000;36:291–301. doi: 10.1037//0012-1649.36.3.291. [DOI] [PubMed] [Google Scholar]
- 15.Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998;21:871–81. doi: 10.1093/sleep/21.8.871. [DOI] [PubMed] [Google Scholar]
- 16.Gaina A, Sekine M, Hamanishi S, Chen X, Kagamimori S. Gender and temporal differences in sleep-wake patterns in Japanese schoolchildren. Sleep. 2005;28:337–42. [PubMed] [Google Scholar]
- 17.Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142:383–9. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. Available at: http://www.cdc.gov/nchs/about/major/nhanes/datalink.htm.
- 19.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–85. [PubMed] [Google Scholar]
- 20.Rechtschaffen A, Kales A. Bethesda, Maryland: U.S. Dept. of Health, Education, and Welfare; 1968. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. [Google Scholar]
- 21.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 22.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–9. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- 23.Girardin JL, Kripke DF, Mason WJ, Elliot JA, Youngstedt SD. Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods. 2001;105:185–91. doi: 10.1016/s0165-0270(00)00364-2. [DOI] [PubMed] [Google Scholar]
- 24.Bland JM, Altman DG. Statistical method for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10. [PubMed] [Google Scholar]
- 25.de Souza L, Benedito-Silva AA, Pires ML, Poyares D, Tufik S, Calil MH. Further validation of actigraphy for sleep studies. Sleep. 2003;26:81–5. doi: 10.1093/sleep/26.1.81. [DOI] [PubMed] [Google Scholar]
- 26.Pollak CP, Tryon WW, Nagaraja H, Dzwonczyk R. How accurately does wrist actigraphy identify the states of sleep and wakefulness? Sleep. 2001;24:871–8. doi: 10.1093/sleep/24.8.957. [DOI] [PubMed] [Google Scholar]
- 27.Sadeh A, Sharkey K, Carskadon M. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17:201–7. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- 28.Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27:774–83. [PubMed] [Google Scholar]
- 29.Gruber R, Sadeh A. Sleep and neurobehavioral functioning in boys with attention-deficit/hyperactivity disorder and no reported breathing problems. Sleep. 2004;27:267–73. doi: 10.1093/sleep/27.2.267. [DOI] [PubMed] [Google Scholar]
- 30.Blackwell T, Yaffe K, Ancoli-Israel S, et al. for the Study of Osteoporotic Fractures Group. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–10. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 31.Tryon WW. Issues of validity in actigraphic sleep assessment. Sleep. 2004;27:158–65. doi: 10.1093/sleep/27.1.158. [DOI] [PubMed] [Google Scholar]
- 32.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–7. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 33.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657–61. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 34.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]