Abstract
Study Objectives:
To resolve inconsistencies in previously reported changes in percentage of rapid eye movement sleep (REM%) over the adult lifespan and to identify gaps in available information about adults' REM sleep.
Design:
A research synthesis approach specifically designed to detect nonlinear change. Cubic B smoothing splines were fitted to scatterplots generated from reported means and variance for REM%, REM minutes, and total sleep time.
Participants:
382 English-language research reports provided REM% values for 4171 subjects; REM minutes values for 2722 subjects; and values of total sleep time for 5037 subjects. Samples were composed of subjects described by authors as normal or healthy. Mean ages of samples ranged from 18.0 to 91.7 years.
Setting:
University research center.
Interventions:
N/A.
Measurements and Results:
Two coders extracted information. Intercoder reliability was above cutoffs for excellent. Authors often failed to describe screening procedures used to determine subjects' health status. Few results were reported separately for women. The functional relationship between age and REM% was essentially linear over much of the adult lifespan, decreasing about 0.6% per decade. The best estimate of when REM% ceased its small linear decline was the mid-70s, after which time a small increase in REM% was observed due to REM minutes increasing while total sleep time declined.
Conclusions:
Ability to detect both linear and nonlinear change in REM%, REM minutes, and total sleep time over the lifespan was useful for resolving inconsistent findings about the existence of changes in REM% with aging. This approach to research synthesis also facilitated identification of ages for which little normative information about REM sleep was available.
Citation:
Floyd JA; Janisse JJ; Jenuwine ES et al. Changes in REM-sleep percentage over the adult lifespan. SLEEP 2007;30(7):829-836.
Keywords: REM sleep, meta-analysis, aging, nonlinear age trends
AN INTEREST IN AGE-RELATED CHANGES IN SLEEP-STAGE PERCENTAGES HAS GENERATED A LARGE BODY OF RESEARCH FINDINGS OVER THE PAST 45 YEARS. TWO groups of researchers have attempted to synthesize the extensive research findings that describe age-related sleep-stage change using meta-analysis. Beginning in 2000, Floyd and her colleagues reported several meta-analytic findings for how sleep changes over the adult lifespan. They found that total sleep time (TST) and percentage of slow-wave sleep decline with age, whereas the percentages of stage 1 and stage 2 sleep increase with age.1–3 In 2004, Ohayon and his colleagues confirmed these findings and extended knowledge about lifespan changes in sleep stages by examining studies of children and adolescents as well as young, middle-aged, and elderly subjects. Also, in addition to the sleep-stage variables described by Floyd et al, Ohayon et al included findings for percentage of rapid eye movement sleep (REM%).4 Both Floyd et al and Ohayon et al analyzed effect sizes that represented the linear components of sleep-stage change.
Ohayon et al's4 inclusion of REM% as a variable of interest in their meta-analysis was a major contribution because the existence of REM% changes over the adult lifespan has been contested. Several researchers have reported that REM% declines as adults age,5–18 whereas several other researchers have reported no age-related decline in REM%.19–25 Collectively, these findings suggest that any decline in REM% with age is either very small, and therefore difficult to detect, or nonexistent. It is also possible that the decline in REM% is nonlinear in nature, in which case the linear component of the decline could be masked in some studies and not others, depending on the age range examined.
Ohayon et al reported a small linear decline in REM% during some phases of adulthood.4 Their discussion suggested that the functional relationship between age and REM% may be nonlinear, although the nature of the nonlinearity remained unclear. For example, Ohayon et al reported that “percentage of REM sleep first increased from childhood to adolescence, then decreased between young and middle-aged adults, and remained unchanged in subjects older than 60 years of age.” 4(p1268); however, they summarized their findings for age-related REM% as follows: “There was a modest but significant increase in the percentage of REM sleep from childhood to the end of adolescence. After that age, percentage of REM sleep remained relatively stable until 60 years of age, when the percentage again began to decline.”4(1270) In addition, Ohayon et al's Figure 1d suggested a steady decline in REM% over the lifespan from ages 5 through 90.4(p1264) Finally, Ohayon et al's Figure 2 showed a decline in minutes of REM from ages 10 to 15 years, then little if any change from ages 15 to 55 years, another decline in REM minutes from ages 55 to75 years, and no decline in REM minutes after age 75.4(p1270) Given the linear decline in TST over the adult lifespan reported by Floyd et al1–3 and confirmed by Ohayon et al to exist until at least age 60,4(p1255,1258,1269) a lack of change in REM minutes from ages 15 to 55 would lead to increases in REM% during young and middle adulthood rather than to no change or a decline in REM%. These disparate presentations suggest REM% changes over the lifespan are not well understood and could benefit from further analysis and synthesis.
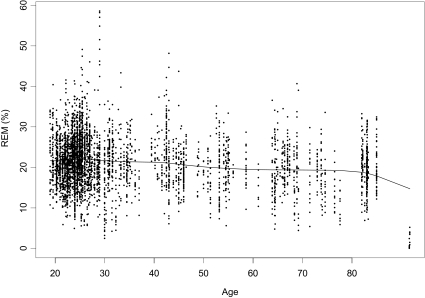
Figure 1.
Example for a smoothing spline for rapid eye movement percentage (REM%) for meta-analytic sample through age 91.7 years (n = 4171).
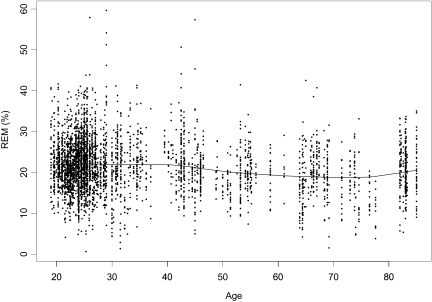
Figure 2.
Example of a smoothing spline for rapid eye movement percentage (REM%) for meta-analytic sample through age 85.0 years (n = 4157).
Purpose
The primary purpose of this study was to apply research synthesis methods specifically designed to detect nonlinearity in age-related sleep change in order to clarify and extend knowledge about the nature and magnitude of change in REM% values over the adult lifespan. To accomplish this, a method of research synthesis was employed that makes use of multiple samples with varying central tendency and dispersion for age to describe age-related change.26 Using this approach, any change in REM% between young and middle-aged adults can be described if it exists; also, changes in the functional relationship reported by Ohayon et al4 as occurring around age 60 can be further explored. A second purpose of this study was to identify age groups for which more normative data about REM sleep would be helpful for clarifying how REM% changes during adulthood. Finally, whenever enough data were available, a third purpose of this project was to examine the effect on REM% of variables that previously had been reported by meta-analysts to mask the magnitude of linear relationships between age and sleep. These variables include control of the first-night effect (controlled/uncontrolled), sex composition of study samples (all men, all women, mixed sex), and objective evidence that subjects who were assumed to be healthy were healthy (present/absent).1,2,4,27–29
METHODS
This report is a part of a program of research focused on the use of various meta-analytic methods to describe how sleep changes over the adult life-span.1–3,29–33 When using traditional meta-analytic methods, effect sizes (values representing odds ratios, correlations, or standardized differences between groups) are calculated from the statistical information provided by each study that addresses the same bivariate relationship between variables (eg, the correlation between REM% and age).34
Newer methods are emerging for the synthesis of univariate sample information when provided for samples with varying mean ages. These methods involve: (a) the parametric simulation of data based on the reported sample statistics from studies in the meta-analysis (constrained by reported sample parameters and population assumptions) and (b) the fitting of smoothing splines to these data.26 Sample statistics for a variable of interest from samples representing narrow bandwidths on age are used in the simulation of data sets. The scatterplots of these data sets show possible values for the variable of interest over the age range of interest. Next, functions are fit that provide an estimate of the magnitude of linear trends and can be tested for nonlinearity. If any of the nonlinearity in the functional relationship between age and REM% for adults suggested by the work of Ohayon et al4 is detected, segmented regression can be used to detect inflection points, ie, the point estimate and confidence interval (CI) for the age at which REM% declines; thus, the methods allow for the examination of linear components of age-related sleep change and further allow for the examination of nonlinear change when it is suspected in sleep values over the lifespan.
Sampling
In meta-analysis, it is never possible to obtain the entire population of studies addressing a research question; thus, it is important to retrieve the sample of existing studies in ways that do not bias the meta-analytic sample, ie, do not systematically exclude a segment of the target population. The target population for the synthesis of knowledge about how REM% changes as adults age was all research reports meeting the following criteria: (1) studies were published in English from 1960 through 2002; (2) studies focused on adults, ie, at least 95% of subjects in each sample were 18 years of age or older; (3) subjects in studies were reported to have been drawn from nonclinical populations and were described as “normal” or “healthy”; (4) authors reported age ranges of samples that were equivalent to a standard deviation of 4.0 or fewer years; and (5) authors reported means and standard deviations for REM% for 1 or more appropriate samples.
The year 1960 was used as the lower chronologic limit for retrieval of studies because the early 1960s mark the point of general acceptance that sleep cycles consist of 4 stages plus REM sleep.29 The maximum within-group standard deviation for age was set at 4.0 years in order to have estimates of REM% for a relatively narrow bandwidth on age. The narrower the age bandwidth for each sample, the greater the sensitivity of the statistical modeling of nonlinear trends across the adult lifespan.
The potentially accessible population was composed of all studies that met the above criteria and were (a) indexed in electronic and paper index and abstract journals; (b) cited on a reference list from retrieved research reports; or (c) identified by archivists in anthropology, health education, nursing, psychology, or sociology departments with accredited graduate programs as an unpublished master's project. Most reports were identified by searching the following databases to obtain reports and reference lists: (1) MEDLESTE: PubMed (1963–2002) and OVID (1990–2002); (2) OLDMEDLINE (1960–1965); (3) CINAHL: OVID (1982–2002) and print (1960–1981); (4) PsycINFO, OCLC First Search (1960–2002); (4) dissertation abstracts, OCLC First Search (1960–2002); (5) IDEAL, Academic Press Journals (1993–2002). The list of keywords used for searches was extensive in order to maximize the number of titles for review that might contain descriptive information about numerous sleep variables, including REM%. Detailed lists of search terms used and comparisons of search strategies for retrieval of sleep studies by age have been previously published.33
Procedures
Titles and abstracts of identified papers were reviewed prior to obtaining a paper copy. This was done in order to exclude papers that seemed clearly devoid of the required information. However, the goal was to locate as much descriptive information as possible about sleep values whether or not the central focus of the study was age-related sleep change. To assess the assumption that exclusion of papers based on their titles and abstracts was valid, systematic sampling with multiple random starts was used to draw samples of papers originally judged “not relevant” from each search in order to reexamine them for relevancy. None of the drawn samples contained papers that met the inclusion criteria for the meta-analysis; thus, there was no evidence that this practice led to the exclusion of appropriate papers.
All papers screened for the study were cataloged alphabetically by author in order to easily detect duplication during the retrieval stage. When data were presented in more than 1 source, the most complete source was chosen, but additional details about sample and study characteristics were incorporated from multiple sources whenever doing so increased the amount of information about a study. Citation information was stored in a reference file created using Pro-Cite software.
Two trained coders extracted the information from relevant papers. Discrepancies between the coders were examined to achieve consensus. Errors were usually due to mistakes in transcription and entry rather than to substantive differences related to coder judgment. Intercoder reliability was stable throughout the study with kappas well above the cutoff for excellent reliability, ie, kappa ≥ 0.75.2,3,30
Analysis and Results
More than 600 papers were identified that might contain REM% values for 1 or more groups of normal or healthy adults of a similar age. Retrieved papers typically belonged to 1 of the following categories: (1) studies of normal sleep; (2) studies of the effect of various conditions, substances, or both on normal sleep, thus usable baseline sleep values were reported for healthy subjects; (3) studies of sleep of subject populations with various physical or psychological conditions compared with groups of healthy subjects, thus usable data from healthy comparison groups were reported.
Research reports were deliberately overretrieved in order to identify as many studies as possible that were suitable for the research synthesis. Approximately 60% of the papers retrieved and reviewed were unusable because they (a) provided descriptive statistics for REM% for groups of subjects whose ages were spread over wide, rather than narrow, ranges; (b) provided measures of central tendency for age but no information from which the standard deviation for age could be estimated; or (c) reported central tendency for REM% for a narrow age range of adults but no variance estimates for REM%.
The final sample of research reports meeting all inclusion criteria for the synthesis of data about REM% over the lifespan consisted of 244 reports. These reports provided means and standard deviations for REM% for 344 samples representing 4171 subjects. Mean ages of samples for REM% data ranged from 19.0 to 91.7 years. For the measurement of REM%, when studies recorded only 1 night of polysomnography, these results were used. When studies recorded 2 or more nights, second-night results were used unless only a later night was recorded. Data for REM% were collected on the first night of polysomnography for 12.5% of the samples (n = 43) and the second or a later night for 86.6% of the samples (n = 298); the night of collection was not specified for 0.9% of the samples (n = 3). When data were presented in both peer-reviewed and non-peer-reviewed formats, peer-reviewed data were used. As a result, 98% of the data came from peer-reviewed journals. Twenty-five percent of the studies (n = 61) were published before 1985; 25% between 1986 and 1992, and half of the studies (n = 122) were published since 1992.
After eligible studies were coded for relevant study elements and study results, descriptive analyses were done using SPSS (SPSS, Inc., Chicago, IL). The reported findings for REM% and information about potential moderator variables were also entered into a data file in S-PLUS (Insightful Corp., Seattle, WA), and a bootstrapping procedure was used to estimate the changes in REM% over the adult lifespan. For each of the 344 samples, the reported mean and standard deviation were used to generate a random set of data points of size equal to the reported sample size for the sample. Because examination of the reported means and standard deviations supported an assumption that REM% values more closely approximated a χ2 distribution than a normal distribution, the reported means and standard deviations were used to generate random χ2 distributions. The REM% values were independently generated based on the mean and standard deviation constraints at fixed mean values of age.26 Next, the newly created distributions from the 344 samples were merged to produce a large dataset for age and REM%. Two thousand bootstrapped datasets were generated.
Estimates of Correlations
In order to report the linear relationship between age and REM% using an effect-size indicator familiar to most researchers, average correlations between age and REM% and its corresponding 95% CI were estimated from the 2000 bootstrapped datasets. The average correlation and 95% CI were established using standard procedures, ie, each estimated correlation was converted to a zr score and then averaged as recommended in the meta-analytic literature.34 The average zr score was then converted back to an average correlation. The 95% CI for this average correlation was obtained as the 2.5 percentile and 97.5 percentile scores from the bootstrapped distribution. Averaged over all 2000 datasets, the slope of the linear function was −0.055 (95% CI = −0.063, −0.046). The correlation between age and REM% was r = −.168 (95% CI = −.193, −.142).
Fitting of REM% Smoothers
Cubic B smoothing splines with 5 degrees of freedom were fitted to the 2000 bootstrapped datasets in order to show the relationship between age and REM%. An example of 1 of the 2000 datasets to which a smoother was fitted is shown in Figure 1. As can be seen, most data came from samples with mean ages between 19 and 30 years, and data were sparse for samples with mean ages between 55 to 64, between 77 to 82, and older than 85 years. Also, a slight decline in REM% that accelerated noticeably around age 85 can be observed. This steep decline in REM% around age 85 was due to gaps in scatterplot data for samples with mean ages between the mid-80s and early 90s in conjunction with atypically small mean values for REM% for the two oldest samples, ie, REM% less than 5%. These two oldest samples came from one study of 14 subjects, which provided one sample 7 women and another sample of 7 men. The mean sample ages were 91.7 for the women and 91.6 years for the men. Because of the gaps in scatterplot data for samples with mean ages between the mid-80s and these two samples, these samples had considerable and undue influence on the fitted smoother. They were removed from the dataset; a second smoother was fitted to the remaining 342 samples ranging on mean age between 19.0 and 85.0 years, see Figure 2. As can be seen, without the influence of data from the two oldest samples, the function had an observable nonlinear component, that is, a slight decrease across the first 5 to 6 decades of the adult lifespan, followed by a leveling off and slight increase in REM% values during the sixth to seventh decades.
Based on the observation of this nonlinearity, a segmented relationship made up of the join of a linear and quadratic curve was explored. The estimated join point from each of these fits was then averaged to provide the age at which the functional form of the relationship between age and REM% began to depart from linear. Based on 2000 bootstrapped datasets, the join point was at age 75.59 (95% CI = 73.80, 77.57). Before the join point, the linear portion of the function had a slope of −0.062 (95% CI = −0.074, −0.051). Following the join point, REM% appeared to level off (no longer decline significantly with age) and then increase slightly. See Table 1 for values of REM% at key ages based on the Cubic B smoothing splines.
Table 1.
REM Percentage Values Based on Fitted Smoothers
| Age | REM% | 95% CI |
|---|---|---|
| 19 | 21.68% | 21.26–22.11 |
| 40 | 21.23% | 20.78–21.70 |
| 75 | 18.78% | 18.30–19.29 |
| 85 | 20.35% | 19.69–21.06 |
Moderator Analysis
Potential moderators of the functional relationship between REM% and age were explored, including (a) control for the first-night effect, (b) sex composition of study samples, (c) objective evidence that investigators excluded subjects with possible mental health problems including depression, and (d) objective evidence that investigators excluded subjects with possible sleep disorders including sleep apnea. The same methods outlined above were used to generate separate scatterplots for each category of a moderator whenever enough data were available.
Removal of the 13.4% of samples (n = 46) for which control of the first-night effect was unknown or lacking did not noticeably affect the smoother fitted to the full meta-analytic sample. Too few all-women samples (12%; n = 41) existed to contrast confidently the shape of the smoother for all-women samples with the full meta-analytic sample. Most all-women samples had mean ages less than 35 years. Given the data available, it appeared that there was a slight increase rather than a decrease in REM% for women from ages 19 to the mid-30s. Data were sparse after the mid-30s, with complete gaps in data for all-women samples between the mid-30s through the early 40s, between the mid-50s to late 60s, and from the mid-70s through the early 80s.
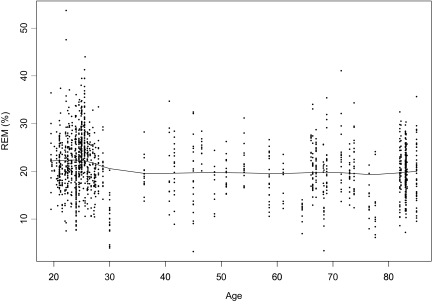
The majority of studies did not describe how the mental health of subjects was ensured, but 30% of the samples (n = 103) came from studies that used reliable psychiatric screening batteries to rule out the possibility of undetected mental health problems, including depression. The decline in REM% between age 40 and the mid-70s seen in the full meta-analytic sample was not observed in these samples (see Figure 3); however, scatterplot data from samples reported to have been strictly screened for mental health problems had large gaps from age 30 until the late 60s.
Figure 3.
Example of a smoothing spline for rapid eye movement percentage (REM%) in samples strictly controlled for depression (n = 1426).
Even fewer samples (21%; n = 73) came from studies in which the authors reported that sleep disorders had been ruled out in the presumably healthy subjects. Examination of scatterplots and smoothing splines using only this subset of studies showed approximately the same slight decline in REM% shown in the full meta-analytic sample until the mid-70s, followed by a slight up-tick in REM% in the early 80s.
Fitting of Additional Smoothers for REM Minutes and TST
In order to provide additional insights into how REM% varies over the adult lifespan, smoothers were fit to scatterplot data for REM minutes and for TST in minutes. The same approaches described above were used for identification, retrieval, and screening of papers and for data management, generation of scatterplots, and fitting of smoothers. A list of papers that provided data for the syntheses of research findings for REM%, REM minutes, and TST, respectively, is available at the SLEEP website.
REM Minutes
Data for REM minutes came from 239 samples with a total of 2722 subjects. Data from the same 2 samples eliminated in the analysis of REM% were eliminated from this analysis of REM minutes given their outlier status on both age and REM values. Mean ages of samples ranged from 18.0 to 85.0 years. Based on sample means and standard deviations, the distribution of REM minutes in the population was assumed to be χ2. Data were unavailable or sparse for ages 37 to 42, 46 to 53, 55 to 62, 68 to 73, 76 to 81, and over 85 years. For data available, the functional relationship between age and REM minutes was quadratic in nature, with the minimum value for REM minutes estimated to occur at age 63.29 years (95% CI = 60.84, 65.98). As occurred when analyzing REM%, relatively few studies (a) failed to control for the first-night effect, (b) provided data for all-women samples, or (c) described procedures used to determine that subjects were healthy.
TST Minutes
Data for TST came from 424 samples with a total of 5037 subjects. Again, data from the two samples previously identified as outliers were not included. Mean ages of samples ranged from 18.0 to 85.5 years. There were no significant gaps in TST data for this age range. Based on sample means and standard deviations, the distribution of TST in the population was assumed to be normal. As expected based on previously published meta-analytic findings,1–4 there was evidence of a substantial decline in TST over the adult lifespan, ie, the correlation between age and TST was r = −.38 (95% CI = −.40, −.36). The slope of the function was −1.22; thus, for each decade in age from the 20s through the mid-80s, the minutes of nighttime sleep decreased 12.2 minutes.
DISCUSSION
The effect size detected for the linear relationship between age and REM% (r = −.17) is considered a “small effect” by Cohen,35 as is the effect size reported by Ohayon et al for the linear decline in REM% with age (d = −0.46),4 Similar results from two different approaches to meta-analysis supports the existence of a small but significant decline in REM% over the adult lifespan.
A significant nonlinear component also was detected in the relationship between age and REM% with (a) the mid-70s identified as the time when REM% most likely ceases its small linear decline and (b) ages 75 to 85 identified as a decade during which there are small increases in REM%. Decline in REM% was estimated to be approximately 2.9% from age 19 to 75. From ages 76 to 85, approximately 1.6% of the decline in REM% was recovered.
Results from analysis and synthesis of REM research findings reported in minutes rather than percentages suggested the age at which REM% begins to increase may be earlier than the mid-70s, ie, age 63, given that this is the age at which REM minutes were found to increase while TST continued to decline. However, means and standard deviations for REM minutes were retrieved from fewer samples than were available for REM% and represented fewer subjects. Based on the data available, it was concluded that the age at which REM% most likely ceases to decline is closer to the mid-70s than age 63. Either way, it appears that Ohayon et al's4 description of REM% as declining from young adulthood to middle age and ceasing to change after age 60 is more congruent with the findings from this study than any of the other possibilities reported by them.
There currently are several hypotheses about the function of REM sleep. Frequently studied hypotheses are those that propose that REM sleep is involved in perceptual learning, memory consolidation, synthesis of new information, or arrangement of information into a network of internal associations.36 Although some support exists for each hypothesis, no unified theory of REM function has emerged at this point.36–40 Progress toward determining the function of REM sleep will be needed to determine the significance of changes in REM% over the adult lifespan.
Discussion of Moderators
Slight variations in the pattern of decline in REM% with age compared to the full meta-analytic sample were observed in the smoothers fitted to data from all-women samples as well as smoothers fitted to samples known to be well screened for mental health problems. These variations may be due entirely to the sparseness of the existing data sets for these two potential moderators. The similarity between the shapes of smoothers fitted to samples reported to be free of sleep disorders compared to smoothers fitted to the full meta-analytic sample may have resulted from researchers routinely ruling out sleep disorders but not reporting that they had done so. This absence of specific statements in the research reports would result in no differences in REM% values between studies by authors who reported that sleep-disordered subjects had been screened out and those authors who did not report their screening procedures. Finally, it is not surprising that removal of reports with no control of first-night effects did not affect the appearance of smoothers given that they constituted only 13.4% of reports.
Previously published meta-analyses have shown that correlations between age and sleep characteristics tend to be diminished in magnitude when findings are included from studies by investigators who did not specifically mention that they controlled for physical and mental health problems.1–4 Specifically, Ohayon et al. reported that REM% was one of the sleep characteristics for which failure to state that subjects had been screened for sleep apnea produced a smaller correlation between sleep and age. To the extent that subjects with sleep apnea were included in the presumably healthy samples, the estimate of the negative correlation between age and REM% may be slightly greater than −.17.
Discussion of Methods
One advantage of the research synthesis method used for this study is its ability to estimate the functional relationship between sleep and age without imposing any a priori relationship. The actual relationship between the variables of interest is used to develop the function. This facilitates the detection of nonlinearity in the relationship without having to know a priori how the nonlinearity will be manifested. Once the form of the relationship is known, parameter estimates for this relationship and for points of interest in the relationship can be estimated by fitting an appropriate segmented regression.
A requirement of the method is that descriptive information be available from samples composed of subjects of a similar age. Minimal age gaps between existing samples are required to detect nonlinearity. Minimal age gaps are especially critical at ages at which the inflection points may be located. Point estimates based on wide age ranges assume linearity across the age range obscuring the nonlinearity that may be present. Gaps in the age ranges at which inflection points are located result in unstable point estimates due to the lack of information within these gaps. The necessity of using samples composed of subjects of a similar age poses a unique challenge to the meta-analyst in the literature-retrieval stage of the research synthesis process. Studies are not indexed based on narrow age ranges; thus, it is necessary to retrieve large numbers of studies that later are eliminated from the meta-analysis because they have samples with age ranges too wide for inclusion in this approach.33
A related issue is the fact that studies providing only correlations between age and sleep characteristics (ie, no means or standard deviations for sleep variables) are excluded in this approach to knowledge synthesis. Although the omission of these studies could be seen as a limitation of the method, in fact, this method allows for inclusion of many more studies than typically meet the inclusion criteria for the meta-analysis of correlations. This research synthesis method draws on information from a different set of studies, ie, those reporting central tendency and variance estimates. When it draws information from studies that meet the inclusion criteria for both approaches to meta-analysis, it makes use of different statistics, ie, sample means and standard deviations rather than correlations; thus, this method provides an additional opportunity to estimate the linear component of age-related change. More importantly, it allows for the exploration of nonlinearity, which is ignored by meta-analysts when they use d to synthesize findings from studies comparing a younger with an older group and when they use r to estimate correlations.
A final requirement when using this approach to research synthesis is that some assumption must be made about the shape of the distribution within each sample for the outcome. This is important both for generating the data for the simulations and for proper modeling of the error structure in the data when estimating the smoothers and segmented regression. Substantive knowledge of the research area, as well as an examination of the available data, should be used to guide these assumptions.
In summary, the methods used in this study to detect linear and nonlinear changes in REM sleep over the adult lifespan (a) confirmed a small average decline in REM% with age; (b) identified ages at which rates of decline in REM sleep increased, decreased, and reversed themselves; and (c) pinpointed age groups for which information about REM-sleep norms is sparse. As more descriptive information becomes available and fills in gaps in the REM-sleep-and-age scatterplots employed with this approach to research synthesis, it will be possible to describe REM-sleep change in the old-old population and identify more precisely the age at which REM minutes begin to increase rather than decrease with age. In addition, more data about the norms for REM sleep in women will allow sex differences in age-related REM sleep to be explored more completely.
ACKNOWLEDGMENTS
Work performed at: Wayne State University
Funded by: NIH R01 NR003880, “Aging-Related Sleep Changes: A Meta-Analysis”
No off-label or investigational use present
Footnotes
Disclosure Statement
This was not an industry supported study. Drs. Floyd, Janisse, Jenuwine, and Ager have reported no financial conflicts of interest.
REFERENCES
- 1.Floyd JA, Medler SM, Ager JA, Janisse JJ. Aging-related changes in sleep initiation, continuity, and length: a meta-analysis. Sleep. 2000;23:S196–7. doi: 10.1002/(sici)1098-240x(200004)23:2<106::aid-nur3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 2.Floyd JA, Medler SM, Ager JA, Janisse JJ. Age-related changes in initiation and maintenance of sleep: a meta-analysis. Res Nurs Health. 2000;23:106–17. doi: 10.1002/(sici)1098-240x(200004)23:2<106::aid-nur3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 3.Floyd JA. Sleep and aging. Nurs Clin North Am. 2002;37:719–31. doi: 10.1016/s0029-6465(02)00034-8. [DOI] [PubMed] [Google Scholar]
- 4.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 5.Collins WE, Iampietro PF. Effects of repeated simulated sonic booms of 1.0 PSF on the sleep behavior of young and old subjects. Aerosp Med. 1973;44:987–95. [PubMed] [Google Scholar]
- 6.Buysse DJ, Reynolds CF, III, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14:331–8. [PubMed] [Google Scholar]
- 7.Carrier J, Monk TH, Buysse DJ, Kupfer DJ. Sleep and morningness-eveningness in the middle years of life (20–59) J Sleep Res. 1997;6:230–7. doi: 10.1111/j.1365-2869.1997.00230.x. [DOI] [PubMed] [Google Scholar]
- 8.Ehlers CL, Kupfer DJ. Effects of age on delta and REM sleep parameters. Electroencephalogr Clin Neurophysiol. 1989;72:118–25. doi: 10.1016/0013-4694(89)90172-7. [DOI] [PubMed] [Google Scholar]
- 9.Prinz PN, Halter J, Benedetti C, Raskind M. Circadian variation of plasma catecholamines in young and old men: relation to rapid eye movement and slow wave sleep. J Clin Endocrinol Metab. 1979;49:300–4. doi: 10.1210/jcem-49-2-300. [DOI] [PubMed] [Google Scholar]
- 10.Williams RL, Karacan I, Hursch CJ. Electroencephalography (EEG) of human sleep: clinical applications. New York: John Wiley & Sons; 1974. [Google Scholar]
- 11.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284:861–8. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 12.Crowley K, Trinder J, Kim Y, Carrington M, Colrain IM. The effects of normal aging on sleep spindle and K-complex production. Clin Neurophysiol. 2002;113:1615–22. doi: 10.1016/s1388-2457(02)00237-7. [DOI] [PubMed] [Google Scholar]
- 13.Hirshkowitz M, Moore CA, Hamilton CR, III, Rando KC, Karacan I. Polysomnography of adults and elderly: sleep architecture, respiration, and leg movement. J Clin Neurophysiol. 1992;9:56–62. [PubMed] [Google Scholar]
- 14.Hoch CC, Dew MA, Reynolds CF, III, et al. A longitudinal study of laboratory-and diary-based sleep measures in healthy “old old” and “young old” volunteers. Sleep. 1994;17:489–96. doi: 10.1093/sleep/17.6.489. [DOI] [PubMed] [Google Scholar]
- 15.Kahn E, Fisher C. The sleep characteristics of the normal aged male. J Nerv Ment Dis. 1969;148:477–94. doi: 10.1097/00005053-196905000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Buysse DJ, Browman KE, Monk TH, Reynolds CF, III, Fasiczka AL, Kupfer DJ. Napping and 24-hour sleep/wake patterns in healthy elderly and young adults. J Am Geriatr Soc. 1992;40:779–86. doi: 10.1111/j.1532-5415.1992.tb01849.x. [DOI] [PubMed] [Google Scholar]
- 17.Monk TH, Reynolds CF, III, Machen MA, Kupfer DJ. Daily social rhythms in the elderly and their relation to objectively recorded sleep. Sleep. 1992;15:322–9. doi: 10.1093/sleep/15.4.322. [DOI] [PubMed] [Google Scholar]
- 18.Webb WB. The measurement and characteristics of sleep in older persons. Neurobiol Aging. 1982;3:311–319. doi: 10.1016/0197-4580(82)90019-7. [DOI] [PubMed] [Google Scholar]
- 19.Landolt HP, Dijk DJ, Achermann P, Borbely AA. Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Res. 1996;738:205–12. doi: 10.1016/s0006-8993(96)00770-6. [DOI] [PubMed] [Google Scholar]
- 20.Murphy PJ, Rogers NL, Campbell SS. Age differences in the spontaneous termination of sleep. J Sleep Res. 2000;9:27–34. doi: 10.1046/j.1365-2869.2000.00185.x. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds CF, III, Kupfer DJ, Taska LS, Hoch CC, Sewitch DE, Spiker DG. Sleep of healthy seniors: a revisit. Sleep. 1985;8:20–9. doi: 10.1093/sleep/8.1.20. [DOI] [PubMed] [Google Scholar]
- 22.Ehlers CL, Kupfer DJ. Slow-wave sleep: Do young adult men and women age differently? J Sleep Res. 1997;6:211–5. doi: 10.1046/j.1365-2869.1997.00041.x. [DOI] [PubMed] [Google Scholar]
- 23.Feinberg I, Keresko RL, Heller N. EEG sleep patterns as a function of normal and pathological aging in man. J Psychiatr Res. 1967;5:107–44. doi: 10.1016/0022-3956(67)90027-1. [DOI] [PubMed] [Google Scholar]
- 24.Haimov I, Lavie P. Circadian characteristics of sleep propensity function in healthy elderly: a comparison with young adults. Sleep. 1997;20:294–300. doi: 10.1093/sleep/20.4.294. [DOI] [PubMed] [Google Scholar]
- 25.Lauer CJ, Riemann D, Wiegand M, Berger M. From early to late adulthood changes in EEG sleep of depressed patients and healthy volunteers. Biol Psychiatry. 1991;29:979–93. doi: 10.1016/0006-3223(91)90355-p. [DOI] [PubMed] [Google Scholar]
- 26.Floyd JA, Janisse JJ, Medler SM, Ager JW. Non-linear components of age-related change in sleep initiation. Nurs Res. 2000;49:290–4. doi: 10.1097/00006199-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Rediehs MH, Reis JS, Creason NS. Sleep in old age: focus on gender differences. Sleep. 1990;13:410–24. [PubMed] [Google Scholar]
- 28.Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: a meta-analysis. Arch Gen Psychiatry. 1992;49:651–68. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- 29.Floyd JA. Sleep promotion in adults. Annu Rev Nurs Res. 1999;17:27–56. [PubMed] [Google Scholar]
- 30.Floyd JA, Moulton RA, Medler SM. Intercoder reliability for a meta-analytic study of age-related sleep changes. Sleep Res. 1997;26:657–8. [Google Scholar]
- 31.Floyd JA, Janisse JJ, Ager JA. (2002). Age and sleep latency: a meta-analysis. Sleep. 2002;25:A202–3. [Google Scholar]
- 32.Floyd JA. Meta-analytic methods that support outcomes management. Outcomes Manag Nurs Pract. 2000;4:58–62. [PubMed] [Google Scholar]
- 33.Jenuwine L, Floyd JA. Comparison of MeSH and text word searches in MEDLINE. J Med Libr Assoc. 2004;92:349–53. [PMC free article] [PubMed] [Google Scholar]
- 34.Cooper H, Hedges LV. The handbook of research synthesis. New York: Russell Sage Foundation; 1994. [Google Scholar]
- 35.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 36.Bonnet MH. Sleep Deprivation. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: WB Saunders; 2000. pp. 53–71. [Google Scholar]
- 37.Stickgold R, Walker MP. Sleep and memory: the ongoing debate. Sleep. 2005;4:1225–7. doi: 10.1093/sleep/28.10.1225. [DOI] [PubMed] [Google Scholar]
- 38.Vertes RP, Siegel JM. Time for the sleep community to take a critical look at the purported role of sleep in memory processing. Sleep. 2005;28:1228–9. doi: 10.1093/sleep/28.10.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stickgold R, Walker MP. Rebuttal. Sleep. 2005;28:1230–1. [Google Scholar]
- 40.Vertes RP, Siegel JM. Rebuttal. Sleep. 2005;28:1232–3. doi: 10.1093/sleep/28.10.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]