Abstract
Study objectives:
Using spectral edge frequency (SEF95) and dimension of activation (DA), a new tool derived from the dimension of correlation, we assessed the activation of thalamus and cortex in the different vigilance states.
Patients:
Results were gathered from intracerebral recordings performed in 12 drug-resistant epileptic patients during video-stereoelectroencephalographic (SEEG) monitoring.
Results:
In the cortex, we observed a progressive decrease of DA from wake to sleep, with minimal DA values characterizing the deep slow wave sleep (dSWS) stage. During paradoxical sleep (PS), cortical level of activity returned to DA values similar to those obtained during wakefulness. In the thalamus, DA values during wakefulness were higher than the values observed during light SWS (ISWS), deep SWS (dSWS) and PS; there were no significant differences between the 3 sleep stages. Similar variations were observed with SEF95.
Conclusion:
DA analysis proved reliable for quantification of cortical activity, in agreement with data issued from classical vigilance states scoring and spectral analysis. At the thalamic level, only 2 levels of activity within a sleep wake cycle were observed, pointing to dissociated levels of activation between the thalamus and the neocortex during ISWS and PS.
Citation:
Rey M; Bastuji H; Garcia-Larrea L et al. Human thalamic and cortical activities assessed by dimension of activation and spectral edge frequency during sleep wake cycles. SLEEP 2007;30(7):907-912.
Keywords: Sleep, cortex, thalamus, human, electrophysiology, dimension of activation
INTRODUCTION
Based on electroencephalographic recordings (EEG), the human sleep/wake cycle has been subdivided in 4 major stages. The passage from wakefulness to light (ISWS) and deep (dSWS) slow wave sleep (SWS) is characterized by a progressive slowing of the EEG, more or less associated with transient elements (spindle; K complex), while the 4th vigilance stage (rapid eye movements or paradoxical sleep - PS) exhibits activity largely comparable to that of wakefulness.1 The high frequency content and the low voltage of the EEG activity during wake and PS are generally viewed as reflecting a high level of cortical activation; conversely low frequency and high voltage EEG activity in ISWS and dSWS stages are thought to be characteristic of lower cortical activation levels. This interpretation is supported by recent metabolic neuroimaging studies showing a decrease in cortical blood flow during SWS.2
The reciprocal activation/deactivation of the cortex and the thalamus fluctuates during the sleep wake cycle. During SWS, 2 EEG features are observed: sleep spindles and slow waves. A large body of evidence suggests that “spindle” oscillations are locally generated in the reticular nucleus of the thalamus.3 Among slow waves, 2 types can be distinguished on the basis of their respective frequencies and putative generating mechanisms. The slow oscillations (<1Hz) can be generated and sustained by the cerebral cortex alone,4,5 while clocklike delta oscillations (1–4 Hz) are generated in thalamo-cortical cells. Thus one can assume that during these 2 types of SWS EEG activity, the deactivation levels could be different in the thalamus and the cortex.
During PS, both the cortex and the thalamus are supposed to be reactivated, as they both show a predominance of low voltage activity at relatively high frequencies.1 However, in at least 2 thalamic nuclei, the ventral posterior lateral nucleus and the medial pulvinar (PuM), PS activity has been shown to be frequently interrupted by long periods of delta waves.6,7 This suggests that some degree of thalamic deactivation and transmission block of sensorial inputs to the cortex, similar to that observed in SWS, is likely to occur also in PS.6,7
The aim of this study was to assess the activation-deactivation pattern at cortical and thalamic levels during the complete sleep wake cycle. For this purpose, we analysed intracranial recordings performed simultaneously at thalamic and cortical levels during the different sleep stages using both a classical spectral parameter (SEF95) and a recently developed type of EEG analysis allowing the quantification of neuronal activity. This new technical approach gives an indicator called “dimension of activation” (DA) which has been already validated for assessing cortical dynamics in different conditions of brain activation.8
MATERIALS AND METHODS
Patients
Thalamic and cortical recordings were obtained from 12 drug-resistant epileptic patients during presurgical stereoelectroencephalographic (SEEG) exploration of their seizures. All of the patients presented with seizures suggesting a rapid propagation of epileptic discharges between limbic areas and posterior temporal neocortex, based on noninvasive video-scalp EEG recordings. Stereotactic implantation of intracranial recording electrodes was performed after all patients have been fully informed and gave their consent about the procedure and aims of this exploration, which have been approved by the local ethic committee. Thalamic exploration was carried out in patients whose seizures were suspected to involve language areas or suprasylvian and/or insular cortex. In some of these patients, SEEG monitoring showing that surgery was contraindicated; they were therefore candidates for chronic PuM stimulation. The decision to explore the medial pulvinar nucleus (PuM) (densely connected with the temporal and parietal lobes9–11) was justified by the possible benefit these patients could draw from chronic PuM stimulation.12
Implantation of SEEG Electrodes
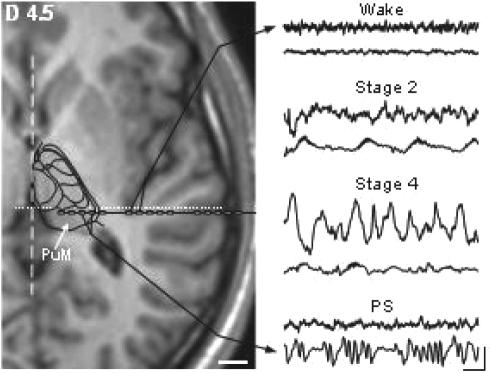
The electrodes implantation procedure was carried out using multiple contact electrodes introduced in the brain perpendicular to the midsagittal plane according to the Stereotactic technique of Talairach and Bancaud.13 Coordinates of relevant targets were determined on the patient's brain magnetic resonance (MR) images according to previously described procedures.14 PuM recordings were performed using the deepest contacts of the electrode exploring the posterior part of the superior temporal gyrus. Anatomical localization of the thalamic and cortical electrode contacts was first counterchecked using fusion of skull X-Ray after electrodes implantation with the appropriate coronal MR slice of patient's brain. The placement of 2 to 4 contacts within the PuM was further assessed by superimposing T1-weighted magnetic resonance (MR) images obtained in 3D mode and cut parallel to the anteroposterior commissure (AC-PC) horizontal plane (slice thickness: 1mm; MRIcro software15) with the corresponding Stereotactic atlas planes of the human thalamus16 (Figure 1).
Figure 1.
right: examples of raw bipolar recordings performed simultaneously in the thalamus and cortex during 4 different vigilance states. Note the peculiar delta oscillation appearing in the thalamus during PS. Scale: 250 μV; 1 s. Left: localisation of recording electrode contacts within the lateral part of PuM and the most posterior extent of the insular cortex. Stereotactic horizontal MRI slice is located 4.5 mm dorsal to the horizontal anterior-posterior commissural plane. Localization of thalamic nuclei is based on the fitting of the corresponding sketch of the human thalamic atlas of Morel et al. (1997) with this MRI section. Broken and dotted lines indicate interhemispheric and vertical posterior commissure planes, respectively. Scale: 10 mm.
Recording Conditions
Night recording under video-SEEG monitoring was conducted after a minimal delay of 5 days post electrode implantation. At that time, anticonvulsant drug intake had been reduced for at least one week in order to record spontaneous epileptic seizures. Signals recorded by intracerebral electrodes in PuM and temporal lateral neocortex, as well as electro-oculograms picked up by periorbital skin electrodes, were amplified, filtered (band pass: 0.33–300 Hz) and stored at a sampling frequency of 128 Hz. The states of vigilance were visually identified on more than 10 intracortical and thalamic contacts with both bipolar and referential traces (reference in the cranial bone) plus EOG, and scored according to the criteria of Rechtschaffen and Kales17 using sequential 30-s epochs (see Magnin et al.7). Stage 1 and 2 correspond to lSWS, stage 3 and 4 to dSWS and REM sleep to PS. In each vigilance state, 2-min periods of PuM and cortical activities picked up by the same multiple contact electrode were selected for analysis after discarding epochs contaminated by interictal epileptic paroxysmal activities (Figure 1).
Signal Analysis
Beside DA analysis a more conventional approach based on the spectral edge frequency (SEF95) variable18 was used for further comparisons. These 2 analyses were performed on bipolar recording of PuM (contact 3–4) and of temporal cortex (contact 10–11) with Synapsys™ software (version 1.3, Synapsys™, Marseille, France).
SEF95
SEF95 is defined as the upper frequency level below which are contained 95% of the total EEG power. Spectral analysis of SEEG signals was performed using fast Fourier transform on 4-s epochs. The 30 individual spectra corresponding to 2 min of SEEG recording were averaged.
DA
Calculation of DA8 has some similarities with that used for determining the dimension of correlation (D2).19 Briefly, both are based on embedding signal time series, and imply computing of the correlation integral C(r). The signal scalar time series is embedded in a high dimensional space (embedding dimension of 20), according to the time delay method but with constant time delay of 1/64. The second step is to calculate the correlation integral Cn(r). In the case of an homogenous attractor, the Cn(r) variation is linear and the slope of log[Cn(r)]/log(r) corresponds to the D2. In SEEG signals, the attractor is not homogeneous and the Cn(r) variation is generally not a linear function. This is why we used a weighted coefficients method to extract a more accurate parameter from the Cn(r) function. To evaluate the DA, calculation of Cn(r) is realized with a local window. The number of the window points is n = 1000, leading to a time window of 1000/64=15.6 seconds. More details on mathematical aspects of DA calculation and its application on EEG signals are given in reference.8 DA reflects the complexity of the working brain considered as a dynamical system. It corresponds to a reduced form of EEG data which allows to characterize and compare the complexity of different brain states. Increased complexity in the brain state is associated with elevated DA levels. During sleep, EEG is less complex than during wakefulness and DA is lower. In a certain sense, DA could be compared to other estimates derived from metabolic neuroimaging studies.
Statistical Analysis
Data were expressed as mean and standard error of the mean (SEM). Statistical analysis of the 2 SEEG parameters (DA and SEF95) was performed using a one-way analysis of variance (ANOVA) with 2 controlled factors: location (PuM, temporal neocortex) and sleep stage (wake, lSWS, dSWS, PS) followed by post hoc Tukey-Kramer test for multiple comparisons. Statistical analysis was performed using SigmaStat 2.03 and SigmaPlot 8.0 software (SSPS Inc., San Rafael, CA, USA). A value of P <0.05 was considered statistically significant.
RESULTS
SEF95
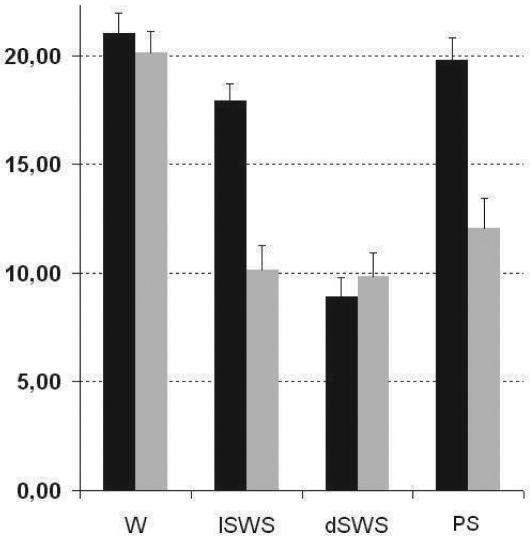
ANOVA indicated a significant effect of vigilance state (F3,88 = 39.4; P <0.0001) and of location (F1,88 = 27.4; P <0.0001) on SEF95 values. In PuM, post hoc pairwise comparisons (Tukey-Kramer test) demonstrated a significant decrease of SEF95 values during the three sleep stages as compared to wakefulness (P <0.001; Figure 2). However, post hoc tests showed that SEF95 values during lSWS, dSWS, and PS did not differ significantly from one another. In temporal cortex, no significant difference between wakefulness and PS was observed whereas a significant decrease of SEF95 values was noted between wakefulness and lSWS (P <0.05) and between lSWS and dSWS (P <0.001) (Figure 2).
Figure 2.
Cortical (black) and thalamic (grey) SEF95 values versus vigilance states
DA
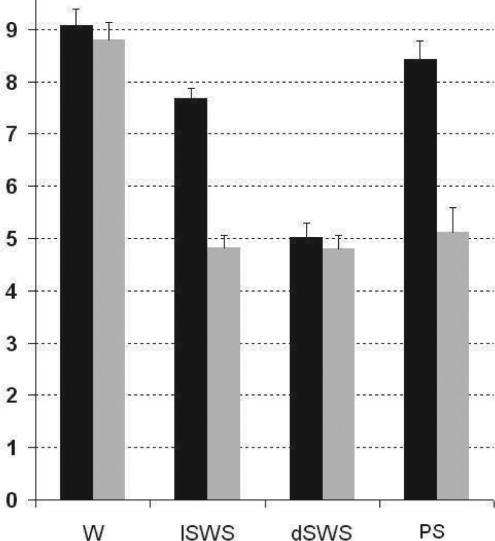
Results were very similar to those observed using the SEF95 technique showing a significant effect of vigilance state (F3,88 = 57.6; P <0.0001) and of position (F1,88 = 56.9; P <0.0001) on DA values. In PuM, post hoc pairwise comparisons (Tukey-Kramer test) demonstrated a significant decrease of DA values during sleep with respect to wakefulness (P <0.001; Figure 3). However the DA values obtained during lSWS, dSWS and PS did not differ significantly. In temporal cortex, no significant difference between wake and PS was observed while there was a significant decrease of DA values from wake to lSWS (P < 0.05) and from lSWS to dSWS (P < 0.001) (Figure 3).
Figure 3.
Cortical (black) and thalamic (grey) DA values versus vigilance states
DISCUSSION
Our data show that during wake, thalamic and cortical SEF95 or DA values are very similar. During dSWS, thalamic values of EEG parameters are also very similar to the cortical values. In contrast, during lSWS and PS, thalamic values depart from cortical ones.
The first question arising is whether this observation could be related to epilepsy or anti-epileptic medication. This is unlikely considering that this phenomenon was observed in all patients, regardless of the site of epileptogenic focus and regardless of the type and daily dosage of anti-epileptic treatment at the time of SEEG recordings (see Table 1). Moreover, while anti-epileptic drugs are known to modify cortical activity, their impact has not been reported to act selectively during one specific vigilance state.20 Finally, our results at the cortical level are similar to those obtained in non-epileptic animals21,22 and in humans23 and epileptic patients.24 We are thus inclined to conclude that our data reflect a genuine physiological process considering also that ictal and interictal activities were discarded from analysis.
Table 1.
Patient Demographics Data, Epilepsy Features (CPS: Complex Partial Seizure) and Pharmacological Treatment Before and After Partial Drug Withdrawal. (CBZ: Carbamazepine, DPH: Diphenylhydantoine, PB: Phenobarbital, LEV: Levtiracetam, TPM: Topiramate, VPA: Valproic Acid, GPN: Gabapentin, GVG: Vigabatrin LTG: Lamotrigine)
| CLINICAL DATA |
MEDICATION |
||||||
|---|---|---|---|---|---|---|---|
| Name | Age | Sex | Epilepsy duration (years) | Origin and type of seizure | Drugs | Amount before SEEG (mg) | Amount at the time of SEEG recordings (mg) |
| 1 | 21 | F | 18 | left temporo-parieto-occipital junction; CPS | CBZ | 1200 | 800 |
| TPM | 500 | 500 | |||||
| Clobazam | 30 | 30 | |||||
| 2 | 47 | M | 17 | left temporal; CPS | LTG | 500 | 300 |
| Clobazam | 10 | 0 | |||||
| 3 | 34 | M | 29 | bilateral temporal; CPS | LTG | 200 | 200 |
| CBZ | 1200 | 600 | |||||
| 4 | 16 | F | 6 | right frontal; CPS | LTG | 200 | 100 |
| VPA | 1000 | 750 | |||||
| GVG | 2500 | 1000 | |||||
| Clobazam | 15 | 0 | |||||
| 5 | 29 | F | 27 | left insulo-temporal; CPS | DPH | 300 | 200 |
| Clobazam 1 | 30 | 0 | |||||
| 6 | 40 | M | 17 | right insulo-temporal; CPS | CBZ | 1000 | 400 |
| Clobazam | 15 | 5 | |||||
| 7 | 31 | F | 17 | right temporal; CPS | PB | 150 | 100 |
| CBZ | 400 | 400 | |||||
| LEV | 1000 | 1000 | |||||
| Clobazam | 20 | 0 | |||||
| 8 | 32 | M | 8 | right temporal; CPS | LEV | 3000 | 2000 |
| CBZ | 1000 | 800 | |||||
| Clobazam | 35 | 10 | |||||
| PB | 150 | 100 | |||||
| 9 | 31 | M | 20 | right temporal; CPS | LEV | 2000 | 1000 |
| CBZ | 1200 | 1200 | |||||
| DPH | 300 | 300 | |||||
| 10 | 25 | M | 3 | left temporal; CPS | CBZ | 1200 | 0 |
| GPN | 1200 | 0 | |||||
| Clobazam | 20 | 0 | |||||
| 11 | 37 | F | 18 | left insulo-temporal; CPS | CBZ | 1200 | 400 |
| LEV | 1000 | 0 | |||||
| 12 | 27 | M | 22 | left insulo-temporal; CPS | LEV | 2000 | 1000 |
| LTG | 400 | 200 | |||||
| PB | 100 | 100 | |||||
A second point concerning the significance of SEF95 and DA values also deserves some comments. These 2 parameters are thought to reflect the level of activation of an EEG recording. SEF95 values are widely used in evaluation of the anaesthetic depth18 and increase when a concomitant increase of high frequencies occurs in the EEG signal. The latter phenomenon, also called desynchronization, is usually considered to reflect brain activation. DA also proved to be a reliable marker of cortical activation in various neurophysiological conditions.8 This is further verified by this study in which DA decreases in relation to the decreasing level of activation in the temporal cortex from wake to dSWS, a phenomenon observed with functional imaging techniques2,25,26 or nonlinear sleep EEG analysis.27,28
In PuM, the DA values obtained during lSWS and dSWS showed no significant difference; this similarity correspond to that observed between raw thalamic signals in these 2 sleep stages (see Figure 1). Thus, the distinction based on visual scoring of cortical EEG activity between these 2 sleep stages is not reflected at the thalamic level. This result agrees with that of Dan Vu29 who failed to detect during NREM sleep, i.e., during lSWS and dSWS, any significant correlation between delta activity and regional cerebral blood flow in the thalamus, contrary to what is observed simultaneously at the cortical level. This result fits with the fact that only 2 kinds of activity have been described at the thalamic level: tonic activity during wake and bursting activity during SWS, without further discrimination within the latter stage1. In contrast, spectral analysis of cortical activity allows an easy discrimination between lSWS and dSWS, as high frequency activities (>14 Hz) are only slightly reduced during lSWS but markedly decreased during dSWS7 (see Figure 2).
During PS, cortical DA value parallels the well-known increase of cortical activation. In contrast, at the PuM level, DA value does not increase above the SWS levels. This low thalamic DA value corresponds to the occurrence of marked delta activity bursts visible on raw recordings6,7 that are generally considered as reflecting a low level of activation. Thus, DA analysis appears a reliable tool for estimating the activation level of brain structures. However, this marker, as many others, presents some limitations. For instance, in thalamus, DA values do not permit to discriminate between PS, lSWS and dSWS in spite of different aspects of raw thalamic signals recorded during these 3 sleep stages. This suggests that a comparable low level of thalamic activation can be achieved via different electrophysiological processes.
Up to now the thalamus has been generally considered to be a structure modulating the information transfer toward the cortex in different vigilance states.1 During SWS, the bursting mode of thalamic activity is thought to prevent transmission of peripheral inputs, thus disconnecting the cortex from external world influences. This view needs however to be tempered by the persistence of cortical sensory and cognitive evoked potentials during SWS.30 Although thalamic structures appear to be activated during both waking state and PS, thus allowing peripheral inputs to reach cortical structures,31 one obviously does not perceive the external world during PS. A theory based on deficient resonance properties in the high frequency range (40 Hz) between thalamocortical loops has been proposed to explain why we could ignore the happenings of the external world during PS.23 Intermittent thalamic delta activity characterized by low values of DA, occurring during PS and disrupting high frequency resonance in thalamocortical loops, could further substantiate this theory. Moreover, a decoupling between thalamic and cortical activities during PS lets suppose that cerebral cortex, although reactivated during this sleep stage, is functioning on a particular action mode, relatively disconnected from thalamic modulation. This situation could account for the strangeness of the content of dreams.32
ACKNOWLEDGEMENTS:
We are indebted to J. Isnard and P. Ryvlin for the opportunity to study their patients and to M. Guénot for stereotactic electrode implantations.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have reported no financial conflicts of interest.
REFERENCES
- 1.Steriade M. Corticothalamic resonance, states of vigilance and mentation. Neuroscience. 2000;101:243–76. doi: 10.1016/s0306-4522(00)00353-5. [DOI] [PubMed] [Google Scholar]
- 2.Maquet P. Functional neuroimaging of normal human sleep by positron emission tomography. J Sleep Res. 2000;9:207–31. doi: 10.1046/j.1365-2869.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- 3.Fuentealba P, Steriade M. The reticular nucleus revisited: intrinsic and network properties of thalamic pacemaker. Progr Neurobiol. 2005;75:125–41. doi: 10.1016/j.pneurobio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Timofeev I, Steriade M. Low frequency rhythms in the thalamus of intact cortex and decorticated cats. J Neurophysiol. 1996;76:4152–68. doi: 10.1152/jn.1996.76.6.4152. [DOI] [PubMed] [Google Scholar]
- 5.Shu Y, Hasentaub A, McCormic DA. Turning on and off recurrent balanced cortical activity. Nature. 2003;423:288–93. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- 6.Velasco M, Velasco F, Cepeda C. A peculiar rhythmic EEG activity from ventrobasal thalamus during paradoxical sleep in man. Electroencephalogr Clin Neurophysiol. 1979;47:119–25. doi: 10.1016/0013-4694(79)90213-x. [DOI] [PubMed] [Google Scholar]
- 7.Magnin M, Bastuji H, Garcia-Larrea L, Mauguière F. Human thalamic medial pulvinar nucleus is not activated during paradoxical sleep. Cereb Cortex. 2004;14:858–62. doi: 10.1093/cercor/bhh044. [DOI] [PubMed] [Google Scholar]
- 8.Guillemant P, Abid C, Rey M. La dimension d'activation de l'EEG: une approche pertinente de la dynamique cérébrale au moyen d'un algorithme de calcul en temps reel. Rev. Traitement du signal. 2005;22(1):7–14. [Google Scholar]
- 9.Baleydier C, Morel A. Segregated thalamocortical pathways to inferior parietal and inferotemporal cortex in macaque monkey. Vis Neurosci. 1992;8:391–405. doi: 10.1017/s0952523800004922. [DOI] [PubMed] [Google Scholar]
- 10.Webster MJ, Bachevalier J, Ungerleider LG. Subcortical connections of inferior temporal areas TE and TEO in macaque monkeys. J Comp Neurol. 1993;335:73–91. doi: 10.1002/cne.903350106. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez C, Cola MG, Seltzer B, Cusick C. Neurochemical and connectional organization of the dorsal pulvinar complex in monkeys. J Comp Neurol. 2000;419:61–86. doi: 10.1002/(sici)1096-9861(20000327)419:1<61::aid-cne4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg D, Mauguière F, Demarquay G, et al. Involvement of medial pulvinar thalamic nucleus in human temporal lobe seizures. Epilepsia. 2006;47:1–10. doi: 10.1111/j.1528-1167.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 13.Talairach J, Bancaud J. Stereotactic approach to epilepsy: methodology of anatomo-functionnal stereotaxic investigations. Prog Neurol Surg. 1973;5:297–354. [Google Scholar]
- 14.Guenot M, Isnard J, Ryvlin P, et al. Neurophysiological monitoring for epilepsy surgery: the Talairach SEEG method Indications, results, complications and therapeutic applications in a series of 100 consecutive cases. Stereotact Funct Neurosurg. 2001;77:29–32. doi: 10.1159/000064595. [DOI] [PubMed] [Google Scholar]
- 15.Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- 16.Morel A, Magnin M, Jeanmonod D. Multiarchitectonic and stereotactic atlas of the human thalamus. J Comp Neurol. 1997;387:588–630. doi: 10.1002/(sici)1096-9861(19971103)387:4<588::aid-cne8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 17.Rechtschafien A, Kales A. Los Angeles: UCLA Brain Information Service; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 18.Rampil IJ. Spectral edge frequency - A new correlate of anaesthetic depth. Anesthesiology. 1980;53:S4. [Google Scholar]
- 19.Grassberger P, Procaccia I. Measuring the stangeness of strange attractors. Physica D. 1983;9:189–208. [Google Scholar]
- 20.Duncan JS. Antiepileptic drugs and the electroencephalogram. Epilepsia. 1997;28:259–66. doi: 10.1111/j.1528-1157.1987.tb04216.x. [DOI] [PubMed] [Google Scholar]
- 21.Franken P, Dijk D, Tobler I, Borbély A. High-frequency components of the rat electrocorticogram are modulated by the vigilance states. Neurosci Lett. 1994;167:89–92. doi: 10.1016/0304-3940(94)91034-0. [DOI] [PubMed] [Google Scholar]
- 22.Maloney KJ, Cape EG, Gotman J, Jones BE. High-frequency gamma electroencephalogram activity in association with sleep-wake states and spontaneous behaviors in the rat. Neuroscience. 1997;76:541–55. doi: 10.1016/s0306-4522(96)00298-9. [DOI] [PubMed] [Google Scholar]
- 23.Llinas R, Ribary U. Coherent 40 Hz oscillation characterizes dream state in humans. Proc Natl Acad Sci USA. 1993;90:2078–81. doi: 10.1073/pnas.90.5.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross DW, Gotman J. Correlation of high-frequency oscillations with the sleep-wake cycle and cognitive activity in humans. Neuroscience. 1999;94:1005–18. doi: 10.1016/s0306-4522(99)00343-7. [DOI] [PubMed] [Google Scholar]
- 25.Braun AR, Balkin TJ, Wesenten NJ, et al. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120:1173–97. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- 26.Maquet P, Péters J-M, Aerts J, et al. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1996;383:163–6. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- 27.Rey M, Guillemant P. Apport des mathématiques non-linéaires (théorie du chaos) à 1'analyse de 1'EEG. Neurophysiol Clin. 1997;27:406–28. doi: 10.1016/s0987-7053(97)88807-7. [DOI] [PubMed] [Google Scholar]
- 28.Shen Y, Olbrich E, Achermann P, Meier PF. Dimensional complexity and spectral properties of human sleep EEG. Clin Neurophysiol. 2003;114:199–209. doi: 10.1016/s1388-2457(02)00338-3. [DOI] [PubMed] [Google Scholar]
- 29.Dang-Vu TT, Desseilles M, Laureys S, et al. Cerebral correlates of delta waves during non-REM sleep revisited. Neuroimage. 2005;28:14–21. doi: 10.1016/j.neuroimage.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 30.Bastuji H, Perrin F, Garcia-Larrea L. Semantic analysis of auditory input during sleep: studies with event related potentials. Int J Psychophysiol. 2002;46:243–55. doi: 10.1016/s0167-8760(02)00116-2. [DOI] [PubMed] [Google Scholar]
- 31.Glenn LL, Steriade M. Discharge rate and excitability of cortically projecting intralaminar thalamic neurons during waking and sleep states. J Neurosci. 1982;2:1387–404. doi: 10.1523/JNEUROSCI.02-10-01387.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz S, Maquet P. Sleep imaging and the neuropsychological assessment of dreams. Trends Cogn Sci. 2002;6:23–30. doi: 10.1016/s1364-6613(00)01818-0. [DOI] [PubMed] [Google Scholar]