Abstract
Study Objectives:
To explore the familial aggregation and HLA susceptibility of narcolepsy in Hong Kong Chinese by objective sleep measurements and HLA typing.
Design:
Case control design
Participants:
Twelve narcoleptic probands, 34 first-degree relatives, and 30 healthy controls.
Interventions:
N/A
Measurements and Results:
Each subject underwent a standardized nocturnal polysomnogram (PSG), followed by a daytime multiple sleep latency test (MSLT). HLA typing was performed for all subjects. One relative (2.9%) was diagnosed as suffering from narcolepsy with cataplexy. Nearly 30% of the relatives fulfilled the criteria of narcolepsy spectrum disorder (shortened mean sleep latency [MSL] and/or the presence of sleep onset REM periods [SOREMPs]). When using the population data for comparison, the relative risk of narcolepsy in first-degree relatives was 85.3. The odds ratio of narcolepsy spectrum disorder in first-degree relatives was 5.8 (95% CI: 1.2 – 29.3) when compared to healthy controls. There existed 6 multiplex families, in which all 10 relatives with narcolepsy spectrum disorders, including all 3 relatives with multiple SOREMPs, were positive for HLA DQB1*0602.
Conclusions:
Our study demonstrated a definitive familial aggregation of narcolepsy, narcolepsy spectrum disorders, and possibly cataplexy in Hong Kong Chinese. This familial aggregation supported an inherited basis for narcolepsy spectrum. The tight co-segregation of HLA DQB1*0602 and narcolepsy spectrum disorders might suggest that HLA typing, especially DQB1*0602, at least partly confer the familial risk of narcolepsy. In addition, our study suggested that the subjective questionnaire measurements including Ullanlinna Narcolepsy Scale and Epworth Sleepiness Scale were unable to detect the presence of narcolepsy spectrum disorders among the relatives. A stringent objective measurement-based design for family studies is suggested for future study. Further studies are indicated for the determination of the mode and molecular level of narcolepsy transmission.
Citation:
Chen L; Fong SYY; Lam CW et al. The familial risk and HLA susceptibility among narcolepsy patients in Hong Kong Chinese. SLEEP 2007;30(7):851-858.
Keywords: Familial risk, HLA susceptibility, narcolepsy, narcolepsy spectrum
INTRODUCTION
NARCOLEPSY IS A CHRONIC DEBILITATING SLEEP DISORDER CHARACTERIZED BY EXCESSIVE DAYTIME SLEEPINESS (EDS), CATAPLEXY, AND RAPID EYE movement (REM) sleep related phenomena, such as sleep-related hallucinations (SRH) and sleep paralysis (SP).1 Narcolepsy has pernicious effects on school performance and family and social activities, and also leads to an increase of accidents and divorce.2,3 Recent research suggested that narcolepsy exerted an even greater negative socioeconomic impact on patients than similar chronic medical disorders such as epilepsy.4
Family Studies on Narcolepsy
The tendency for narcolepsy to run in families has long been recognized.5 The familial risk of first-degree relatives was 0.43%–14.04% for narcolepsy, which was 10–280 times higher than the prevalence in the general population6–15 (Table 1). Family studies of narcolepsy also reported an increased prevalence of EDS and idiopathic hypersomnia, which suggested that a spectrum of phenotypes existed across narcolepsy with cataplexy, narcolepsy without cataplexy, and idiopathic hypersomnia.6–15 However, the majority of these family studies were based on the sole information provided by narcoleptic probands or questionnaires from their relatives. Furthermore, the diagnoses in most cases were not confirmed by objective measurements such as polysomnogram (PSG) or multiple sleep latency test (MSLT) (Table 1).6–15
Table 1.
Family Studies of Narcolepsy Worldwide
| Reference (Published year) | Subjects and methods | % of first-degree relatives affected | HLA typing in probands | PSG & MSLT for relatives | Exclusion of other EDS-related disorders | Control group |
|---|---|---|---|---|---|---|
| Kessler et al6 (1976) | Family history questionnaires from 130 probands | 2.1% (18/839) |
Not done | No | - | No |
| Kales et al7 (1982) | 50 probands were clinically examined and each completed a family history questionnaire together with at least one relative | 1.2% (5/408) |
Not done | No | - | No |
| Honda et al8 (1983) | Family histories from 308 probands | 1.12% (14/1249) |
Not done | No | - | No |
| Guilleminault et al9 (1989) | Family histories from 334 probands. 32 probands and 18 relatives had HLA typing. | 0.9% (14/1603) |
91% (29/32) |
7% (109/1603) |
Yes | No |
| Hublin et al10 (1991) | 5 probands and 47 relatives were interviewed. All probands and 16 relatives were clinically examined | 6.38% (3/47) |
100% DR2 + |
34% (16/47) for MSLT |
Yes | No |
| Billiard et al11 (1994) | 188 probands and 25 relatives were interviewed; 170 probands and 11 relatives had PSG; 162 probands and 22 relatives had HLA typing. | 1.06% (9/847) |
Done but not reported. | 1% (11/847) for PSG |
Yes | No |
| Hayduk et al12 (1997) | 32 probands and 57 relatives were clinically examined. | 14.04% (8/57) |
66% (21/32) |
All (data from 3 families were reported) | - | No |
| Nevsimalovaet al13 (1997) | Questionnaire to 153 probands; 38 probands were interviewed; 11 relatives were clinically examined; HLA typing in 6 multiplex families. | 2.28% (17/747) by questionnaire 1.2% (9/747) by clinical examination |
83% (5/6) |
1% (9/747) |
- | No |
| Mayer et al14 (1998) | Family histories from 411 probands; 47 relatives completed sleep questionnaires; 24 relatives were clinically examined | 0.43% (9/2466) |
Done but not reported. | 1% (24/2466) |
- | No |
| Ohayon et al15 (2005) | 157 probands, 261 relatives, 68 spouses and 3970 subjects from general population were phone interviewed with Sleep-EVAL system. | 7.3% (19/261) |
Not done | No | - | Yes |
Studies on Narcolepsy in Chinese
The study of narcolepsy in Chinese was even more limited.16–20 In 1998–2000, we conducted a cross-sectional study with a 2-phase design, estimating the prevalence rate of narcolepsy in the general population in Hong Kong Chinese.20 We interviewed 9851 subjects, and the prevalence rate of narcolepsy was found to be 0.034% (95% CI: 0.010%-0.117%).20
The aim of this study was to explore the familial pattern and HLA susceptibility of narcolepsy in Hong Kong Chinese.
METHODS
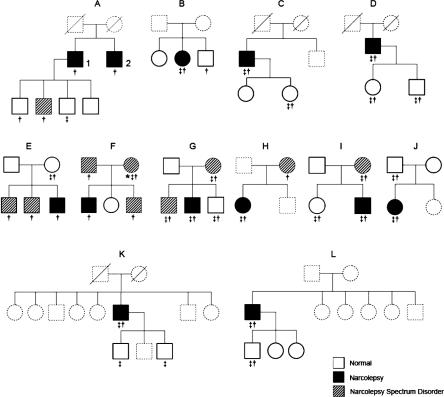
Twelve narcolepsy probands were chosen from our sleep clinic and were invited for this family study. Seven of them (58.3%) were diagnosed as having narcolepsy with cataplexy by the following criteria according to ICSD-2 21: (1) EDS for more than 3 months; and (2) a definite history of cataplexy; and/or (3) a mean sleep latency (MSL) of ≤ 8 min with ≥2 sleep onset REM periods (SOREMP) in 5 naps during MSLT; and (4) hypersomnia is not better explained by other sleep disorders, medical, mental, medication, or substance use disorders. Five probands (41.7%) who fulfilled the above criteria except for the presence of definite cataplexy were diagnosed as having narcolepsy without cataplexy. There were 9 males and 3 females. The mean duration of illness for these 12 narcoleptic probands was 11.5 years. Thirty-four first-degree relatives of the narcoleptic probands who were available and consented to the study were recruited, including 69% (11/16) parents, 40% (10/25) siblings and 93% (13/14) offspring. Eight relatives were not recruited because they were not living in Hong Kong, and 13 refused because of scheduling issues. In addition, 8 relatives had died before the study. Thus, the response rate of the relatives who were available in Hong Kong for the study was 72.3% (34/47). The detailed pedigrees of these 12 families were shown in Figure 1. Thirty unrelated healthy controls were chosen from the 113 controls from 2 previous studies, which were carried out in our sleep center.20,22 Thirteen of them were selected from the narcolepsy prevalence study (based on the general population), and the rest of them were recruited from the habitual sleep study (sampling frame with 13 primary schools; the parents of the school children were invited for the habitual sleep study). Subjects with EDS and other symptoms of narcolepsy, sleep disorders, circadian, medical, mental, medication, or substance use disorders were carefully excluded. All probands, relatives, and controls underwent a standardized overnight nocturnal PSG followed by MSLT on the following day. In addition, each subject underwent a detailed clinical interview and completed a set of sleep questionnaires including Chinese version of Ullanlinna Narcolepsy Scale (CUNS),23 Epworth Sleepiness Scale (ESS),24 and questionnaires about the self-reported sleep habits and problems.20
Figure 1.
Family pedigrees of the twelve narcolepsy probands. Relatives recruited were shown in solid lines and relatives who were not recruited were shown in dotted lines; Case 1 in family (A) was the proband and case 2 was diagnosed as cataplectic-narcolepsy clinically. * probable cataplexy ‡ DRB1*1501 positive †DQB1*0602 positive
We used the ICSD-2 criteria for diagnosing the presence of narcolepsy with cataplexy and narcolepsy without cataplexy in relatives and controls. Based on previous studies and our clinical experiences, there seemed to be the presence of broad narcoleptic phenotypes among family members of narcolepsy.6–15 Thus, we used the following additional criteria for diagnosing narcolepsy spectrum disorder: (1) those subjects with a mean sleep latency of >8 min and ≥2 SOREMPs, (2) those with a MSL of ≤8 min and 1 SOREMP, or (3) those with a MSL of ≤8 min without SOREMPs; in these cases, hypersomnia was not better explained by other sleep disorders, circadian, medical, mental, medication, or substance use disorders.
The ethical approval for this study was obtained from the university ethics committee. All subjects gave written informed consents.
Sleep Assessments
The PSG and daytime MSLT were performed with a CNS SL-1000p polygraph (CNS, Chanhassen, MN). The PSG included the measurement of central (C3-A2, C4-A1) and occipital (O2-A1) electroencephalogram (EEG), bilateral electrooculogram (EOG), electromyogram (EMG) of mentalis and bilateral anterior tibialis muscles, electrocardiogram (ECG), and respiratory airflow. The MSLT containing 5 nap tests was performed according to the standard recommendation to determine the sleep latency and SOREMPs.25 Sleep stages of both PSG and MSLT were scored in 30-second epochs following the Rechtschaffen and Kales criteria.26 The sleep latency was defined as the elapsed time from lights-out to the first epoch scored as sleep. The REM sleep latency was defined as the time from the beginning of sleep onset to the first epoch of REM sleep. All computerized sleep data were further reviewed by experienced polysomnographic technicians and clinicians.
HLA Genotyping
Blood samples were drawn from all subjects. Sequencing Based Typing (SBT) of HLA DRB and DQB were performed according to the protocols established by the International Histocompatibility Working Group (IHWG). Sequencing data was analyzed using the SBTengine software (Genome Diagnostics, Netherlands).
Statistical Analysis
Comparisons of probands, their relatives, and healthy controls on age, BMI, and other continuous sleep parameters were made through one-way analyses of variance (ANOVA) with Bonferroni post hoc comparisons. Binary variables were analyzed using chi-square test and Fisher's exact test for pairwise comparisons. Relative risk (RR) was defined as the ratio of disease prevalence in first-degree relatives to that in the general population. Odds ratios (OR) were calculated with 95% confidence intervals. All statistical tests were computed using SPSS 13.0 for windows (SPSS Inc, Chicago, IL).
RESULTS
Subjects
Twelve narcoleptic probands (M/F ratio: 9/3), 34 relatives (M/F ratio: 20/14) and 30 healthy controls (M/F ratio: 14/16) were recruited. The 3 groups were not significantly different in sex ratio.
Sleep Characteristics
The detailed clinical characteristics of the probands were summarized in Table 2. All probands had at least one SOREMP and the mean MSL was 2.9 min. Ten of 12 probands (83.3%) had a MSL <5 min with ≥2 SOREMPs.
Table 2.
Clinical and Polysomnographic Characteristics of the Narcoleptic Probands
| Proband No. | Sex | Age (yr) | Am | SE % | SL (min) | REM SL (min) | S1 % Sleep | S2 % Sleep | SWS % Sleep | REM % Sleep | MSLT (min) | SOREMP | Cataplexy | SRH | SP | DRB1 *1501 | DQB1 *0602 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 7 | 0 | 83.9 | 5.5 | 47.0 | 20.1 | 9.4 | 45.8 | 24.8 | 0.9 | 5 | − | − | − | + | + |
| 2 | M | 11 | 2.3 | 89.3 | 6.5 | 3.5 | 7.3 | 27.0 | 41.7 | 24.0 | 1.8 | 5 | + | + | + | − | + |
| 3 | M | 13 | 11.7 | 83.8 | 2.0 | 0.5 | 12.6 | 17.6 | 46.8 | 23.0 | 0.1 | 5 | + | + | + | + | + |
| 4 | M | 13 | 0 | 83.0 | 4.0 | 115.0 | 5.0 | 43.6 | 32.0 | 19.4 | 0.8 | 5 | − | − | − | + | + |
| 5 | M | 15 | 0.5 | 81.6 | 30.5 | 9.5 | 18.4 | 41.1 | 14.0 | 26.5 | 1.5 | 5 | − | − | − | − | + |
| 6 | F | 16 | 10.8 | 87.2 | 29.0 | 136.5 | 15.1 | 45.8 | 11.6 | 18.9 | 6.2 | 1 | + | − | − | + | + |
| 7 | F | 23 | 3.0 | 88.3 | 23.0 | 56.5 | 11.5 | 29.8 | 23.8 | 34.9 | 7.7 | 2 | + | + | + | + | + |
| 8 | M | 45 | 5.1 | 88.9 | 1.0 | 12.5 | 20.9 | 45.4 | 2.9 | 30.8 | 4.5 | 5 | + | + | + | + | + |
| 9 | M | 45 | 3.4 | 87.9 | 13.0 | 1.5 | 19.4 | 55.6 | 1.6 | 23.4 | 0.9 | 5 | − | + | + | + | + |
| 10 | M | 48 | 3.4 | 80.1 | 4.5 | 4.5 | 26.9 | 53.8 | 5.6 | 13.7 | 1.9 | 4 | − | − | + | + | + |
| 11 | M | 52 | 15.9 | 93.0 | 10.5 | 50.5 | 25.9 | 50.8 | 1.1 | 22.2 | 3.0 | 4 | + | + | − | + | + |
| 12 | M | 62 | 12.3 | 75.4 | 8.5 | 5.5 | 38.5 | 31.1 | 0.4 | 30.0 | 4.9 | 4 | + | + | + | − | + |
AHI: Apnea-hypopnea index; SE: sleep efficiency; SL: sleep latency; MSLT: multiple sleep latency test; SOREMP: Sleep onset REM period; SRH: sleep related hallucinations; SP: sleep paralysis.
The time in bed, total sleep time, sleep efficiency, AHI (apnea-hypopnea index), sleep latency, and percentage of REM sleep were comparable among probands, relatives, and controls (Table 3). The narcoleptic probands had an increase in stage 1 sleep, which suggested disturbed nocturnal sleep. They also had prominently shortened REM latency while maintaining normal amounts of REM sleep, which was consistent with previous reports.27,28 As expected, they had a shortened MSL and multiple SOREMPs in MSLT tests, and higher total scores and cataplexy subscores in CUNS, in comparison with relatives and controls. No significant differences were observed on the above parameters between relatives and controls. However, the controls had higher percentage of stage 2 but less slow wave sleep than the relatives. This might be related to the older age of the controls.
Table 3.
Demographic Characteristics and Sleep Variables Among Narcoleptic Probands, Their Relatives, and Healthy Controls
| Narcoleptic probands (N=12) (mean ± SD) | Relatives (N=34) (mean ± SD) | Controls (N=30) (mean ± SD) | P value | |
|---|---|---|---|---|
| Age | 29.2 ± 19.6b | 28.1 ± 14.5b | 42.6 ± 8.9 | 0.000 |
| BMI | 22.6 ± 3.0 | 22.0 ± 4.3 | 23.7 ± 2.4 | NS |
| Time in bed (min) | 491.6 ± 76.2 | 500.4 ± 45.7 | 494.3.3 ± 47.8 | NS |
| Total Sleep Time (min) | 417.7 ± 64.0 | 423.5 ± 55.4 | 403.2 ± 70.2 | NS |
| Sleep Efficiency (%) | 85.2 ± 4.8 | 84.7 ± 7.9 | 81.4 ± 9.9 | NS |
| AHI | 6.2 ± 5.4 | 4.4 ± 5.0 | 4.6 ± 5.5 | NS |
| Sleep Latency (min) | 11.5 ± 10.4 | 23.2 ± 18.3 | 16.9 ± 11.2 | NS |
| REM Sleep Latency (min) | 36.9 ± 46.5 a,b | 125.4 ± 105.3 | 115.2 ± 43.6 | 0.004 |
| % Sleep of Stage 1 | 18.5 ± 9.2 a,b | 8.5 ± 5.8 | 11.2 ± 4.7 | <0.001 |
| % Sleep of Stage 2 | 37.6 ± 14.6b | 42.9 ± 15.6b | 60.7 ± 9.0 | <0.001 |
| % Sleep of Stage 3 | 5.5 ± 4.8 | 9.0 ± 4.7b | 2.4 ± 3.9 | <0.001 |
| % Sleep of Stage 4 | 13.4 ± 14.2b | 16.3 ± 15.8b | 1.3 ± 3.0 | <0.001 |
| % Sleep of REM | 24.3 ± 5.8 | 23.4 ± 4.8 | 23.6 ± 4.4 | NS |
| MSLT (min) | 2.9 ± 2.4a,b | 13.2 ± 5.3 | 12.2 ± 3.3 | <0.001 |
| SOREMP | 4.2 ± 1.3a,b | 0.3 ± 0.8 | 0 ± 0.2 | <0.001 |
| CUNS Score | 18.3 ± 6.5a,b | 4.6 ± 2.5 | 4.5 ± 1.2 | <0.001 |
| Cataplexy Subscore of CUNS | 3.3 ± 4.6a,b | 0.2 ± 0.8 | 0 | <0.001 |
| ESS Score | 15.5 ± 5.7a,b | 7.1 ± 3.8 | 5.4 ± 4.4 | <0.001 |
AHI: Apnea-hypopnea index; MSLT: multiple sleep latency test; SOREMP: sleep onset REM period; CUNS: Chinese version of Ullanlinna Narcolepsy Scale; ESS: Epworth Sleepiness Scale; NS: Not Significant.
Significantly different from relatives, Bonferroni P <0.05
Significantly different from controls, Bonferroni P <0.05
Higher prevalence of EDS (shortened MSL), SOREMPs and cataplexy were observed in narcoleptic probands compared with controls (Table 4). While isolated SOREMP was also seen in the control group, relatives had a higher prevalence of multiple SOREMPs (8.8% vs. 0). Similarly, while shortened MSL was observed in controls, the prevalence of narcolepsy spectrum disorders in relatives was significantly higher than controls (29.4% vs 6.7%; OR 5.8, 95% CI: 1.2–29.3). The prevalence of sleep-related hallucinations or sleep paralysis was not higher in relatives than controls (Table 4). A lower rate of EDS was observed in offspring of narcoleptic probands (7.7%), compared to the rate in parents (45.5%) and siblings (20%). None of the offspring had SOREMP, while 9.1% of parents and 40% of siblings had at least one SOREMP in MSLT tests. The mean CUNS scores in those relatives who were positive for narcolepsy spectrum disorders were 4.4 ± 2.5 and did not differ from those without narcoleptic spectrum disorders (4.5 ± 2.4). Similarly, the mean ESS scores in these 2 groups were not significantly different from each other (7.0 ± 4.2 for positive group vs 6.2 ± 3.7 for negative group).
Table 4.
Presence of Narcoleptic Spectrum Among Narcoleptic Probands, Their First-degree Relatives, and Controls
| Narcoleptic probands (N=12) | First-degree relatives (N=34) Odds Ratio (95% CI) | Controls (N=30) | |||
|---|---|---|---|---|---|
| % of positive cases | Odds Ratio (95% CI) | % of positive cases | Odds Ratio (95% CI) | % | |
| EDS | |||||
| MSLT ≤ 10 min | 100.0a (n=12) | - | 26.5 (n=9) | 1.0 (0.3–3.0) | 26.7 (n=8) |
| MSLT ≤ 8 min | 100.0a (n=12) | - | 23.5 (n=8) | 4.3 (0.8–22.1) | 6.7 (n=2) |
| MSLT ≤ 5 min | 83.3a (n=10) | 145.0 (12–1777) | 11.8 (n=4) | 3.9 (0.4–36.7) | 3.3 (n=1) |
| SOREMP ≥ 2 | 91.7a(n=11) | - | 8.8 (n=3) | - | 0 |
| SOREMP = 1 | 8.3 (n=1) | 2.6 (0.2–45.9) | 5.9 (n=2) | 1.8 (0.2–21.1) | 3.3 (n=1) |
| SOREMP ≥ 1 | 100.0a (n=12) | - | 14.7 (n=5) | 5.0 (0.6–45.5) | 3.3 (n=1) |
| MSLT ≤ 8 min & SOREMP ≥ 2 | 91.7a (n=11) | - | 0 | - | 0 |
| MSLT > 8 min & SOREMP ≥ 2 | 0 | - | 8.8 (n=3) | - | 0 |
| MSLT ≤ 8 min & SOREMP = 1 | 8.3 (n=1) | 0 | 5.9 (n=2) | - | 0 |
| MSLT ≤ 8 min & SOREMP = 0 | 0 | - | 17.6 (n=6) | 3.0(0.6–16.2) | 6.7 (n=2) |
| Narcoleptic spectrum | - | - | 29.4a (n=10) | 5.8 (1.2–29.3) | 6.7 (n=2) |
| Cataplexy | 58.3a (n=7) | - | 5.9 (n=2) | - | 0 |
| Sleep related hallucinations | 58.3a (n=7) | 19.6 (3.1–123.1) | 2.9 (n=1) | 0.4 (0.0–4.9) | 6.7 (n=2) |
| Sleep paralysis | 58.3 (n=7) | 1.8 (0.5–7.1) | 17.6a (n=6) | 0.3 (0.1–0.9) | 43.3 (n=13) |
Significantly different from controls, P <0.05
One proband with cataplexy had a relative who reported long-standing sleepiness, definite and recurrent cataplexy. This relative had a mean sleep latency of 11 minutes and 3 SOREMPs and was diagnosed as having narcolepsy with cataplexy by criteria of ICSD-2.21 Another relative of a non-cataplectic proband was considered to have probable cataplexy, as she reported emotion-induced knee unlocking that led to falling onto the ground once in her lifetime.
Altogether, the prevalence was 2.9% (n = 1) for cataplectic-narcolepsy and 29.4% (n = 10) for narcolepsy spectrum disorder. Based on the prevalence data of the general population,20 the relative risk (lambda) of narcolepsy in first-degree relatives was 2.9/0.034 = 85.3.
HLA Genotyping
All narcoleptic probands were DQB1*0602 positive and 75% (9/12) of them were both DRB1*1501 and DQB1*0602 positive. Altogether 61.8% of the first-degree relatives were DQB1* 0602 positive while 44.1% of them were DRB1*1501 positive. There existed 6 multiplex families, in which all 10 first-degree relatives with narcolepsy spectrum disorder were DQB1*0602 positive (Figure 1). Moreover, all 3 relatives with multiple SOREMPs were DQB1*0602 positive. Although shortened MSL and SOREMP were also seen in the control group, all of them were negative for DQB1*0602.
DISCUSSION
The genetic predisposition for narcolepsy has been studied for several decades. However, the exact risk of this disease among close relatives of narcoleptic probands remained uncertain, which varied greatly from one ethnic group to another.6–15 The differences may be the result of a lack of uniform criteria of narcolepsy/narcolepsy spectrum disorder and a standardized methodology in these family studies, especially for those studies without objective sleep and HLA assessments. The study of narcolepsy in Chinese was rather limited, and the family study was conspicuously absent. Our laboratory-based study of narcoleptic probands and their first-degree relatives in comparison with healthy controls provided a reliable preliminary understanding of the familial pattern and HLA susceptibility of Chinese narcoleptic patients.
Heritability of Narcolepsy and Associated Symptoms
The frequency of narcolepsy in first-degree relatives of narcoleptic probands in our study was 2.9%, which was comparable to those found in American and Czech studies.6,13 The relative risk (lambda) for narcolepsy in first-degree relatives, compared to the general population, was 85.3 in our study, which was comparable to that of 40–115 in the Czech study,13 but much higher than that of the German (RR=16.5) study14 and the Japanese (RR=7) study.8 Our results suggested that the varying heritability of narcolepsy among different studies might also be explained by methodological discrepancy other than ethnic differences. A stringent objective measurement-based design for family studies is suggested for future family studies and cross-ethnic comparisons.
Narcolepsy spectrum disorder was identified by MSLT in the relatives of half of the probands studied. Multiple SOREMPs and narcolepsy spectrum disorders had a much higher frequency in first-degree relatives than in controls, suggesting that multiple SOREMPs, narcolepsy spectrum disorders, and narcolepsy might share a common genetic component.7,10,29,30 We also observed a slight increase of the prevalence of cataplexy (5.9%, n=2 including the probable one) in relatives than in controls (0% of cataplexy). Definite or probable cataplexy was present among the relatives of probands with and without cataplexy. Further family studies with cataplectic probands are needed to clarify the heritability issue of cataplexy, as this might have a huge impact on the diagnostic concept of narcolepsy. On the contrary, there was no familial tendency of sleep related hallucinations or sleep paralysis in first-degree relatives of narcoleptic probands (odds ratio: sleep related hallucinations: 0.4, 95% CI: 0.0–4.9; sleep paralysis: 0.3, 95% CI: 0.1–0.9). These results were consistent with a recent Italian study through telephone interviews with the Sleep-EVAL system.15 In fact, sleep paralysis seemed to be less common among relatives (which might be related to the older age of controls). As the involvement of environmental risk factors was suggested.5,31 the familial tendency of narcolepsy may also be explained by the fact that family members may share similar living conditions and environmental exposures. More systematic studies should be carried out to clarify genetic-environmental influence on narcolepsy susceptibility.
The percentage of EDS as reflected by a shortened MSL (23.5%) among first-degree relatives was much higher than studies carried out in other parts of the world including Japanese (4.47%),8 Czech (4.28%),13 German (5.4%),14 and French studies (3.65%).11 Our study differed from all these studies by having objective MSL data and the inclusion of a control group. In fact, our study suggested that the subjective measurements of EDS based on self-report even with the use of standardized questionnaires like CUNS and ESS were unable to detect the presence of narcolepsy spectrum disorder among relatives. In other words, these relatives might not complain of EDS despite the fact that they were carrying the objective phenotype of narcolepsy spectrum. According to recent studies by Mignot et al and Meeta Singh et al, multiple SOREMPs and shortened MSL (≤8 mins) were observed in about 4% of the general population, suggesting either a large false-positive result in MSLT or a striking prevalence of narcoleptic spectrum in the general population.32,33 Similarly, the presence of shortened MSL was not uncommon among our healthy controls, despite careful screening to rule out concomitant sleep, medical, and mental disorders. The prevalence of narcolepsy spectrum disorders was up to 6.7% among controls. Thus, the estimated odds ratio of having narcolepsy spectrum disorder in first-degree relatives was 5.8 (95% CI: 1.2–29.3) when compared to controls. In other words, narcolepsy spectrum seemed to breed true even if we used a rather stringent comparison control group. The diagnostic concept of narcolepsy has been evolving and there is a timely need for more extensive phenotype studies for better understanding of narcoleptic spectrum.
The much lower rate of EDS and SOREMP in offspring of narcoleptic probands might be related to an age effect. Follow-up studies would allow the ascertainment of narcoleptic features appearing in the future in these young children. Age effect has also been taken into account when recruiting our healthy controls. Controls with an older age were included to allow the emergence and expression, if any, of potential narcoleptic phenotypes.
HLA Typing and Narcolepsy Transmission
DQB1*0602 was considered to play a major role in conferring narcolepsy susceptibility among HLA alleles across different ethnic groups.34 Our study showed that all narcolepsy probands were 100% DQB1*0602 positive, and 75% of them were both HLA-DRB1*1501 and DQB1*0602 positive, in concordance with our previous finding16 and that reported in Japanese studies.35 The result indicated that DRB1*1501 was less tightly associated with DQB1*0602 in our Chinese population sample. Within the 6 multiplex families, all 10 relatives with narcolepsy spectrum disorder and all 3 relatives with multiple SOREMPs were DQB1*0602 positive, suggesting that HLA typing, especially DQB1*0602, at least partly, confer the familial risk of narcolepsy (Figure 1). Interestingly, the “narcoleptic spectrum” as seen in the control group were all DQB1*0602 negative. In this regard, the exact reason for the presence of non-DQB1*0602 “narcolepsy spectrum” in the supposed “healthy” general population was unclear. A recent study suggested that people who reported habitual sleep duration might also harbour sleep debt,36 but further study will be needed to ascertain this interesting phenomenon. In fact, the positive DQB1*0602 (3.3%) frequency in controls were slightly lower than that of the local Hong Kong Chinese general population (7.7–9.2%).37–38 The simplest explanation was the limited sample size of controls, but it might also be explained by the “super-healthy” status of controls and suggesting that HLA DQB1*0602 might confer certain risk for daytime sleepiness, shortened MSL, and multiple SOREMPs in the general population.30,39 In this sense, narcolepsy could be understood as a spectrum of phenotypes with various degrees of severity, ranging from shortened MSL and multiple SOREMPs to fully expressed narcolepsy with cataplexy.
Limitations of the Study
A number of factors have limited the result of the current study to be preliminary and exploratory. These include the small number of families and cases; potential selection bias in the recruitment of probands, relatives, and controls; unequal application of inclusion and exclusion criteria to relatives and controls; lack of full independence among relatives (some relatives came from the same family); and multiple comparisons. In addition, there were no data on CSF hypocretin status. Nonetheless, our study has detailed clinical, polysomnographic examination, and HLA typing, as well as a control group for the clarification of the phenotypic characteristics. In the future, a larger sample is mandatory to replicate the results and to allow the calculation of segregation ratio. A larger sample will also allow more in-depth investigation, including the molecular pathogenic study for multiplex narcoleptic families in order to determine the mode and genetics of narcolepsy transmission.
CONCLUSION
Our study, which was the first family study of narcolepsy in the Chinese population, demonstrated a definitive familial aggregation of narcolepsy, multiple SOREMPs, narcolepsy spectrum, and possibly cataplexy in Hong Kong Chinese.
ACKNOWLEDGEMENT
This study was supported by a RGC direct grant 4450036. We want to thank the technical assistance of all the technical staff of Sleep Assessment Unit, logistic assistance of Mandy Yu and Raymond HY Li, the collaboration of all patients, relatives, and controls.
Footnotes
Disclosure Statement
This was not an industry supported study. Mr. Ho has participated in speaking engagements for Tyco Healthcare and University Hospital, Macau University of Science and Technology. Dr. Wing has received research support from Eli Lilly Asia, Inc. Drs. Chen, Fong, Lam, Tang, Ng, Li, Cheng, and Lau have reported no financial conflicts of interest.
REFERENCES
- 1.Thorpy MJ, Goswami M. Treatment of narcolepsy. In: Thorpy M, editor. Handbook of sleep disorders. New York: Marcel Dekker; 1990. pp. 235–57. [Google Scholar]
- 2.Daniels E, King MA, Smith IE, Shneerson JM. Health-related quality of life in narcolepsy. J Sleep Res. 2001;10:75–81. doi: 10.1046/j.1365-2869.2001.00234.x. [DOI] [PubMed] [Google Scholar]
- 3.Broughton WA, Broughton RJ. Psychosocial impact of narcolepsy. Sleep. 1994;17(8) Suppl:S45–49. doi: 10.1093/sleep/17.suppl_8.s45. [DOI] [PubMed] [Google Scholar]
- 4.Broughton RJ, Guberman A, Roberts J. Comparison of the psychosocial effects of epilepsy and narcolepsy/cataplexy: a controlled study. Epilepsia. 1984;25:423–33. doi: 10.1111/j.1528-1157.1984.tb03438.x. [DOI] [PubMed] [Google Scholar]
- 5.Mignot E. Genetic and familial aspects of narcolepsy. Neurology. 1998;50(2) Suppl 1:S16–21. doi: 10.1212/wnl.50.2_suppl_1.s16. [DOI] [PubMed] [Google Scholar]
- 6.Kessler S, Guilleminault C, Dement W, Passouant P. Narcolepsy. New York: Spectrum; 1976. Genetic factors in narcolepsy; pp. 285–302. [Google Scholar]
- 7.Kales A, Cadieux RJ, Soldatos CR, et al. Narcolepsy-cataplexy. I. Clinical and electrophysiologic characteristics. Arch Neurol. 1982;39:164–8. doi: 10.1001/archneur.1982.00510150034008. [DOI] [PubMed] [Google Scholar]
- 8.Honda Y, Asaka A, Tanimura M, Furusho T, Guilleminault C, Lugaresi E. Sleep/wake disorders: natural history, epidemiology and long-term evolution. New York: Raven Press; 1983. A genetic study of narcolepsy and excessive daytime sleepines in 308 families with a narcolepsy or hypersomnia proband. [Google Scholar]
- 9.Guilleminault C, Mignot E, Grumet FC. Familial patterns of narcolepsy. Lancet. 1989;2(8676):1376–9. doi: 10.1016/s0140-6736(89)91977-6. [DOI] [PubMed] [Google Scholar]
- 10.Hublin C, Partinen M, Koskimies S. Familial narcolepsy in Finland. Acta Neurol Scand. 1991;83:388–93. doi: 10.1111/j.1600-0404.1991.tb03969.x. [DOI] [PubMed] [Google Scholar]
- 11.Billiard M, Pasquie-Magnetto V, Heckman M, et al. Family studies in narcolepsy. Sleep. 1994;17(8) Suppl:S54–59. doi: 10.1093/sleep/17.suppl_8.s54. [DOI] [PubMed] [Google Scholar]
- 12.Hayduk R, Flodman P, Spence MA, Erman MK, Mitler MM. HLA haplotypes, polysomnography, and pedigrees in a case series of patients with narcolepsy. Sleep. 1997;20:850–7. doi: 10.1093/sleep/20.10.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nevsimalova S, Mignot E, Sonka K, Arrigoni JL. Familial aspects of narcolepsy-cataplexy in the Czech Republic. Sleep. 1997;20:1021–26. doi: 10.1093/sleep/20.11.1021. [DOI] [PubMed] [Google Scholar]
- 14.Mayer G, Lattermann A, Mueller-Eckhardt G, Svanborg E, Meier-Ewert K. Segregation of HLA genes in multicase narcolepsy families. J Sleep Res. 1998;7:127–33. doi: 10.1046/j.1365-2869.1998.00105.x. [DOI] [PubMed] [Google Scholar]
- 15.Ohayon MM, Ferini-Strambi L, Plazzi G, Smirne S, Castronovo V. Frequency of narcolepsy symptoms and other sleep disorders in narcoleptic patients and their first-degree relatives. J Sleep Res. 2005;14:437–45. doi: 10.1111/j.1365-2869.2005.00476.x. [DOI] [PubMed] [Google Scholar]
- 16.Wing YK, Chen CN, Ho CK. HLA DR2 and DQ1 frequency among narcoleptic patients in Hong Kong Chinese. Psychiatr Clin Neurosci. 1998;52:523–7. doi: 10.1046/j.1440-1819.1998.00423.x. [DOI] [PubMed] [Google Scholar]
- 17.Wing YK, Chiu HF, Ho CK, Chen CN. Narcolepsy in Hong Kong Chinese--a preliminary experience. Aust N Z J Med. 1994;24:304–6. doi: 10.1111/j.1445-5994.1994.tb02177.x. [DOI] [PubMed] [Google Scholar]
- 18.Wing YK, Lee S, Chiu HF, Ho CK, Chen CN. A patient with coexisting narcolepsy and morbid jealousy showing favourable response to fluoxetine. Postgrad Med J. 1994;70:34–6. doi: 10.1136/pgmj.70.819.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han F, Chen E, Wei H, et al. Childhood narcolepsy in North China. Sleep. 2001;24:321–4. doi: 10.1093/sleep/24.3.321. [DOI] [PubMed] [Google Scholar]
- 20.Wing YK, Li RHY, Lam CW, Ho CKW, Fong SY, Leung T. The prevalence of narcolepsy among Chinese in Hong Kong. Ann Neurol. 2002;51:578–84. doi: 10.1002/ana.10162. [DOI] [PubMed] [Google Scholar]
- 21.2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders: diagnostic and coding manual. [Google Scholar]
- 22.Zhang B. The Chinese University of Hong Kong; 2005. Habitual short and long sleepers in middle-aged Hong Kong Chinese: epidemiological, clinical and polysomnographic study. PhD thesis. [Google Scholar]
- 23.Wing YK, Li RHY, Ho CKW, Fong SYY, Chow LY, Leung T. A Validity study of Ullaninna Narcolepsy Scale in Hong Kong Chinese. J Psychosom Res. 2000;49:355–61. doi: 10.1016/s0022-3999(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 24.Fong SYY, Ho CKW, Wing YK. Comparing MSLT and ESS in the measurement of excessive daytime sleepiness in obstructive sleep apnea syndrome. J Psychosom Res. 2005;58:55–60. doi: 10.1016/j.jpsychores.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–24. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 26.Rechtschaffen A, Kales A. Los Angeles: Brain Research Institute; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects UCLA. [Google Scholar]
- 27.Okun ML, Lin L, Pelin Z, Hong S, Mignot E. Clinical aspects of narcolepsy-cataplexy across ethnic groups. Sleep. 2002;25:27–35. doi: 10.1093/sleep/25.1.27. [DOI] [PubMed] [Google Scholar]
- 28.Broughton R, Dunham W, Newman J, Lutley K, Duschesne P, Rivers M. Ambulatory 24 hour sleep-wake monitoring in narcolepsycataplexy compared to matched controls. Electroencephalogr Clin Neurophysiol. 1988;70:473–81. doi: 10.1016/0013-4694(88)90145-9. [DOI] [PubMed] [Google Scholar]
- 29.Honda Y, Matsuki K. Genetic aspects of narcolepsy. In: Thorpy M, editor. Handbook of sleep disorders. New York: Marcel Dekker; 1990. pp. 217–34. [Google Scholar]
- 30.Kessler S, Guilleminault C, Dement W. A family study of 50 REM narcoleptics. Acta Neurol Scand. 1974;50:503–12. doi: 10.1111/j.1600-0404.1974.tb02796.x. [DOI] [PubMed] [Google Scholar]
- 31.Orellana C, Villemin E, Tafti M, Carlander B, Besset A, Billiard M. Life events in the year preceding the onset of narcolepsy. Sleep. 1994;17(8) Suppl:S50–53. doi: 10.1093/sleep/17.suppl_8.s50. [DOI] [PubMed] [Google Scholar]
- 32.Mignot E, Lin L, Finn L, et al. Correlates of sleep-onset REM periods during the Multiple Sleep Latency Test in community adults. Brain. 2006;129(Pt 6):1609–23. doi: 10.1093/brain/awl079. [DOI] [PubMed] [Google Scholar]
- 33.Singh M, Drake CL, Roth T. The prevalence of multiple sleep-onset REM periods in a population-based sample. Sleep. 2006;29:890–95. doi: 10.1093/sleep/29.7.890. [DOI] [PubMed] [Google Scholar]
- 34.Mignot E, Lin L, Rogers W, et al. Complex HLA-DR and -DQ interactions confer risk of narcolepsy-cataplexy in three ethnic groups. Am J Hum Genet. 2001;68:686–99. doi: 10.1086/318799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Juji T, Matsuki K, Tokunag K, Naohara T, Honda Y. Narcolepsy and HLA in the Japanese. Ann NY Acad Sci. 1988;540:106–14. doi: 10.1111/j.1749-6632.1988.tb27056.x. [DOI] [PubMed] [Google Scholar]
- 36.Klerman EB, Dijk DJ. Interindividual variation in sleep duration and its association with sleep debt in young adults. Sleep. 2005;28:1253–59. doi: 10.1093/sleep/28.10.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang YW, Hawkins BR. HLA class I and class II frequencies of a Hong Kong Chinese population based on bone marrow donor registry data. Hum Immunol. 1997;56:125–35. doi: 10.1016/s0198-8859(97)00108-0. [DOI] [PubMed] [Google Scholar]
- 38.Li PK, Leung NW, Poon AS, Wong KC, Chan TH, Lai KN. Molecular genetics of major histocompatibility complex class II genes in hepatocellular carcinoma. Dig Dis Sci. 1995;40:1542–46. doi: 10.1007/BF02285206. [DOI] [PubMed] [Google Scholar]
- 39.Mignot E, Young T, Lin L, Finn L, Palta M. Reduction of REM sleep latency associated with HLA-DQB1*0602 in normal adults. Lancet. 1998;351(9104):727. doi: 10.1016/S0140-6736(05)78496-8. [DOI] [PubMed] [Google Scholar]