Abstract
Study Objectives:
Ponto-geniculo-occipital (PGO) waves are phasic pontine, lateral geniculate, and cortical field potentials occurring during and before REM sleep that are proposed to mediate a wide variety of sleep related neural processes. We sought to identify and characterize human PGO waves.
Design:
We recorded simultaneously from intrapontine depth electrodes and scalp electrodes in a human subject across sleep states.
Setting:
Tertiary care neurological and neurosurgical referral center.
Patients or Participants:
We studied a patient involved in a study of the clinical effects of unilateral pedunculopontine nucleus (PPN) stimulation on Parkinson disease (PD).
Interventions:
No interventions.
Measurements and Results:
We recorded phasic potentials from the human pons occurring during and before REM sleep with a morphology, temporal distribution, and localization similar to those of PGO waves in other mammals. The source of these potentials was localized to a circumscribed region of the pontomesencephalic tegmentum. These potentials were only incompletely associated with eye movements. They were followed by characteristic cortical potentials with a latency of 20–140 msec.
Conclusions:
We conclude that PGO waves are a feature of human REM sleep, that they are generated or propagated in the pontomesencephalic tegmentum, that they are only partially associated with eye movements, and that they are associated with characteristic changes in cortical activity.
Citation:
Lim AS; Lozano AM; Moro E et al. Characterization of REM-sleep associated ponto-geniculo-occipital waves in the human pons. SLEEP 2007;30(7):823-827.
Keywords: PGO Waves, human, REM sleep, Pons
INTRODUCTION
PONTO-GENICULO-OCCIPITAL (PGO) WAVES, A HALLMARK OF MAMMALIAN RAPID EYE MOVEMENT (REM) SLEEP, ARE PHASIC PONTINE, LATERAL GENICULATE, and cortical field potentials occurring during, and immediately before, REM sleep.1
Evidence supports an important role for PGO waves in neural processes as diverse as learning,2 brain maturation,3 network organization in REM sleep,4 brainstem activation,5 and the transmission of eye movement information to the cortex.6 Moreover, PGO waves are noted in most models of REM sleep,7 and represent the input component of the activation-synthesis dream hypothesis, which proposes that dreams result from cortical interpretation of phasic ascending brainstem input.8,9
Others have observed focal phasic potentials during human REM sleep in striate cortex depth recordings,10 occipito-parietal scalp transients time-locked to REMs,11 and increased blood flow to the lateral geniculate body and occipital cortex in relation to REMs,12,13 providing evidence for phasic cortical activity in human REM sleep. However, PGO waves have never been directly recorded from the human pons or thalamus.
Using intrapontine and scalp EEG recordings across sleep states in a human subject, we tested the hypotheses that REM sleep associated phasic activity similar to PGO waves would be present in the human pons, that such activity would be closely related to REMs, and that these waveforms would be associated with characteristic cortical potentials.
METHODS
We investigated a 67-year-old right-handed man with a 10-year history of Parkinson disease (PD) and no other medical conditions, who was participating in a study of the clinical effects of unilateral pedunculopontine nucleus (PPN) deep brain stimulation (DBS) in PD. This study was approved by the local research ethics board and signed informed consent was obtained.
A quadripolar DBS electrode (model 3387, Medtronic, Minneapolis, MN) was implanted into the left pontine tegmentum using previously described methods.14 The electrode was implanted stereotactically under local anesthesia, using PPN coordinates determined by preoperative magnetic resonance imaging (MRI). Postoperative MRI images were reconstructed on a surgical navigation workstation (Stealth Station; Medtronic, Minneapolis, MN). Electrode contact locations were plotted on a human brainstem atlas.15 The 4 contacts, separated by 1.5 mm, were numbered 0–3 from most to least caudal.
Two days postoperatively we obtained 24-h video-polysomnography, recording simultaneously from scalp and intrapontine DBS electrodes. Scalp electrodes were positioned according to the standard ten-twenty electrode system.16
Sleep was staged according to standard methods;17 however, REM sleep was defined only in the presence of REMs and allowances were made for REM sleep without atonia, given that this is common in PD.18 In accordance with previous studies,19 we defined pre-REM as the 120 s before the 1st REM in each bout of REM sleep.
The pontine recordings were examined for sleep stage dependent activity, looking specifically for waves similar to PGO waves in other mammals: biphasic, 60–200 msec, sharply contoured, and occurring predominantly during REM sleep and the minutes preceding it. We labeled transients meeting these criteria as P-waves and tallied them with clues to sleep stage removed. The rate of occurrence of P-waves in each stage was calculated, and homogeneity of P-wave occurrences across pre-REM/REM or NREM sleep was assessed using the χ2 test.
The peak of each P-wave was marked visually, as was the beginning of each REM. REMs and P-waves were deemed associated if they occurred within 1 second of each other. We calculated the proportion of REMs associated with P-waves and vice-versa, tallying rightward and leftward REMs separately.
P-waves were identified visually, and marked at their peaks. Cortical activity in the 500 msec preceding and following each peak was averaged using Insight software (Persyst Development Corp, Prescott, AZ, USA) and voltage topographic maps were generated.
RESULTS
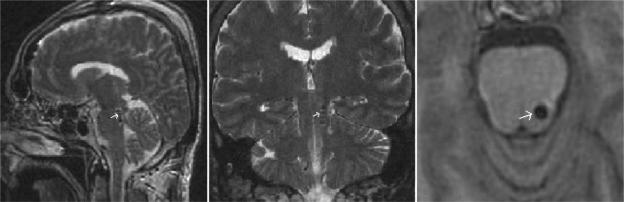
Using postoperative MRI, we confirmed localization of the DBS electrodes as follows: contact 0 was localized to the region of the locus ceruleus at a level 7–8mm below the caudal aspect of the inferior colliculus (IC); contact 1 was localized to a region just mesial to the superior cerebellar peduncle, 5–6 mm below the caudal aspect of the IC; contact 2 was localized to the region of the PPN at the level of the caudal IC; and contact 3 was localized to the region of the cuneiform nucleus at a level midway between the rostral and caudal extents of the IC (Figure 1).
Figure 1.
Postoperative coronal, sagittal, and axial MRI showing the location of the electrode in the posterolateral left pons. The white arrow indicates the location of contact 2.
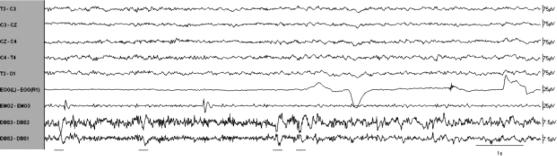
Reliable recordings were obtained from contacts 1, 2, and 3 of the intracranial array. Examination of the pontine EEG revealed repeated sharp wave transients, phase-reversing at contact 2, occurring singly or in clusters during REM and pre-REM sleep. These pontine waves (P-waves) were biphasic, 150–200 ms in duration, and 10–15μV in amplitude. They bore an unmistakable morphologic resemblance to pontine waves in rats (Figure 2 and Figure 3).19
Figure 2.
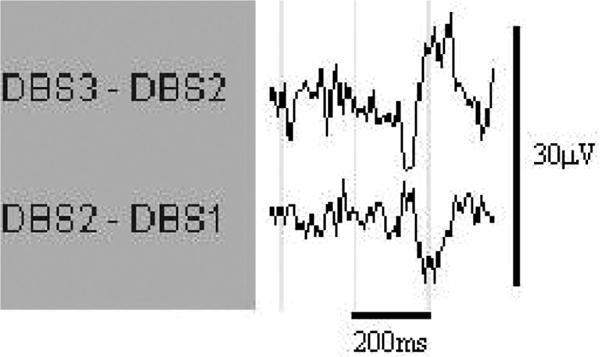
Representative P-wave in REM Sleep. DBSx refers to contact x of the electrode chain as outlined in the text; contact 3 is the most rostral. The contacts are referenced to each other in a bipolar manner.
Figure 3.
Segment of REM sleep with P-waves (underlined). Low frequency filter = 1 Hz, except EMG channel where low frequency filter = 7.5 Hz. High frequency filter = 70 Hz.
P-waves were inhomogeneously distributed across sleep stages (χ2=494, P=1.9×10−9). REM/pre-REM sleep accounted for only 13.1% of total sleep time but 59% of P-waves. P-wave density in REM/pre-REM sleep was 12.3/min and in NREM sleep was 1.3/min.
We saw an incomplete correspondence between REMs and P-waves: during REM sleep, only 41% of REMs were associated with a P-wave and only 22% of P-waves were associated with a REM. Leftward REMs (43%) were no more likely than rightward REMs (33%) to be associated with a left pontine P-wave (χ2=0.44, P=0.50). We saw few P-waves during wakefulness (0.01/min) despite many eye movements.
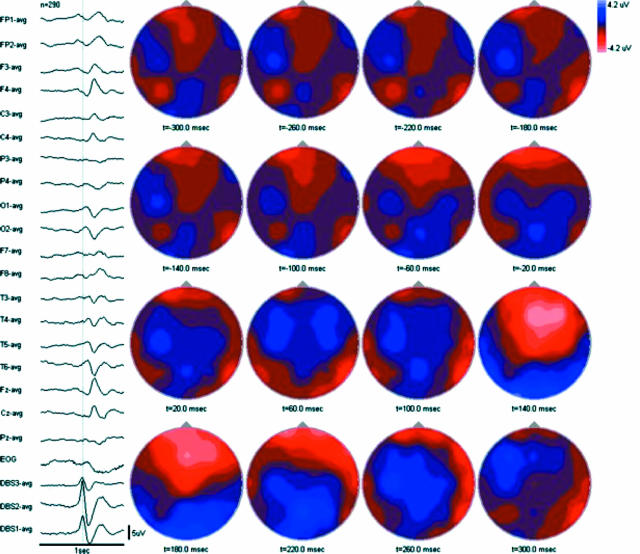
P-waves were followed by scalp voltage changes consisting of an early (∼20–60 msec latency) low amplitude negative field maximal over the posterior cortex (electrodes O1/2 and T5/6) followed by a later (∼140 msec latency) higher amplitude negative field maximal over the central and midfrontal cortex (electrodes Fz> F3/4>Cz>C3/4) with a corresponding positive field maximal over the posterior cortex (electrodes O1/2 and T5/6) (Figure 4). Overall, scalp voltage changes were appreciated from 20 to 300 msec following the peak of each P-wave, for a total duration of roughly 280 msec.
Figure 4.
Averaged cortical potentials associated with P-waves (n=290) with associated topographic maps. All electrodes referenced to common average. Low frequency filter = 1 Hz. High frequency filter = 70 Hz.
DISCUSSION
In this study, we demonstrated the occurrence of REM sleep associated phasic activity in the human pons associated with characteristic cortical potentials. The morphology, localization, temporal distribution, and rate of occurrence of the P-waves we observed are similar to those of PGO waves in other animals, supporting the assertion that these waveforms represent the pontine component of human PGO waves.
The amplitude of the P-waves we observed was 10–15 μV, which is somewhat lower than the 200–300 μV seen in cats.20 This may represent differences in electrode positioning relative to the P-wave source, as the recording of P-waves is known in animals to be highly dependent on electrode position.21 This may also represent differences in recording technique (for instance in the electrodes used) or interspecies variation.
The P-wave phase reversal at contact 2 of our intracranial electrode array localizes this local field potential to a volume of the pontomesencephalic tegmentum bracketed by the axial planes of contacts 3 and 1. This region encompasses the pontomesencephalic tegmentum between the level of the inferior colliculus and a level 7–8 mm caudal to this, which includes several nuclei of the peribrachial area such as the PPN, locus ceruleus, as well as caudolateral peribrachial region. Based on experiments in animals, the caudolateral peribrachial region in cats and the subceruleal region in rats are proposed to represent a PGO trigger zone,20 while structures in the rostral peribrachial region, including the PPN, are thought to represent PGO transferring regions.20 Our findings are compatible with this. Unfortunately, given that our intracranial array extends in only 1 dimension and that the electrodes are spaced at relatively wide (1.5 mm) intervals, more precise localization of the human P-wave source is not possible with our technique.
In cats, approximately 80% of REMs are associated with a P-wave.22 Moreover, in single cell recordings, PGO burst cells in the peribrachial region, ipsilateral but not contralateral to a horizontally directed REM, increase firing before and during the REM.23 Finally, when bilateral lateral geniculate body (LGB) field recordings are obtained, the waveform in the lateral geniculate body ipsilateral to an associated REM is consistently shorter in latency and higher in amplitude than that seen in the contralateral LGB.6 Taken together, these have led to the hypothesis that PGO waves represent corollary discharges for eye movements.6,23 In our study, only 41% of REMs were associated with a P-wave, and there were few P-waves in the waking state despite many eye movements. Using local field potential recordings, we saw no relationship between the directionality of eye movements and the likelihood of an associated P-wave in the left pons, suggesting that at the macroscopic field potential level, P-wave occurrence is independent of eye movement direction. Our techniques do not permit comment on whether individual PGO burst cells may be firing in a REM direction specific manner. Moreover, in the absence of bilateral recordings, we cannot comment on whether human REM directionality might be encoded by lateralized occurrence of primary (shorter latency, higher amplitude) vs. secondary (longer latency, lower amplitude) P-waves.
One proposed function of REM sleep is to stimulate the brain to reverse the effects of NREM sleep on immediately subsequent waking behavior.24 In this vein, PGO waves are proposed to represent a nonspecific orienting response to brainstem activation, either endogenously generated, or in response to exogenous stimuli.5 This is supported by the finding that potentials nearly identical to PGO waves can be generated in cats5 and rats25 in response to auditory stimulation. In this study, the observed P-waves were not correlated to any known ambient sensory stimuli we were aware of. However, the findings of this study invite further investigation of this hypothesis by relating human P-wave occurrence to various exogenous stimuli, or by comparing arousal thresholds to graded auditory stimuli during NREM, pre-REM and REM sleep with and without P-waves.
In this study, P waves were followed by characteristic changes in scalp voltage topography with a latency of 20–140 msec. This latency of these potentials is too long for them to be accounted for by volume conduction of the original P-wave. We think these potentials represent the cortical manifestation of P-waves, and provide evidence for phasic ponto-cortical input during human REM sleep.
The latency of 20–140 msec overlaps incompletely with the 10–40 msec latency observed in cats using simultaneous pontine and striate cortex depth recordings.22 Some of this difference may be accounted for by technical differences (surface vs. depth recording) while some may represent interspecies variation.
It is impossible to infer the true localization of the sources underlying the cortical potentials using visual analysis alone. Notwithstanding this uncertainty, the demonstration in principle of P-wave induced changes in human cortical activity highlights the pons as an endogenous source of phasic cortical input in human sleep. Such phasic cortical activation has been proposed in animals to contribute to sleep related memory consolidation, by reactivating or otherwise modulating areas in which information is “temporarily" stored and “consolidating" it into a more permanent form. This process has been proposed to involve the induction of long-term potentiation amidst the synaptic downscaling of slow wave sleep,26 as well as the modulation of transcription and neurotrophic factors in areas of the brain important for memory.2,27,28 P-waves have been proposed to be an important source of phasic cortical activation in REM sleep. Indeed, selective lesions of P-wave generating centers in rats have been shown to affect subsequent REM sleep associated memory formation, and chemical stimulation of the same areas has been shown to reverse the deleterious effects of REM sleep deprivation on learning in an aversive task paradigm.2,29 The demonstration of P-waves in humans invites exploration of the importance of these waveforms to human memory consolidation by physical or pharmacologic manipulation of P-waves and measurement of cognitive correlates.
The activation-synthesis dream hypothesis proposes that dreams result from cortical interpretation of phasic ascending brainstem input.8,9 The finding of P-wave related changes in cortical activity confirms the existence in humans of a key element of this hypothesis and suggests a way to test it by selective assessment of dream recall during periods of P-wave activity, particularly in the pre-REM period, when P-waves and REM sleep are dissociated.
Although we have demonstrated that P-waves can in principle be recorded directly from the human pons, noninvasive markers of P-wave activity are still needed given the invasiveness of our techniques and the limited period during which any intracranial electrode can remain safely externalized. This study opens the door to validation of such markers against a gold standard.
Our observations are limited by 2 factors. First, we studied an individual with PD, a disease with known brainstem and sleep pathology.18 A similar study in a normal subject, while desirable, would be ethically impossible. Second, we acknowledge that the surgical procedure itself may have altered the usual dynamics of P-waves.
Notwithstanding these limitations, we conclude that PGO waves are a feature of human REM sleep, that these potentials are generated or propagated in a circumscribed region of the pontomesencephalic tegmentum, that they are incompletely associated with eye movements, and that they are associated with changes in cortical activity. These findings support the commonality of mammalian sleep control mechanisms, and invite further in vivo exploration of the importance of phasic ponto-cortical interactions in human REM sleep control, dream generation, and learning.
ACKNOWLEDGEMENTS
We acknowledge Mr. Dana Jewell, RPSGT, Ms. Yu-Yan Poon, RN, and Ms. Filomena Mazzella, RN, for their invaluable assistance.
Footnotes
Disclosure Statement
This was not an industry supported study. Drs. Lozano and Moro have consulted for Medtronic, Inc. Dr. Lang has received research support from Amgen, Cepholon, Eisai, Novartis, Teva, Solvary, and Taro and has consulted for Medtronics, Inc., Amgen, Allergan, Ceregene, Boehringer Ingelheim, Eisai, Novartis, Teva, Solvay, Taro, and Valeant. Dr. Murray has received an educational grant from AstraZeneca and has consulted for Pfizer, Shire Pharmaceuticals, McNeil, Bayer, and Boehringer-Ingelheim. Drs. Lim, Hamani, Hutchison, Dostrovsky, and Wennberg have reported no financial conflicts of interest.
REFERENCES
- 1.Callaway CW, Lydic R, Baghdoyan HA, Hobson JA. Pontogeniculooccipital waves: spontaneous visual system activity during rapid eye movement sleep. Cell Mol Neurobiol. 1987;7:105–49. doi: 10.1007/BF00711551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Datta S. Activation of phasic pontine-wave generator: A mechanism for sleep-dependent memory processing. Sleep Biol Rhythms. 2006;4:16–26. [Google Scholar]
- 3.Shaffery JP, Roffwarg HP, Speciale SG, Marks GA. Ponto-geniculo-occipital-wave suppression amplified lateral geniculate nucleus cell-size changes in monocularly deprived kittens. Dev Brain Res. 1999;114:109–19. doi: 10.1016/s0165-3806(99)00027-9. [DOI] [PubMed] [Google Scholar]
- 4.Amzica F, Steriade M. Progressive cortical synchronization of ponto-geniculo-occipital potentials during rapid eye movement sleep. Neuroscience. 1996;72:209–14. doi: 10.1016/0306-4522(96)00012-7. [DOI] [PubMed] [Google Scholar]
- 5.Bowker RM, Morrison AR. The startle reflex and PGO spikes. Brain Res. 1976;102:185–90. doi: 10.1016/0006-8993(76)90586-2. [DOI] [PubMed] [Google Scholar]
- 6.Nelson JP, McCarley RW, Hobson JA. REM sleep burst neurons, PGO waves, and eye movement information. J Neurophys. 1983;50:784–97. doi: 10.1152/jn.1983.50.4.784. [DOI] [PubMed] [Google Scholar]
- 7.Siegel JM. REM Sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia: Elsevier; 2005. pp. 120–35. [Google Scholar]
- 8.Hobson JA, McCarley RW. The brain as a dream state generator: an activation-synthesis hypothesis of the dream process. Am J Psychiatry. 1977;134:1335–48. doi: 10.1176/ajp.134.12.1335. [DOI] [PubMed] [Google Scholar]
- 9.Hobson JA, Pace-Schott EF. The cognitive neuroscience of sleep: neuronal systems, consciousness, and learning. Nat Rev Neurosci. 2002;3:679–93. doi: 10.1038/nrn915. [DOI] [PubMed] [Google Scholar]
- 10.Salzarulo P, Lairy GG, Bancaud J, Munari C. Direct depth recording of the striate cortex during REM sleep in man: are there PGO potentials? Electroen Clin Neuro. 1975;38:199–202. doi: 10.1016/0013-4694(75)90231-x. [DOI] [PubMed] [Google Scholar]
- 11.McCarley RW, Winkelman JW, Duffy FH. Human cerebral potentials associated with REM sleep rapid eye movements: links to PGO waves and waking potentials. Brain Res. 1983;274:359–64. doi: 10.1016/0006-8993(83)90719-9. [DOI] [PubMed] [Google Scholar]
- 12.Wehrle R, Dzisch M, Kaufmann C, et al. Rapid eye movement-related brain activation in human sleep: a functional magnetic resonance imaging study. Neuroreport. 2005;16:853–7. doi: 10.1097/00001756-200505310-00015. [DOI] [PubMed] [Google Scholar]
- 13.Peigneux P, Lauerys S, Fuchs S, et al. Generation of rapid eye movements during paradoxical sleep in humans. Neuroimage. 2001;14:701–8. doi: 10.1006/nimg.2001.0874. [DOI] [PubMed] [Google Scholar]
- 14.Saint-Cyr JA, Hoque T, Pereira LC, et al. Localization of clinically effective stimulating electrodes in the human subthalamic nucleus on magnetic resonance imaging. J Neurosurg. 2002;97:1152–66. doi: 10.3171/jns.2002.97.5.1152. [DOI] [PubMed] [Google Scholar]
- 15.Paxinos G, Huang XF. Atlas of the human brainstem. San Diego: Academic Press; 1995. [Google Scholar]
- 16.Jasper HH. The ten-twenty system of the International Federation. Electroen Clin Neuro. 1958;10:371–73. [PubMed] [Google Scholar]
- 17.Rechtschaffen A, Kales A. A manual of standardized techniques, terminology, and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Service; 1968. [Google Scholar]
- 18.Cornelia CL. Sleep disturbances and excessive daytime sleepiness in Parkinson disease: an overview. J Neural Transm Suppl. 2006;70:349–55. doi: 10.1007/978-3-211-45295-0_53. [DOI] [PubMed] [Google Scholar]
- 19.Farber J, Marks GA, Roffwarg HP. Rapid eye movement sleep PGO-type waves are present in the dorsal pons of the albino rat. Science. 1980;209:615–7. doi: 10.1126/science.6994229. [DOI] [PubMed] [Google Scholar]
- 20.Datta S. Cellular basis of pontine ponto-geniculo-occipital wave generation and modulation. Cell Mol Neurobiol. 1997;17:341–65. doi: 10.1023/A:1026398402985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Datta S, Siwek DF, Patterson EH, Cipolloni PB. Localization of pontine PGO wave generation sites and their anatomical projections in the rat. Synapse. 1998;30:409–23. doi: 10.1002/(SICI)1098-2396(199812)30:4<409::AID-SYN8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Sakai K, Cespuglio R. Evidence for the presence of eye movement potentials during paradoxical sleep in cats. Electroen Clin Neuro. 1976;41:37–48. doi: 10.1016/0013-4694(76)90213-3. [DOI] [PubMed] [Google Scholar]
- 23.Datta S, Hobson JA. Neuronal activity in the caudolateral peribrachial pons: relationship to PGO waves and rapid eye movements. J Neurophys. 1994;71:95–109. doi: 10.1152/jn.1994.71.1.95. [DOI] [PubMed] [Google Scholar]
- 24.Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437:1264–71. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufman LS, Morrison AR. Spontaneous and elicited PGO spikes in rats. Brain Res. 1981;214:61–72. doi: 10.1016/0006-8993(81)90438-8. [DOI] [PubMed] [Google Scholar]
- 26.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Saha S, Datta S. Two-way active avoidance training-specific increases in phosphorylated cAMP response element-binding protein in the dorsal hippocampus, amygdala, and hypothalamus. Eur J Neurosci. 2005;21:3403–14. doi: 10.1111/j.1460-9568.2005.04166.x. [DOI] [PubMed] [Google Scholar]
- 28.Ulloor J, Datta S. Spatio-temporal activation of cyclic AMP response element-binding protein, activity regulated cytoskeletal-associated protein, and brain derived nerve growth factor: a mechanism for pontine-wave generation activation-dependent two-way active-avoidance memory processing in the rat. J Neurochem. 2005;95:418–28. doi: 10.1111/j.1471-4159.2005.03378.x. [DOI] [PubMed] [Google Scholar]
- 29.Datta S, Mavanji V, Ulloor J, Patterson E. Activation of phasic pontine-wave generator prevents rapid eye movement sleep deprivation-induced learning impairment in the rat: a mechanism for sleep-dependent plasciticy. J Neurosci. 2004;24:1416–27. doi: 10.1523/JNEUROSCI.4111-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]