Abstract
Study objectives:
Sleep disturbances and their sequelae are the most common complaints of patients with restless legs syndrome (RLS). We compared polysomnography (PSG) findings in a large cohort of patients with idiopathic RLS and of healthy subjects.
Design:
Comparative observational study.
Setting:
University hospital sleep laboratory.
Patients:
Age- and sex-matched patients with idiopathic but untreated RLS versus healthy controls.
Interventions:
N/A
Results:
Each group consisted of 29 females and 16 males. RLS subjects and controls were 47.4 ± 10.9 and 47.3 ± 10.5 years old, respectively. RLS severity was 24.0 ± 6.2 points on the IRLS scale, indicating moderately severe RLS symptoms. We found strong multivariate group effects on PSG parameters (Wilks' lambda, P <0.001): RLS patients exhibited prolonged sleep onset latencies (according to the 10-min criterion but not to the one-epoch criterion), shorter total sleep time, lower sleep efficiency, higher arousal index, higher number of stage shifts, and longer REM sleep latency. During the sleep period time, percentage of wake and sleep stage 1 were increased, and sleep stage 2 and REM sleep were decreased in RLS patients. The PLMS indices and the sleep fragmentation index were markedly increased in the RLS group.
Conclusions:
We present the largest polysomnography study to date that compares patients with idiopathic RLS with age- and sex-matched healthy subjects. The findings demonstrate markedly fragmented sleep with deterioration of both NREM and REM sleep in RLS patients.
Citation:
Hornyak M; Feige B; Voderholzer U et al. Polysomnography findings in patients with restless legs syndrome and in healthy controls: a comparative observational study. SLEEP 2007;30(7):861–865.
Keywords: Polysomnography, restless legs syndrome, sleep fragmentation index, healthy controls, comparative study
INTRODUCTION
RESTLESS LEGS SYNDROME (RLS) DESCRIBES A COMMON SENSORIMOTOR DISORDER WHICH IN MOST CASES TAKES A CHRONIC COURSE. THE DIAGNOSIS OF RLS relies on the patient's history.1 Due to the nocturnal occurrence of symptoms, patients with moderate or severe RLS usually suffer from sleep disruption. In patients seeking medical help, sleep disturbances and their consequences are the primary morbidity of the disorder and have a negative impact on quality of life.1,2
While RLS severity is usually evaluated with questionnaires like the International RLS Study Group Rating Scale (IRLS),3 polysomnography (PSG) recordings may support diagnosis.4 Periodic leg movements (PLM),5,6 sleep efficiency, and sleep onset latency are the most frequently used assessment parameters for determining symptom severity and treatment efficacy.7 In a typical RLS patient, one expects to find an increase of PLM during sleep (PLMS), increased sleep onset latency, increased wake periods after sleep onset, increased sleep stage shifts, as well as an increase of sleep stage 1 and a decrease in slow wave sleep.8 Although alterations of sleep are a common finding, no study has yet been published that compares polysomnographic parameters in a larger cohort of RLS patients and healthy subjects. The only comparable PSG study examined 12 patients and 12 matched controls and reported NREM sleep abnormalities, with increased sleep stage 1 and awakenings during sleep and decreased sleep efficiency in RLS;9 the spectral analysis of sleep EEGs did not find differences in the sleep EEG spectra.10
We compared the PSG findings from patients with idiopathic RLS with those of age- and sex-matched controls in order to determine characteristic differences between them and to uncover any macroarchitectural pattern in RLS. We hypothesized that RLS patients would exhibit lower sleep efficiency, longer sleep onset latency, and more fragmented sleep than controls. For assessment of sleep fragmentation, we applied the sleep fragmentation index (SFI).11,12 The SFI was introduced as an estimate of sleep disruption in patients with sleep disordered breathing.11 The main advantage of the SFI is that it can be determined without having to score arousals. The assessment of arousals is considered to be the “gold standard” in detecting sleep fragmentation, but arousal scoring is time consuming and requires trained observers. The SFI has shown a good correlation with the arousal index and indices of sleep discontinuity and high inter-night reliability in previous studies.11,12
METHODS
Patients and healthy controls
Only data from subjects with simultaneous PSG and PLM recordings on 2 consecutive nights were analyzed. Forty-five of the 100 consecutive idiopathic RLS patients investigated between 1999 and 2005 (64 females, 36 males, mean age 55.3 ± 12.4 yrs) were pair-matched to healthy controls derived from our laboratory database, using a computer program. The selected subgroup was younger on average (see below) because of the lack of older healthy controls, while the sex distribution did not differ from the original population of 100 patients (chi-square test, P = 0.956). Each patient and healthy subject underwent a semi-structured interview to ascertain history of sleep disturbance, physical and psychiatric examination, laboratory examination, electrocardiography (ECG), electroencephalography (EEG), and polysomnography (PSG).
RLS Patients
RLS was diagnosed according to the criteria of the International Restless Legs Syndrome Study Group.1 All patients suffered from idiopathic RLS and were unmedicated for at least 2 weeks prior to PSG. Exclusion of secondary RLS was based on laboratory analysis (blood cell count, serum ferritin, serum creatinine), and assessment of patient history and physical examination. One patient without REM sleep during the first night (having slept for only 84 min of the 8-hour bedtime period) and the matching control were excluded from the statistical analysis.
Healthy Subjects
Healthy subjects were recruited through relatives and friends of the clinic staff over a period of 12 years by word of mouth. None had a history of sleep or psychiatric disorders, substance abuse, or intake of hypnotics or other substances known to influence sleep (e.g., antihistamines). Exclusion of any relevant medical condition potentially influencing sleep was based on a semi-structured interview to ascertain history of sleep disturbance, physical and psychiatric examination, serum biochemistry, and polysomnography.
Polysomnographic and PLM recordings
Polysomnographic assessments included EEG (C3-A2, C4-A1), EOG, submental EMG, ECG, and superficial EMG of both anterior tibial muscles. Oronasal air flow, thoracic and abdominal breathing efforts, and transcutaneous oximetry were monitored in all patients, usually in the first night. Patients with clinically relevant sleep disordered breathing (apnea-hypopnea index >10 per hour) were excluded. PSG recordings were performed for an 8-h bedtime period, usually from 23:00 (“lights out”) to 07:00 (“lights on”); an adjustment of ± 30 min was allowed. Sleep recordings were visually analyzed according to Rechtschaffen and Kales by experienced raters.13 Arousals were scored as described by the American Sleep Disorders Association.14 PLMS were scored according to standard criteria.15
PSG parameters were calculated as follows: sleep onset latency (time from “lights out” until the first epoch of any sleep stage excluding sleep stage 1); sleep onset latency-10 (“10-minute criterion,” latency to persistent sleep) defined as time from “lights out” until any sleep stage, excluding sleep stage 1, lasting at least 10 min; sleep period time (SPT) defined as the time between sleep onset (according to the one-epoch criterion) and last sleep epoch of the recording (not regarding sleep stage 1); total sleep time (TST) defined as time spent in any sleep stage during SPT; sleep efficiency (SE) defined as percentage of TST during time in bed; arousal index (number of arousals per hour of TST); REM sleep latency (time from sleep onset until the first epoch of REM sleep); REM sleep latency-3 (“3-minute criterion”) defined as time from sleep onset until the first REM sleep lasting at least 3 min (6 epochs); REM density defined as (number of eye movements during REM/number of REM epochs) × 10; number of stage shifts (number of stage shifts during SPT); and number of wake periods (number of wake periods during SPT). The following PLMS indices were calculated: 1) the PLMS index (number of all PLMS per hour of TST) and 2) the PLMS-arousal index (PLMS associated with arousals per hour of TST). As PLMS data was only available in the first night for all controls, only PLMS data from the first night of PSG recording are presented. The sleep fragmentation index (SFI) was determined as previously described11 (total number of awakenings and shifts to stage 1 sleep divided by the TST) but modified to include any sleep stage shift and the total number of awakenings divided by TST in hours.12
Scales
RLS severity was assessed with the International RLS Severity Score (IRLS), a validated questionnaire developed by the International RLS Study Group.3
Statistics
Sleep variables were statistically analyzed using repeated measures MANOVA (SPSS 9.0 GLM, SPSS Inc, Chicago, IL) with the factor GROUP. MANOVAs were employed to avoid alpha error inflation. Correspondingly, the univariate ANCOVA F is considered only when the corresponding multivariate test was significant, and single factors or covariates were considered only when the corresponding univariate ANCOVA F was significant. As an additional exploratory analysis, nonparametric Spearman rank order correlations were performed. The level of significance (two-tailed if not indicated otherwise) was set at P ≤0.05. For descriptive purposes, mean ± standard deviation was calculated.
RESULTS
Patients
For each group, 45 patients and healthy controls were matched for age and sex. Patients and controls were 47.4 ± 10.9 years old and 47.3 ± 10.5 years old, respectively. Each group consisted of 29 females and 16 males. Females were aged 45.6 ± 11.3 years (range: 19–68 years) in the RLS group and 45.5 ± 11.0 years (range: 19–69 years) in the control group. Males were 50.6 ± 9.6 years old (range: 31–66 years) in the RLS group and 50.7 ± 8.7 years old (range: 32–63 years) in the control group. RLS severity as assessed with the IRLS scale was 24.0 ± 6.2 points, thus indicating moderately severe symptoms.-The apnea index of both groups was the same (0.5 ± 0.8/h in the RLS and 0.3 ± 0.5/h in the control group, P = 0.092), while the apnea-hypopnea index was 1.0/h of sleep higher in RLS patients (1.8 ± 2.1 in the RLS and 0.8 ± 1.7 in the control group, P = 0.017).
Polysomnography parameters
Group Effects
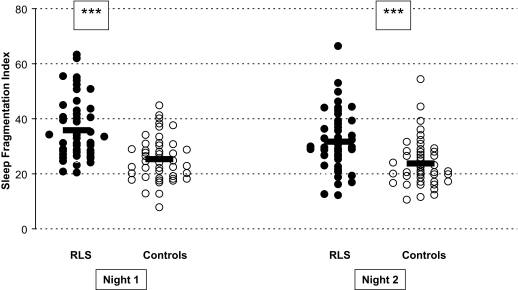
Data for sleep variables and statistical results are presented in Table 1. We found strong overall group effects (P <0.001): RLS patients exhibited longer sleep onset latencies (according to the 10-min criterion but not to the one-epoch criterion), shorter total sleep times, lower sleep efficiencies, higher arousal indices, more stage shifts, and longer REM sleep latency (for the one-epoch criterion). During sleep period time, percentage of wake, sleep stage 1, sleep stage 2, and REM sleep were different in patients compared with controls (see Table 1). The sleep fragmentation index (SFI) was significantly higher in the RLS group (Figure 1). The PLMS index and the PLMS-arousal index, assessed in the first night, were elevated in patients, as expected. As PLMS monitoring was not performed in every subject in the second night, an analysis of the PLMS parameters has been done only for the first (adaptation) night.
Table 1.
Polysomnography Parameters in RLS Patients and the Healthy Control Group. SPT: Sleep Period Time.
| Adaptation night |
Baseline night |
Group P | Night P | Night*Group P | |||
|---|---|---|---|---|---|---|---|
| Controls | RLS | Controls | RLS | ||||
| Multivariate test (Wilks' lambda) | <0.001 | <0.001 | 0.058 | ||||
| Sleep onset latency (min) | 24.3 ± 23.3 | 26.2 ± 24.3 | 17.1 ± 12.2 | 19.9 ± 24.5 | 0.536 | 0.013 | 0.859 |
| Sleep onset latency-10 (min) | 34.2 ± 32.8 | 56.2 ± 41.3 | 25.4 ± 17.8 | 41.6 ± 44.6 | 0.004 | 0.005 | 0.469 |
| REM sleep latency (min) | 100.7 ± 61.9 | 123.1 ± 73.0 | 70.0 ± 30.3 | 92.4 ± 76.3 | 0.044 | <0.001 | 0.998 |
| REM sleep latency-3 (min) | 117.7 ± 65.7 | 142.2 ± 86.5 | 84.2 ± 32.7 | 97.6 ± 80.4 | 0.135 | <0.001 | 0.461 |
| Sleep period time (min) | 454.2 ± 38.8 | 447.0 ± 29.7 | 462.8 ± 30.1 | 454.9 ± 26.6 | 0.186 | 0.023 | 0.932 |
| Total sleep time (min) | 403.5 ± 51.2 | 345.2 ± 80.2 | 425.4 ± 34.3 | 383.9 ± 67.0 | <0.001 | <0.001 | 0.172 |
| Sleep efficiency (%) | 82.5 ± 10.8 | 72.4 ± 16.5 | 87.6 ± 7.6 | 80.0 ± 13.9 | <0.001 | <0.001 | 0.305 |
| Arousal index (/h) | 13.9 ± 9.1 | 25.9 ± 13.0 | 12.4 ± 7.2 | 23.4 ± 12.6 | <0.001 | 0.007 | 0.497 |
| Number of stage shifts | 144.2 ± 40.8 | 168.0 ± 44.9 | 145.8 ± 44.6 | 168.3 ± 47.9 | 0.007 | 0.826 | 0.882 |
| Number of awakenings | 22.3 ± 11.3 | 30.8 ± 14.1 | 20.8 ± 16.2 | 26.8 ± 11.7 | 0.005 | 0.038 | 0.347 |
| Sleep fragmentation index | 25.3 ± 8.1 | 35.8 ± 11.1 | 23.8 ± 8.7 | 31.6 ± 11.0 | <0.001 | 0.004 | 0.174 |
| Wake (% of SPT) | 11.1 ± 9.0 | 23.0 ± 16.1 | 7.9 ± 7.0 | 15.6 ± 13.8 | <0.001 | <0.001 | 0.069 |
| Sleep stage 1 (% of SPT) | 9.0 ± 5.5 | 11.6 ± 4.9 | 8.6 ± 5.1 | 9.8 ± 3.6 | 0.042 | 0.010 | 0.087 |
| Sleep stage 2 (% of SPT) | 56.1 ± 8.2 | 46.0 ± 10.8 | 56.2 ± 7.8 | 50.6 ± 10.8 | <0.001 | 0.013 | 0.017 |
| Slow wave sleep (% of SPT) | 4.3 ± 5.6 | 4.2 ± 6.0 | 5.1 ± 6.8 | 4.9 ± 7.1 | 0.922 | 0.017 | 0.766 |
| REM sleep (% of SPT) | 19.1 ± 5.7 | 15.0 ± 6.1 | 21.7 ± 4.6 | 19.0 ± 6.0 | 0.001 | <0.001 | 0.259 |
| REM density (%) | 22.8 ± 8.0 | 25.8 ± 10.7 | 24.2 ± 9.1 | 25.2 ± 8.5 | 0.248 | 0.628 | 0.210 |
| PLMS index (/h) | 0.6 ± 1.2 | 22.9 ± 31.8 | - | - | <0.001 | - | - |
| PLMS-arousal index (/h) | 0.1 ± 0.3 | 8.1 ± 12.1 | - | - | <0.001 | - | - |
Figure 1.
Scatterplots of the sleep fragmentation indices of the first and second nights. Thick lines indicate means. ***: P < 0.001.
Night Effects
The multivariate repeated measures factor “NIGHT” was significant for both groups. In the second night, patients and controls showed decreased sleep onset latencies for both the one-epoch criterion and the 10-min criterion, increased total sleep time and sleep period time, improved sleep efficiency, and decreased arousal index. During sleep period time, fractions of waking and sleep stage 1 were significantly reduced, and REM sleep increased in night 2 compared with the first night recording. REM latency was shorter in the second night in both groups. Sleep fragmentation, as assessed with the SFI, decreased on the second night compared with the adaptation night.
Relationship of the Sleep Fragmentation Index, PLMS, and Arousal Parameters
In an additional exploratory analysis, we investigated the relationship between the SFI, the PLMS indices, and the arousal index. The SFI correlated with the arousal index in both groups and in both nights (RLS group: 1st night: r = 0.562, P <0.001; 2nd night: r = 0.544, P <0.001; control group: 1st night: r = 0.646, P <0.001, 2nd night: r = 0.611, P <0.001). There was a slight but significant correlation between the SFI and the PLMS-arousal index in the 1st night in both groups (RLS: r = 0.307, P = 0.040; controls: r = 0.296, P = 0.048). The apnea index (AI) and the apnea-hypopnea index (AHI) did not correlate with the SFI in the RLS (AI: r = −0.202, P = 0.184; AHI: r = −0.029, P = 0.851) or in the control group (AI: r = 0.170, P = 0.264; AHI: r = 0.273, P = 0.070).
DISCUSSION
In the present study, we compared the polysomnography data of a large cohort of patients with idiopathic RLS with that of ageand sex-matched healthy subjects. As hypothesized, RLS patients showed numerous periods of waking, increased arousals, diminished sleep duration and sleep efficiency, and an elevated sleep fragmentation index. In addition, we found evidence of REM sleep disturbance, with increased REM sleep latency and decreased percentage of REM sleep for both nights in the RLS group.
In a previous study, Saletu and coworkers 9 described similar changes in NREM sleep in a group of 12 RLS patients and age- and sex-matched healthy subjects. In their study, the patients showed increased REM sleep latency and decreased REM duration, however, REM parameters were not significantly different from those of healthy controls. This may be due to the small number of subjects investigated. Interestingly, sleep onset latency and sleep period time were comparable in RLS patients and healthy controls in both our study and in the aforementioned study.9 This finding is surprising, as most patients complain about difficulties in initiating sleep. The discrepancy might be explained by the clinical observation that RLS patients fall asleep rapidly but cannot maintain sleep. This is supported by our finding that it is not the sleep onset latency with the one-epoch criterion but the sleep onset latency with the 10-min criterion that is significantly prolonged in RLS patients. Therefore, we propose the routine assessment of this parameter in studies investigating RLS.
In our study, we also calculated the sleep fragmentation index (SFI). To date, only one study has investigated the SFI in a mixed group of RLS and PLMD (periodic leg movement disorder) patients.12 This study reported somewhat higher indices than in our RLS group, possibly because of the study's heterogenous patient population.12 In our study, the slightly elevated apnea-hypopnea index in the RLS group (1.8 ± 2.1/h vs. 0.8 ± 1.7 /h) might have contributed to the difference in SFI. A substantial influence is unlikely as the apnea-hypopnea index was only minimal, (1/h higher) and its magnitude was not comparable to the main correlate of SFI, the arousal index. As shown in Figure 1, SFI values of the RLS and the healthy group overlap to some extent. The overlap might stem from the calculation method used. This method has been validated12 and was developed to provide a measure of the fragmented sleep in sleep apnea syndrome, but it might not entirely capture the fragmented sleep in RLS patients.
A difference in the patients' preferred bedtimes and the bedtimes in the sleep laboratory might have influenced the PSG parameters. RLS patients tend to go to bed later and to get up later because they have the most restorative sleep period during the early morning hours. But this explanation is unlikely because most of our patients were employed and had to get up regularly in the early morning during the week. A possible limitation of the results is the younger age of the analyzed population compared with the original population of 100 patients. Also, the subgroups of females tended to be younger. However, because of the relatively small number of the patients in the subgroups we did not perform an additional subgroup analysis.
The alteration of REM sleep, with reduction of REM sleep duration and increase of REM sleep latency, is a novel finding. It might have occurred as a consequence of sleep interruptions which were in turn due to the nocturnal occurrence of RLS symptoms. We cannot however exclude the possibility that in some patients, bedtimes in the sleep laboratory began earlier and that REM phases subsequently occurred later. The REM sleep abnormalities we described may also be related to the pathology of RLS. However, no data is available which would explain such a relationship.
The consequences of chronic sleep loss have been investigated intensively in recent years. Chronic sleep deprivation, as in untreated RLS, may lead to increased risk of insulin resistance and type 2 diabetes17 or to impairment of sleep-dependent memory consolidation.18 Prefrontal cognitive deficits similar to those reported for loss of one night of sleep were shown recently in patients with RLS.19 Interestingly, modest reductions of sleep time and specific loss of REM sleep in healthy subjects appear to be related to hyperalgesia the following morning,20 an observation which may be especially relevant in RLS patients in light of the mechanical hyperalgesia described in this disorder.21
In summary, we present the first comparative analysis of polysomnography parameters of RLS and age- and sex-matched healthy subjects in a larger cohort. Our findings show markedly fragmented sleep with deterioration of both NREM and REM sleep in RLS. The long-term consequences of sleep loss in this patient population should be investigated further.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have reported no financial conflicts of interest.
REFERENCES
- 1.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institute of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 2.Allen RP, Walter AS, Montplaisir J, Hening W, Myers A, Bell TJ, Ferini-Strambi L. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165:1286–92. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 3.The International Restless Legs Syndrome Study Group. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 4.Hornyak M, Kotterba S, Trenkwalder C. Members of the Study Group “Motor Disorders” of the German Sleep Society: Indications for performing polysomnography in the diagnosis and treatment of restless legs syndrome. Somnologie. 2001;5:159–62. doi: 10.1016/s1389-9457(02)00080-1. [DOI] [PubMed] [Google Scholar]
- 5.Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lesperance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997;12:61–5. doi: 10.1002/mds.870120111. [DOI] [PubMed] [Google Scholar]
- 6.Hornyak M, Kopasz M, Voderholzer U, Riemann D. Variability of periodic leg movements in sleep in various sleep disorders: Implications for clinical and pathophysiological studies. Sleep. 2005;28:331–5. [PubMed] [Google Scholar]
- 7.Hening WA, Allen RP, Earley CJ, Picchietti DL, Silber MH. Restless Legs Syndrome Task Force of the Standards of Practice Committee of the American Academy of Sleep Medicine. An update on the dopaminergic treatment of restless legs syndrome and periodic limb movement disorder. Sleep. 2004;27:560–83. doi: 10.1093/sleep/27.3.560. [DOI] [PubMed] [Google Scholar]
- 8.Hening W. The clinical neurophysiology of the restless legs syndrome and periodic limb movements. Part I: diagnosis, assessment, and characterization. Clin Neurophysiol. 2004;115:1965–1974. doi: 10.1016/j.clinph.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 9.Saletu B, Anderer P, Saletu M, Hauer C, Lindeck-Pozza L, Saletu-Zyhlarz G. EEG mapping, psychometric, and polysomnographic studies in restless legs syndrome (RLS) and periodic limb movement disorder (PLMD) patients as compared with normal controls. Sleep Med. 2002;(3) Suppl:S35–42. doi: 10.1016/s1389-9457(02)00147-8. [DOI] [PubMed] [Google Scholar]
- 10.Hornyak M, Feige B, Voderholzer U, Riemann D. Spectral analysis of sleep EEG in patients with restless legs syndrome. Clin Neurophysiol. 2005;116:1265–72. doi: 10.1016/j.clinph.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Morrell MJ, Finn L, Kim H, Peppard PE, Badr MS, Young T. Sleep fragmentation, awake blood pressure, and sleep-disordered breathing in a population-based study. Am J Respir Crit Care Med. 2000;162:2091–96. doi: 10.1164/ajrccm.162.6.9904008. [DOI] [PubMed] [Google Scholar]
- 12.Haba-Rubio J, Ibanez V, Sforza E. An alternative measure of sleep fragmentation in clinical practice: the sleep fragmentation index. Sleep Med. 2004;5:577–81. doi: 10.1016/j.sleep.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Rechtschaffen A, Kales A. Los Angeles: Brain Information Service/Brain Research Institute; 1968. A Manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 14.American Sleep Disorders Association Report. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 15.American Sleep Disorders Association report: atlas and scoring rules. Recording and scoring leg movements. Sleep. 1993;16:749–59. [Google Scholar]
- 16.ICSD-2. American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders, 2nd Ed. (ICSD-2): Diagnostic and coding manual. [Google Scholar]
- 17.Spiegel K, Knutson K, Leproult R, Tasali E, van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 18.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437(7063):1272–8. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 19.Pearson VE, Allen RP, Dean T, Gamaldo CE, Lesage SR, Earley CJ. Cognitive deficits associated with restless legs syndrome (RLS) Sleep Med. 2006;7:25–30. doi: 10.1016/j.sleep.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Roehrs T, Hyde M, Blasdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29:145–151. doi: 10.1093/sleep/29.2.145. [DOI] [PubMed] [Google Scholar]
- 21.Stiasny-Kolster K, Magerl W, Oertel WH, Moller JC, Treede RD. Static mechanical hyperalgesia without dynamic tactile allodynia in patients with restless legs syndrome. Brain. 2004;127(Pt 4):773–82. doi: 10.1093/brain/awh079. [DOI] [PubMed] [Google Scholar]