Abstract
Background:
Obstructive sleep apnea (OSA) is associated with cardiovascular disease through incompletely understood mechanisms. Urinary albumin excretion is a surrogate for endothelial dysfunction and a potent cardiovascular disease risk predictor. We sought to determine whether urinary albumin excretion is increased in subjects with OSA.
Methods:
Four hundred ninety-six adults, representing a spectrum of OSA severity, underwent overnight polysomnography and urine collection. OSA severity was assessed using the apnea-hypopnea index (AHI). The primary outcome measure was the adjusted albumin-to-creatinine ratio (aACR). Linear mixed models were used to assess the association between AHI category and aACR, adjusted for confounders and renal dysfunction.
Results:
Subjects had a mean age of 44 ± 17 (SD) years and approximately half were men (44%) and African American (56%). The percentages of subjects with mild (AHI 5–14), moderate (AHI 15–29), and severe (AHI ≥ 30) OSA were 23%, 15%, and 15%, respectively. The median aACR for the entire sample was 4.3 mg/g (interquartile range: 2.9, 7.5). Adjusted linear mixed-model analyses showed a significant association between AHI category and aACR, with the AHI ≥ 30 group having the highest aACR levels (7.87 ± 1.02 mg/g vs 5.08 ± 0.41 mg/g for those with AHI < 5; P < 0.006). Similar findings were observed after excluding subjects with renal dysfunction.
Conclusion:
OSA is significantly associated with increased urine albumin excretion, especially among those with more severe disease. These data provide further evidence supporting endothelial dysfunction as a mediating pathway between cardiovascular disease and OSA.
Citation:
Faulx MD; Storfer-lsser A; Kirchner HL et al. Obstructive sleep apnea is associated with increased urinary albumin excretion. SLEEP 2007;30(7):923–929.
Keywords: Sleep, risk factors, epidemiology, endothelium
OBSTRUCTIVE SLEEP APNEA (OSA) IS A GROWING PUBLIC HEALTH PROBLEM IN THE UNITED STATES AND OTHER DEVELOPED COUNTRIES. THE NIGHTTIME apneas, hypoxemia, and arousals that characterize OSA are associated with daytime consequences that extend beyond the subjective fatigue and reduced quality of life commonly experienced by patients with OSA. Increasing research supports a causal role for OSA in diurnal hypertension,1 and the importance of this relationship is illustrated by the inclusion of sleep apnea as an identifiable cause of hypertension in current management guidelines.2 Additionally, individuals with OSA are more likely to suffer from heart failure, ischemic heart disease, stroke, and recurrent atrial fibrillation than are subjects without OSA.3–6 Individuals with untreated severe OSA also experience increased total mortality, including cardiovascular disease (CVD) mortality and sleep-related deaths.7,8
Although OSA is clearly associated with CVD, the pathophysiology that underlies this association remains unclear. Several physiologic links between OSA and CVD have been observed in recent years, including increased sympathetic nerve traffic, impaired vagal activity, and insulin resistance.9,10 Recent advances in our understanding of CVD risk at the molecular level suggest that regulation of homeostatic mechanisms such as systemic inflammation,11 coagulation,12 and endothelial function is altered in subjects with CVD, and such alterations may represent a state of “preclinical” CVD.13–15 Similar abnormalities have been described in OSA subjects even in the absence of known CVD or contributing comorbidities.16 Many of these abnormalities improve with continuous positive airway pressure (CPAP) therapy, suggesting that, if sleep-related pathophysiology does directly contribute to CVD risk, this risk may be attenuated to some degree by OSA treatment.15–17
Albuminuria independently predicts cardiovascular events in subjects with and without established cardiovascular risk factors.18,19 Albuminuria in subjects with diabetes may reflect a state of microvascular endothelial dysfunction, and therapies directed at reducing albumin excretion rates have been shown to slow the progression to overt kidney failure and attenuate cardiovascular disease risk in this population.20,21 Increased rates of urinary albumin excretion may also portend a greater risk for cardiovascular events in hypertensive individuals.22 The urinary albumin-to-creatinine ratio (ACR) has been shown to reliably estimate urine albumin excretion in a broad range of subjects.23 Furthermore, race- and sex-specific methods of urinary ACR calculation exist that adjust for baseline variation in creatinine excretion between these populations.24 While frank proteinuria has been previously observed in small, select populations of adult and pediatric subjects with OSA, the presence of overt glomerular dysfunction in many of these individuals confounds this observation.25,26 The relationship between OSA and microalbuminuria in adult individuals with intact glomerular filtration has not been previously described.
The purpose of this study is to define the relationship between OSA severity and race- and sex-adjusted ACR (aACR). We hypothesize that increased OSA severity will be independently associated with increased urinary aACR levels. Additionally, we will explore the extent to which associations with urinary albumin excretion and OSA are moderated by diabetes and hypertension.
METHODS
Sample
Subjects were participants in the Cleveland Family Study, an ongoing cohort study exploring the natural history and genetic epidemiology of OSA. Recruitment methods and data collection have been previously described.27 Briefly, subjects were recruited as members of families of probands with laboratory-confirmed OSA and from neighborhood control families. Out of 2462 cohort members, 735 were selected to undergo in-depth cardiovascular and metabolic risk factors assessment based on expected informativity for genetic analyses, as described elsewhere.28
Of the 735 participants, 644 were adults (≥ 16 years of age; data from subjects younger than 16 years were not included in this study), and 574 were not currently receiving CPAP treatment. We excluded 78 individuals due to: missing aACR data (n = 3); pregnancy or active menstruation at the time of sample collection (n = 37); and/or medical conditions, including macroalbuminuria (aACR ≥ 250 mg/g) (n = 18), systemic inflammatory illnesses (i.e., connective tissue diseases, sarcoidosis, multiple sclerosis, lupus), and kidney disease; and/or use of medications that may influence urinary albumin excretion or renal function (i.e., steroids, lithium) (n = 20). To avoid indication bias, we included subjects taking medications that could potentially influence urinary albumin excretion, such as HMG-CoA reductase inhibitors (n = 57, 11.6%) and angiotensin converting enzyme inhibitors (n = 68, 13.7%). These medication classes were examined in multivariable models, and none were significantly associated with aACR, nor were they confounded with AHI. The sample included 18 subjects who had reported CPAP use in the past. They were included in the analysis, since it was difficult to quantify the regularity of their use of CPAP and sensitivity analyses showed no appreciable differences in the results when these 18 subjects were excluded. Thus, the final analytic sample consisted of 496 subjects.
Protocol
The protocol was approved by the University Hospitals of Cleveland Institutional Review Board, and written informed consent was obtained for all participants. Participants were studied in a dedicated clinical research facility and underwent overnight polysomnography and overnight collection of voided urine, venipuncture, and physiologic and anthropometric assessments. Height was measured using a rigid stadiometer, and weight with a calibrated digital scale. Three blood pressure readings at each of 3 times (2200, 0700, and 1000) were obtained following standardized guidelines using a calibrated sphygmomanometer.29 Information on medical illnesses, medications, and symptoms was obtained from a standardized questionnaire.30
Sleep Data
The polysomnography recording consisted of 2 electroencephalograms, bilateral electrooculograms, a bipolar submental electromyogram, thoracic and abdominal respiratory inductance plethysmography, airflow (by a nasal-oral thermocouple and nasal pressure recording), finger pulse oximetry, electrocardiogram, body position, and bilateral leg movements. Studies were scored by 1 research technologist. Sleep staging and arousals were based on standard criteria.31,32 Apneas and hypopneas were defined using Sleep Heart Health Study criteria, modified to include the nasal pressure signal.33 Respiratory events were identified as a clear (> 30%) decline in airflow (from thermocouple or nasal pressure signals) or respiratory effort (from inductive respiratory bands) for at least 10 seconds associated with an oxygen desaturation of at least 3%. Corroborative arousal was not used in the scoring of respiratory events.
Urinary Albumin Excretion
The methods for measurement of urinary albumin and creatinine levels and for estimation of albumin excretion rates have been previously described.24 Overnight urine was collected between 2200 and 0600. Aliquots were adjusted to maintain a pH between 6.8 and 7.2. Albumin levels were detected by nephelometry using monoclonal antibodies to human albumin. Creatinine levels were determined using the Jaffe method and adjusted for sex and race using published formulae.24,35 The aACR was defined as [urine albumin (mg)]/k[urine creatinine (g)]], where k represents a sex- and race-dependent correction factor.24 Microalbuminuria was defined as an aACR between 25 and 250 mg/g.
Exposure
The primary exposure was the apnea-hypopnea index (AHI; number of respiratory events [apneas or hypopneas] recorded per sleep hour). AHI was categorized into 4 groups: low (AHI < 5), mild (AHI 5–14), moderate (AHI 15–29) and severe (AHI ≥ 30).
Covariate Data
Blood pressure was determined as the average of 9 measurements. Individuals were considered hypertensive if average systolic blood pressure was 140 or greater, diastolic blood pressure was 90 or greater, or the use of blood pressure medications in the past year was reported. Body mass index (BMI) was computed as the ratio of weight to the square of height (kg/m2). Individuals with a fasting glucose of 126 or greater, who were taking medicine for diabetes, or who had a doctor's diagnosis of diabetes were classified as diabetic. Current smokers reported smoking at least 1 cigarette per day over the past month. Cystatin-C, a serum measure of glomerular filtration rate, was assayed with a BNII nephelometer (Dade Behring Inc., Deerfield, Ill.) using a particle-enhanced immunonepholometric assay (N Latex Cystatin-C).34 Glomerular filtration rate (GFR) was calculated from serum cystatin-C levels and sex-corrected as described by Grubb et al.36
Statistical Analysis
Subject characteristics, sleep indices, and aACR were summarized using means, standard deviations, and medians for continuous variables and counts and proportions for categorical variables. Univariate analyses showed that aACR level distribution was right-skewed; therefore the outcome was transformed using the natural logarithm (ln) to achieve approximate normality. Spearman correlations were used to provide a preliminary assessment of the linear relationship between AHI, BMI, GFR, and aACR, ignoring the familial correlation. Generalized estimating equations with an exchangeable within-family correlation structure and robust variance estimate were used to examine relationships between continuous and categorical covariates (e.g., microalbuminuria). Linear mixed models, with family as a random effect to account for the intrafamilial correlation, was used to examine relationships with ln(aACR). To assess the relationship between AHI category and aACR, 3 sets of linear mixed models were fitted: unadjusted for covariates (model 1), adjusted for subject characteristics (age, sex, race, BMI, and GFR) (model 2), and adjusted for subject characteristics as well as comorbidities (hypertension and diabetes) (model 3). Exploratory analyses and residual diagnostics showed a quadratic relationship between age and aACR; thus, an age-squared term was added to the adjusted models. Continuous covariates were centered to their mean value to enhance interpretability and decrease collinearity between the age and age-squared terms. Additional covariates, including smoking, known CVD, angiotensin converting enzyme inhibitor use, and HMG-CoA reductase inhibitor use, were evaluated in model 2, and statistically significant effects were retained. Since hypertension and diabetes both promote urinary albumin excretion, we wished to determine whether sleep apnea severity influenced urinary albumin excretion differently in the presence of either of these 2 diagnoses. Thus, if the main effect of AHI was statistically significant in model 3, 2-way interactions between hypertension and AHI, as well as between diabetes and AHI, were evaluated. Secondary sensitivity analyses included refitting the 3 models excluding the 30 participants with moderate kidney dysfunction (GFR < 60). To control for the family-wise error rate, the Bonferroni-Holm step-down procedure was used for pairwise comparisons of AHI group.37 The results of the linear mixed models are summarized via regression coefficients and standard errors on the In-scale in the tables, and adjusted geometric means with 95% confidence intervals are displayed for the AHI groups in text and Figure 1. The data were analyzed using SAS version 9 (SAS Institute Inc., Cary, NC).
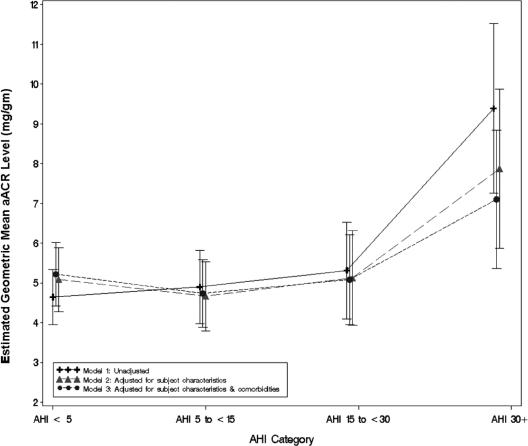
Figure 1.
Adjusted albumin-to-creatinine ratio (aACR): unadjusted and adjusted linear regression results. AHI refers to apnea-hypopnea index.
RESULTS
Subject characteristics are shown in Table 1, stratified by AHI category. The sample consisted of 496 subjects with a mean age of 44.5 (17.3 SD) years and a mean BMI of 32.5 (8.2) kg/m2. Approximately half of the sample were men (44.4%), and half were African American (56.3%). Roughly one-third (34.7%) had hypertension, with a smaller proportion of subjects having diabetes (12.7%). A wide range of OSA severity was observed; approximately half (47.0%) of the sample had an AHI less than 5, 23.4% were in the mild range (AHI 5–14), 14.7% were in the moderate range (AHI 15–29), and 14.9% were in the severe range (AHI ≥ 30). Not unexpectedly, participants with more severe OSA tended to be older and heavier, with higher proportions of men, those with diabetes, and those with hypertension.
Table 1.
Sample Characteristics by AHI Category
| Analytic Sample (n = 496) |
AHI < 5 (n = 233) |
AHI 5–14 (n = 116) |
AHI 15–29 (n = 73) |
AHI ≥ 30 (n = 74) |
|
|---|---|---|---|---|---|
| Subject Characteristics | |||||
| Age, y | 44.5 ± 17.3 (45.3) |
37.6 ±16.9 (37.1) |
49.0 ± 15.6 (49.8) |
50.9 ±13.9 (49.5) |
52.7 ± 16.3 (51.0) |
| Male sex | 220 (44.4) | 80 (34.3) | 50(43.1) | 34 (46.6) | 56 (75.7) |
| African American race | 279 (56.3) | 122 (52.4) | 71 (61.2) | 42 (57.5) | 44 (59.5) |
| BMI, kg/m2 | 32.5 ± 8.2 (31.3) |
28.9 ± 7.2 (27.2) |
34.2 ± 7.1 (32.9) |
34.8 ± 7.3 (34.5) |
38.6 ±8.3 (37.6) |
| Obesea | 281 (56.7) | 80 (34.3) | 81 (69.8) | 54 (74.0) | 66 (89.2) |
| Current smoker | 128 (25.8) | 62 (26.6) | 27 (23.3) | 16(21.9) | 23 (31.1) |
| Hypertensive | 172 (34.7) | 40 (17.2) | 50(43.1) | 37 (50.7) | 45 (60.8) |
| Diabetic | 63 (12.7) | 14 (6.0) | 17 (14.7) | 11 (15.1) | 21 (28.4) |
| Sleep Characteristics | |||||
| AHI, events/h | 14.8 ±21.2 (5.9) |
1.7 ±1.3 (1.5) |
9.5 ± 3.0 (9.0) |
21.3 ±4.3 (20.7) |
57.8 ± 22.5 (52.7) |
| Arousal Index, arousals/h | 16.9 ±10.3 (14.4) |
12.8 ± 6.0 (11.9) |
15.3 ± 7.1 (13.9) |
18.5 ±9.1 (16.4) |
31.4 ±13.5 (30.1) |
| % time < 90% o2 saturation | 4.2 ±11.6 (0.0) |
0.8 ± 6.8 (0.0) |
3.0 ±10.6 (0.2) |
4.0 ±5.8 (2.0) |
17.2 ±18.6 (10.0) |
| (0.0) | (0.0) | (0.2) | (2.0) | (10.0) |
Data are presented as number (%) for categorical variables and mean ± SD (median) for continuous variables.
Obese is defined as a body mass index (BMI) ≥ 30 kg/m2. AHI refers to apnea-hypopnea index.
The distribution of urinary albumin levels and estimates of GFR for the analytic sample and by AHI category are provided in Table 2. aACR correlated positively with AHI (r = 0.19, 95% confidence interval [CI]: 0.11,0.27), P < 0.001). Participants with an AHI of at least 30 had significantly higher mean aACR levels, compared with each of the other 3 AHI groups (all P values < 0.0003). Similarly, participants with an AHI of at least 30 had the highest prevalence of microalbuminuria, whereas those with an AHI less than 5 had the lowest prevalence (20.3% vs 4.7%, P = 0.0002). Subjects in the most severe AHI group also had significantly lower mean GFR levels compared with those in the AHI less than 5 group (P = 0.0080). Spearman correlations, however, showed that aACR and GFR were not significantly associated (r = 0.03, 95% CI: −0.06, 0.11), P = 0.567).
Table 2.
Distribution of Urinary Albumin Levels and Glomerular Filtration Rate Estimates by AHI Category
| Analytic Sample (n = 496) |
AHI < 5 (n = 233) |
AHI 5–14 (n = 116) |
AHI 15–29 (n = 73) |
AHI ≥ 30 (n = 74) |
|
|---|---|---|---|---|---|
| aACR, mg/gm | 10.7 ±23.7 (4.3) |
7.4 ± 15.4 (3.9) |
9.7 ± 22.2 (4.1) |
11.9 ± 25.3 (5.1) |
21.5 ±38.6 (7.6) |
| Ln aACR, ln mg/gm | 1.64 ±1.00 (1.46) |
1.48 ±0.85 (1.37) |
1.55 ± 1.02 (1.42) |
1.69 ± 1.06 (1.62) |
2.22 ± 1.18 (2.03) |
| Microalbuminuria, aACR 25–249 | 41 (8.3) | 11 (4.7) | 10 (8.6) | 5 (6.9) | 15 (20.3) |
| GFR, mL/min | 117.1 ±40.0 (115.6) |
123.5 ± 36.7 (119.6) |
113.0 ±42.5 (112.3) |
110.6 ± 37.6 (107.3) |
109.8 ±45.8 (110.3) |
Data are presented as number (%) for categorical variables and mean ± SD (median) for continuous variables. Adjusted albumin-to-creatinine ratio (aACR) and glomerular filtration rate (GFR) based on Cystatin C levels, as described in the methods).
The results of the primary analysis assessing the association between AHI category and ln (aACR) in unadjusted and adjusted linear mixed models are shown in Table 3 and Figure 1. AHI level was significantly associated with ln(aACR) in all 3 regression models. In the unadjusted model (model 1) as well as the model adjusted for subject characteristics and GFR (model 2), pair-wise comparisons revealed that subjects with an AHI of at least 30 had significantly higher mean aACR levels, compared with each of the other AHI categories (e.g., model 2: AHI ≥ 30: 7.87 ± 1.02 mg/g vs AHI < 5: 5.08 ± 0.41 mg/g, P < 0.0057). Results of model 3, which additionally adjusted for diabetes and hypertension, showed a similar pattern. In this model, subjects with an AHI of at least 30 had significantly higher aACR levels, compared with each of the other AHI groups without correcting for multiple comparisons. After correction, those with an AHI of at least 30 had significantly higher mean aACR levels, compared with subjects with mild OSA (AHI 5–14.9) (7.10 ± 0.89 vs 4.74 ± 0.43, P = 004). Significant interactions between AHI and hypertension and AHI and diabetes were not found (P > 0.10).
Table 3.
Linear Mixed-Model Analysis of ln aACR
| Model 1: Unadjusted |
Model 2: Adjusted for subject characteristics – GFR |
Model 3: Adjusted for subject characteristics, comorbidities – GFR |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Covariates | β | SE | P value | β | SE | P value | β | SE | P value |
| Intercept | 1.535 | 0.076 | < 0.0001 | 1.285 | 0.113 | < 0.0001 | 1.147 | 0.111 | < 0.0001 |
| AHI Category | |||||||||
| <5 | -- | -- | < 0.0001 | -- | -- | 0.0043 | -- | -- | 0.0338 |
| 5–14.9 | 0.054 | 0.107 | < 0.0001 | -0.086 | 0.117 | 0.0043 | -0.098 | 0.111 | 0.0338 |
| 15–29.9 | 0.135 | 0.128 | < 0.0001 | 0.009 | 0.140 | 0.0043 | -0.027 | 0.134 | 0.0338 |
| 30+ | 0.705 | 0.128 | < 0.0001 | 0.438 | 0.158 | 0.0043 | 0.308 | 0.152 | 0.0338 |
| Age | 0.053 | 0.014 | 0.0003 | 0.019 | 0.015 | 0.1993 | |||
| Age2 | 0.010 | 0.004 | 0.0043 | 0.009 | 0.003 | 0.0061 | |||
| Male sex | 0.083 | 0.093 | 0.3728 | 0.082 | 0.088 | 0.3551 | |||
| African American race | 0.226 | 0.114 | 0.0502 | 0.118 | 0.113 | 0.2970 | |||
| Current smoker | 0.206 | 0.102 | 0.0426 | 0.253 | 0.097 | 0.0093 | |||
| BMI, per 5-unit increase | 0.056 | 0.032 | 0.0861 | 0.010 | 0.032 | 0.7393 | |||
| GFR, per 20-unit increase | 0.047 | 0.025 | 0.0658 | 0.058 | 0.024 | 0.0168 | |||
| Diabetes | 0.724 | 0.132 | < 0.0001 | ||||||
| Hypertension | 0.385 | 0.102 | 0.0002 | ||||||
aACR refers to adjusted albumin-to-creatinine ratio; AHI, apnea-hypopnea index; BMI, body mass index; GFR, glomerular filtration rate.
Secondary sensitivity analyses explored the association between AHI and aACR excluding the 30 participants with a GFR less than 60 mL/minute, indicative of moderate kidney dysfunction. Although the effect of AHI was somewhat attenuated, the pattern of results was consistent with those of the primary analyses. AHI was significantly associated with aACR in unadjusted and adjusted models (β coefficient 0.22, P = 0.034 for AHI ≥ 30 after adjusting for subject characteristics, comorbidities and GFR), and participants in the most severe AHI category had the highest mean levels of aACR.
DISCUSSION
We have demonstrated that the presence of severe OSA is significantly associated with increased urine albumin excretion, and this association, while modest, remains significant after adjusting for the presence of confounding factors such as obesity, diabetes, or hypertension. More modest levels of OSA were not associated with increased albuminuria. The observed associations were independent of GFR, as demonstrated by analyses that adjusted for estimated GFR or that excluded individuals with a GFR less than 60 mL/minute. To illustrate our findings in a more clinical light, we compared the magnitude of the association between OSA and urinary albumin excretion to that of hypertension and urinary albumin excretion. Our models estimate that the potential influence of severe OSA on aACR is essentially equivalent to that of hypertension. Specifically, adjusted mean aACR levels are estimated to be 1.36 mg/g higher in subjects with OSA, compared with subjects with an AHI less than 5, and 1.47 mg/g higher in subjects with hypertension, as compared with subjects without hypertension.
Urinary albumin excretion in individuals with OSA may result from the influence of sleep-related pathophysiologic changes (i.e., intermittent hypoxemia, increased sympathetic nerve traffic) on glomerular endothelial function. Albuminuria has been shown to correlate with indices of systemic endothelial dysfunction, and the presence of albumin in the urine, even in nondiabetic, nonhypertensive individuals with normal renal function, may be considered a measure of systemic endothelial dysfunction detected at the glomerular level.38 Since prior studies have demonstrated that endothelial dysfunction exists in otherwise healthy subjects with moderate to severe OSA,39,40 the association between albuminuria and OSA in this study is not particularly surprising. Although evidence implicating OSA as a direct cause of CVD is lacking, this study adds plausibility to the notion that sleep apnea-related pathophysiologic changes directly influence systemic CVD risk markers and illustrates the need for further mechanistic study in this area.
One might question whether OSA promotes urinary albumin excretion through its association with hypertension and diabetes. OSA is now considered a treatable cause of hypertension, and hypertensive subjects with urinary albumin excretion rates as low as 5 meg/minute (7.2 mg/day) carry a significantly increased risk for incident coronary artery disease and death.2,41,42 There is increasing evidence that OSA is associated with impaired glucose tolerance, as well as with diabetes.43 Urinary albumin excretion is prevalent in the diabetic population in which microalbuminuria is considered not only a risk factor, but also a target for therapeutic intervention.21,42 Additionally the presence of the metabolic syndrome, common in subjects with OSA, appears to accelerate urine albumin excretion and magnifies cardiovascular risk in hypertensive subjects.43,44 In the present study, we show that urinary aACR levels are significantly increased in subjects with severe OSA after adjusting for the presence of hypertension and diabetes, suggesting that OSA may directly influence urinary albumin loss. Furthermore, the association of OSA with urinary albumin excretion does not appear to be moderated by the presence of hypertension or diabetes. Thus, our findings suggest that the increased urinary aACR levels observed in subjects with severe OSA may be due to the presence of OSA itself rather than confounding comorbidities.
Although microalbuminuria (typically defined as the daily excretion of 30 to 300 mg of albumin, or ACR 25–250 mg/g) is strongly associated with CVD in the medical literature,19,46 urinary ACR levels and albumin excretion rates well below the microalbuminuria level are associated with an increased risk for hypertension, CVD, stroke, and major adverse cardiovascular events.18,19,47 Although most of our study subjects had aACR levels below the microalbuminuria range, current literature suggests that albumin excretion rates comparable to those seen in our subjects with more severe OSA reflect the presence of endothelial dysfunction and imply some degree of increased CVD risk. Furtner and colleagues explored the relationship between urine albumin excretion and arterial atherosclerosis in the general population in Italy.18 Subjects in the upper quintile for urine ACR (range 8.27–17.41 mg/g) were twice as likely to have carotid and/or femoral artery atherosclerosis detected by ultrasound than those in the first quintile (0.28–3.47 mg/g). In another study, healthy men and women with urine ACR levels greater than 6.67 and 7.49 mg/g, respectively (fourth quartile) were twice as likely as individuals with ACR levels in the first quartile to develop hypertension within 3 years.47 In our study, the median aACR for subjects with severe OSA was 7.6 mg/g; 75% of these subjects had aACR levels greater than 4.1 mg/g and 25% had ACR levels greater than 15.7 mg/g. Albumin excretion rates in this range are clearly associated with an adverse cardiovascular risk profile.
Our study has several strengths. First, we studied individuals with a wide range of OSA severity that allowed for the exploration of a dose-response relationship with aACR. Additionally, we rigorously collected information on exposure and outcome measures, as well as potential confounders, in a dedicated clinical research facility staffed by trained personnel following a standardized protocol. We used as our primary outcome measure the aACR, a simple index of cardiovascular risk that has been validated as a potent predictor of incident cardiovascular disease in several study populations.18,19,47 Our ability to adjust ACR to account for race- and sex-related differences in creatinine clearance made us more confident that our results were not biased by race and sex differences.24 We also accounted for the potential influence of unrecognized kidney disease on albumin excretion rates by estimating GFR from plasma cystatin-C levels. The latter is a reliable validated assay that is felt to provide a more accurate assessment of glomerular function than serum creatinine alone or GFR estimated from serum and urine creatinine using the Cockcroft-Gault formula.36,48 In our sample, the mean GFR for each AEH group was well within the normal range, and the number of study subjects with significant kidney disease was small. When these subjects were excluded from analysis, our findings did not appreciably change.
There are several limitations that should be addressed. One limitation is that the participants in our study with more severe OSA were generally obese men, and more than half had hypertension. However, this is the population commonly encountered in the clinical setting, so our results may have broader clinical applicability than if we had studied a more select group. Although individuals with severe OSA were older, were more obese, and had a higher prevalence of hypertension and diabetes, there was sufficient overlap of these characteristics across our relatively large sample to allow for the statistical adjustment of these factors. We also included community control subjects without OSA. Further, by excluding individuals with low GFR estimates, we were able to eliminate occult renal disease as an explanatory factor for our observed findings.
Our findings have potential clinical implications. Cardiovascular disease affects many individuals with OSA, and the onset of clinically apparent cardiovascular disease may be preceded by a prolonged period of “preclinical” disease characterized by asymptomatic endothelial dysfunction.14 Furthermore, CVD risk appears to vary considerably among subjects with OSA.4 Early detection of end-organ (e.g., endothelial) dysfunction with measures such as urinary aACR may allow for the selection of higher risk populations who may benefit from closer medical follow-up or preventive pharmacotherapy. Although randomized, controlled intervention trials targeting microalbuminuria in subjects with OSA are lacking, data from such a trial in subjects with the metabolic syndrome showed significant clinical benefit in subjects with albuminuria who received pravastatin.49 Urine aACR measurement is a safe, inexpensive, readily available test that can be performed in an office setting. If clinical benefit from preventive therapy for microalbuminuria in OSA is confirmed in clinical trials, urine albumin measurement may play a role in the management of OSA comparable to the role it presently plays in the management of diabetes mellitus.
In summary, we have demonstrated that severe OSA is independently associated with increased urine albumin excretion, a marker of both endothelial dysfunction and cardiovascular disease risk. This study provides further evidence supporting the hypothesis that sleep apnea-related pathophysiology independently contributes to systemic cardiovascular risk and extends prior research by suggesting the presence of glomerular endothelial dysfunction in subjects with OSA. Overnight urinary albumin excretion may serve as a marker of OSA severity and CVD risk in patients with sleep apnea. Further study of endothelial dysfunction, including assessment of renal vascular changes, in subjects with OSA may provide additional insights into the high prevalence of cardiovascular disease in this population.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Redline has received research support from Medical Devices, Inc. and has served as an advisor for Cypress Biosciences and Organon. Dr. Faulx, Ms. Storfer-Isser, Dr. Kirchner, Dr. Jenny, and Dr. Tracy have reported no financial conflicts of interest.
REFERENCES
- 1.Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA. 2003;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 3.Bradley TD, Floras JS. Sleep apnea and heart failure part I: obstructive sleep apnea. Circulation. 2003;107(12):1671–8. doi: 10.1161/01.CIR.0000061757.12581.15. [DOI] [PubMed] [Google Scholar]
- 4.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163(1):1925. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 5.Yaggi H, Mohsenin V. Obstructive sleep apnoea and stroke. Lancet Neurol. 2004;3(6):333–42. doi: 10.1016/S1474-4422(04)00766-5. [DOI] [PubMed] [Google Scholar]
- 6.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589–94. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 7.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hyponoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 8.Gami AS, Howard DE, Olson EJ, et al. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352(12):1206–14. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 9.Usui K, Bradley TD, Spaak J, Ryan CM, Kubo T, Kanecko Y, Floras JS. Inhibition of awake sympathetic nerve activity of heart failure patients with obstructive sleep apnea by nocturnal continuous positive airway pressure. J Am Coll Cardiol. 2005;45(12):2008–11. doi: 10.1016/j.jacc.2004.12.080. [DOI] [PubMed] [Google Scholar]
- 10.Naughton MT. The link between obstructive sleep apnea and heart failure: underappreciated opportunity for treatment. Curr Cardiol Rep. 2005;7(3):211–5. doi: 10.1007/s11886-005-0079-2. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM. High-sensitivity C-reactive protein, inflammation, and cardiovascular risk: from concept to clinical practice to clinical benefit. Am Heart J. 2004;148(Suppl l):S19–26. doi: 10.1016/j.ahj.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Loscalzo J. Regulation of tissue factor expression in human microvascular endothelial cells by nitric oxide. Circulation. 2000;101(18):2144–8. doi: 10.1161/01.cir.101.18.2144. [DOI] [PubMed] [Google Scholar]
- 13.Ganz P, Vita JA. Testing endothelial vasomotor function. nitric oxide, a multipotent molecule. Circulation. 2003;108(17):2049–53. doi: 10.1161/01.CIR.0000089507.19675.F9. [DOI] [PubMed] [Google Scholar]
- 14.Faulx MD, Wright AT, Hoit BD. The Detection of Endothelial Dysfunction Using Brachial Artery Ultrasonography. Am Heart J. 2003;145(6):943–51. doi: 10.1016/S0002-8703(03)00097-8. [DOI] [PubMed] [Google Scholar]
- 15.Harsch IA, Schahin SP, Radespiel-Troger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169(2):156–62. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 16.Quan SF, Gersh BJ National Center on Sleep Disorders Research; National Heart, Lung, and Blood Institute. Cardiovascular consequences of sleep-disordered breathing: past, present, and future. Report of a workshop from the national center on sleep disorders research and the national heart, lung, and blood institute. Circulation. 2004;109(8):951–7. doi: 10.1161/01.CIR.0000118216.84358.22. [DOI] [PubMed] [Google Scholar]
- 17.Ip MS, Lam B, Chan LY, et al. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000;162(6):2166–71. doi: 10.1164/ajrccm.162.6.2002126. [DOI] [PubMed] [Google Scholar]
- 18.Furtner M, Kiechl S, Mair A, et al. Urinary albumin excretion is independently associated with carotid and femoral artery atherosclerosis in the general population. Eur Heart J. 2005;26(3):279–87. doi: 10.1093/eurheartj/ehi014. [DOI] [PubMed] [Google Scholar]
- 19.Gerstein HC, Mann JFE, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286(4):421–6. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 20.Schalkwijk CG, Stehouwer CD. Vascular complications in diabetes mellitus:the role of endothelial dysfunction. Clin Sci (Lond) 2005;109(2):143–59. doi: 10.1042/CS20050025. [DOI] [PubMed] [Google Scholar]
- 21.Jerums G, MacIsaac RJ. Treatment of microalbuminuria in patients with type 2 diabetes mellitus. Treat Endocrinol. 2002;1(3):163–73. doi: 10.2165/00024677-200201030-00004. [DOI] [PubMed] [Google Scholar]
- 22.Romundstad S, Holmen J, Kvenild K, et al. Microalbuminuria and all-cause mortality in 2,089 apparently healthy individuals: a 4.4-year follow-up study. The Nord-Trondelag Health Study (HUNT), Norway. Am J Kidney Dis. 2003;42(3):466–73. doi: 10.1016/s0272-6386(03)00742-x. [DOI] [PubMed] [Google Scholar]
- 23.Schwab SJ, Christensen RL, Dougherty K, et al. Quantitation of proteinuria by the use of protein-to-creatinine ratios in single urine samples. Arch Intern Med. 1987;147(5):943–4. [PubMed] [Google Scholar]
- 24.Jacobs DR, Jr, Murtaugh MA, Steffes M, et al. Gender- and race-specific determination of albumin excretion rate using albumin-to-creatinine ratio in single, untimed urine specimens. The coronary artery risk development in young adults study. Am J Epidemiol. 2002;155(12):1114–9. doi: 10.1093/aje/155.12.1114. [DOI] [PubMed] [Google Scholar]
- 25.Chaudhary BA, Sklar AH, Chaudhary TK, Kolbeck RC, Speir WA. Sleep apnea, proteinuria, and nephrotic syndrome. Sleep. 1988;11(1):69–74. doi: 10.1093/sleep/11.1.69. [DOI] [PubMed] [Google Scholar]
- 26.Krishna J, Shah ZA, Merchant M, Klein JB, Gozal D. Urinary protein expression patterns in children with sleep-disordered breathing: preliminary findings. Sleep Med. 2006;7(3):221–7. doi: 10.1016/j.sleep.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Redline S, Tishler PV, Tosteson TD, et al. The familial aggregation of obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151(3 Pt 1):682–7. doi: 10.1164/ajrccm/151.3_Pt_1.682. [DOI] [PubMed] [Google Scholar]
- 28.Palmer LJ, Buxbaum SG, Larkin E, et al. A whole genome scan for obstructive sleep apnea and obesity. Am J Hum Genetic. 2003;72(2):340–50. doi: 10.1086/346064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dallas, TX: 1980. American Heart Association recommendations for human blood pressure determination by sphygmomanometers: Report of a Subcommittee of the Postgraduate Education Committee. [Google Scholar]
- 30.Kump K, Whalen C, Tishler PV, et al. Assessment of the validity and utility of a sleep symptom questionnaire. Am J Respir Crit Care Med. 1994;150(3):735–41. doi: 10.1164/ajrccm.150.3.8087345. [DOI] [PubMed] [Google Scholar]
- 31.EEG arousals: scoring rules and examples. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 32.Rechtschaffen A, Kales A. Washington, D.C.: U.S. Government Printing Office; 1968. A manual of standardized techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 33.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep. 1998;21(7):759–67. [PubMed] [Google Scholar]
- 34.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II system. Scand J Clin Lab Invest. 1999;59(1):1–8.1. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 35.Slot C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest. 1965;17(4):381–7. doi: 10.3109/00365516509077065. [DOI] [PubMed] [Google Scholar]
- 36.Grubb A, Björk J, Lindström V, et al. A cystatin C-based formula without anthropometric variables estimates glomerular filtration rate better than creatinine clearance using the Cockroft-Gault formula. Scand J Clin Lab Invest. 2005;65:153–62. doi: 10.1080/00365510510013596. [DOI] [PubMed] [Google Scholar]
- 37.Holm S. A simple sequentially rejective multiple test procedure. Scand J Statistics. 1979;6:65–70. [Google Scholar]
- 38.Clausen P, Jensen JS, Jensen G, et al. Elevated urinary albumin excretion is associated with impaired arterial dilatory capacity in clinically healthy subjects. Circulation. 2001;103(14):1869–74. doi: 10.1161/01.cir.103.14.1869. [DOI] [PubMed] [Google Scholar]
- 39.Faulx MD, Larkin EK, Hoit BD, et al. Sex Influences Endothelial Function in Sleep-Disordered Breathing. Sleep. 2004;27(6):1113–20. doi: 10.1093/sleep/27.6.1113. [DOI] [PubMed] [Google Scholar]
- 40.Kato M, Roberts-Thompson P, Phillips BG, et al. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102(21):2607–10. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- 41.Dhillon S, Chung SA, Fargher T, et al. Sleep apnea, hypertension, and the effects of continuous positive airway pressure. Am J Hypertens. 2005;18(5 Pt 1):594–600. doi: 10.1016/j.amjhyper.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 42.Klausen KP, Scharling H, Jensen G, et al. New definition of microalbuminuria in hypertensive subjects: association with incident coronary artery disease and death. Hypertension. 2005;46(1):33–7. doi: 10.1161/01.HYP.0000169153.78459.50. [DOI] [PubMed] [Google Scholar]
- 43.Punjabi NM, Shahar E, Redline S, et al. Sleep Heart Health Study Investigators. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160(6):521–30. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 44.Resnick HE, Redline S, Shahar E, et al. Diabetes and sleep disturbances: findings from the Sleep Heart Health Study. Diabetes Care. 2003;26(3):702–9. doi: 10.2337/diacare.26.3.702. [DOI] [PubMed] [Google Scholar]
- 45.Weir MR. Microalbuminuria in type 2 diabetics: an important, overlooked cardiovascular risk factor. J Clin Hypertens (Greenwich) 2004;6(3):134–41. doi: 10.1111/j.1524-6175.2004.02524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mule G, Nardi E, Cottone S, et al. Influence of metabolic syndrome on hypertension-related target organ damage. J Intern Med. 2005;257(6):503–13. doi: 10.1111/j.1365-2796.2005.01493.x. [DOI] [PubMed] [Google Scholar]
- 47.Wang TJ, Evans JC, Meigs JB, et al. Low-grade albuminuria and the risks of hypertension and blood pressure progression. Circulation. 2005;111:1370–6. doi: 10.1161/01.CIR.0000158434.69180.2D. [DOI] [PubMed] [Google Scholar]
- 48.Bicik Z, Bahcebasi T, Kulaksizoglu S, et al. The efficacy of cystatin C assay in the prediction of glomerular filtration rate. Is it a more reliable marker for renal failure? Clin Chem Lab Med. 2005;43(8):855–61. doi: 10.1515/CCLM.2005.144. [DOI] [PubMed] [Google Scholar]
- 49.Geluk CA, Asselbergs FW, Hillege HL, et al. Impact of statins in microalbuminuric subjects with the metabolic syndrome: a substudy of the PREVEND Intervention Trial. Eur Heart J. 2005;26(13):1314–20. doi: 10.1093/eurheartj/ehi253. [DOI] [PubMed] [Google Scholar]