Abstract
Study Objectives:
Morphometric analysis of magnetic resonance imaging brain scans was used to investigate possible neuroanatomic differences between patients with primary insomnia compared to good sleepers.
Design:
MRI images (1.5 Tesla) of the brain were obtained from insomnia patients and good sleepers. MRI scans were analyzed bilaterally by manual morphometry for different brain areas including hippocampus, amygdala, anterior cingulate, orbitofron-tal and dorsolateral prefrontal cortex.
Setting:
University Hospital Sleep Center and Radiology Department
Participants:
8 unmedicated physician-referred patients with chronic primary insomnia (3 males, 5 females; 48.4 + 16.3 years) and 8 good sleepers matched for age, sex, body mass index, and education.
Interventions:
N/A
Measurements and Results:
Patients with primary insomnia demonstrated significantly reduced hippocampal volumes bilaterally compared to the good sleepers. None of the other regions of interest analyzed revealed differences between the 2 groups.
Conclusions:
These pilot data raise the possibility that chronic insomnia is associated with alterations in brain structure. Replication of the findings in larger samples is needed to confirm the validity of the data. The integration of structural, neuropsychological, neuroendocrine and polysomnographic studies is necessary to further assess the relationships between insomnia and brain function and structure.
Citation:
Riemann D; Voderholzer U; Spiegelhalder K; Hornyak M; Buysse DJ; Nissen C; Hennig J; Perlis ML; van Elst LT; Feige B. Chronic insomnia and MRI-measured hippocampal volumes: a pilot study. SLEEP 2007;30(8):955–958.
Keywords: Insomnia, hippocampus, MRI, memory, learning
INTRODUCTION
COMPELLING EVIDENCE SUGGESTS THAT SLEEP MODULATES BRAIN FUNCTION AND DEVELOPMENT INCLUDING GENE EXPRESSION, PROTEIN TRANSLATION AND learning and memory formation.1,2 Studies in animals have shown that sleep loss has a detrimental impact on functional brain plasticity, including synaptic long term potentiation.3 Other studies in animals demonstrated that sleep deprivation/ sleep loss attenuates neurogenesis, especially in hippocampal regions, suggesting that sleep loss also affects structural brain plasticity.4–6 These functional and structural findings are consistent with human behavioural studies demonstrating that experimental sleep curtailment disrupts neurocognitive performance,7 including the nocturnal consolidation of hippocampal dependent memories.8
To date no human studies provide evidence that sleep loss affects the structure of the brain. In part, this is due to the impossibility of studying experimental sleep deprivation in humans for long enough durations to demonstrate structural brain alterations. Primary insomnia (PI) is a naturally occurring clinical condition characterized by the subjective experience of chronically disturbed sleep and sleep loss. PI is defined in the Diagnostic and Statistical Manual of the American Psychiatric Association9 by complaints of prolonged sleep onset, difficulty maintaining sleep, or nonrestorative sleep, together with waking symptoms such as mood disturbance, increased anxiety, fatigue, and cognitive difficulties, lasting ≥4 weeks. While not identical to sleep deprivation, PI may serve as a useful human model to test the hypothesis that chronically disturbed sleep is associated with changes in brain structure. PI seems particularly well-suited as a research model because the sleep disturbance is not due to any other confounding medical or psychiatric disorder.
Recent neuropsychological10 and memory studies8,11 indicate that patients with PI show daytime cognitive impairments and deficits of memory formation during sleep compared to good sleepers. This might be related to altered hippocampal function or structures since the hippocampus is critically involved in cognitive performance and memory formation.12
Neuroimaging techniques have been used increasingly to characterize basic sleep-wake states in normal sleep and specific sleep disorders in humans. However, few studies have been conducted in PI. One positron emission tomography (PET) study demonstrated13 that subjects with PI, compared to good sleepers, had higher global cerebral glucose metabolism during sleep and wakefulness. PI had a smaller decline than controls in relative metabolism during NREM sleep in wake promoting regions. These data support the neurocognitve-hyperarousal model14of PI, which is based on electrophysiological and neuroendocrine studies indicating increased levels of arousal both during sleep and wakefulness in these patients.
Based on the aforementioned evidence we tested the hypothesis that brain structures, especially hippocampus, would show reduced volume in patients with chronic PI compared to good sleeper controls, as measured by magnetic resonance imaging (MRI).
METHODS AND MATERIALS
Participants
We studied 8 physician-referred patients (3 males, 5 females) with PI and compared them to 8 good sleepers carefully matched by age, gender, educational status, and body mass index (BMI). Mean age (± SD = standard deviation) was 48.4 ± 16.3 yrs. in PI and 46.3 ± 14.3 yrs. in controls (Wilcoxon test, two-tailed, P = 0.958). Patients had 11.0 ± 1.6 yrs. of school education compared to 12.1 ± 1.6 yrs. in controls (Wilcoxon test, two-tailed, P = 0.275). With the exception of one PI patient, all subjects were right-handed. Body mass index was 22.7 ± 2.1 kg/m2 in the PI sample and 22.5 ± 2.8 kg/m2 in the control group (Wilcoxon test, two-tailed, P = 0.879) Patients had suffered from insomnia for a mean duration of 11.6 ± 8.9 yrs. Their mean score on the Pittsburgh Sleep Quality Index,15 a measure of the sleep quality during the previous 2 weeks, was 11.1 ± 2.9 versus 3.5 ± 2.1 in the control group (Wilcoxon test, two-tailed, P <0.001; scores >5 indicate poor sleep quality). All patients were medication-free for at least 2 weeks prior to the MRI study. PI was diagnosed according to DSM-IV criteria9 based on a structured clinical interview.16 Acute and chronic medical and psychiatric disorders were thoroughly investigated and ruled out by a structured psychiatric diagnostic interview,17 physical examination, clinical daytime EEG, routine ECG, blood tests (analyzed for blood cell count, thyroid, renal and hepatic function) and urine drug testing (for benzodiazepines, opiates, barbiturates, amphetamines, and cannabis). PI subjects had no current or life-time comorbid psychiatric disorders (as assessed by a structured clinical interview17). The control group of good sleepers was recruited from the community and had no acute medical, sleep or psychiatric disorder, determined by the same procedure as above. None of the control subjects had a lifetime history of a psychiatric disorder. The study was approved by the Ethics Committee of the University of Freiburg and all subjects provided written informed consent.
MRI Measurements and Analyses
MRI images were obtained at the Department of Radiology at the University Hospital of Freiburg Medical Center on a 1.5 T Magnetom Sonata scanner (Siemens Medical Solutions, Eliangen, Germany). T1 and T2 weighted coronal images were acquired to screen for brain pathology. For volumetric assessment a T1-weighed anatomical magnetization-prepared rapid gradient-echo sequence was recorded18 (160 sagittal slices with 256 × 256 voxels, 1.0 × 1.0 × 1.0 mm3). Volumetric measurements were performed using the interactive software program MReg after nonuniformity correction and reorienting to coronal slices (MINC software, http://www.bic.mni.mcgill.ca/). Within MRreg, the images were zoomed to a magnification of 4X for outlining the regions of interest (ROI). The ROIs were outlined manually using a mouse driven cursor following the established protocol described by Watson et al.19 Total brain volume was measured at every 10 slices with a magnification of 2X. The volume of each structure in each slice (the in-slice volume) was calculated by multiplying the number of voxels contained within each trace by the voxel volume, 1 mm3, and dividing by the magnification factor. Total volume of each structure was the sum of all in-slice volumes. The substructure volumes were corrected for total brain size by multiplying the ROI volume by the ratio of the mean brain volume over the individual's brain volume.11 Delineation of each ROI followed a well established and validated protocol19,21,22 and included the dorsolateral prefrontal cortex, the orbitofrontal cortex, the anterior cingulate cortex, amygdala, and hippocampus. We selected these regions because of their roles in the regulation of executive function, memory, and affect; PI patients complain of impairments in each of these functions. Images of patients and controls were presented to one independent rater in a random sequence without any cues to patient/control status to ensure that the rater was blinded to the identity and clinical status of the subject and the laterality of the images. The rater demonstrated reliability in a separate subset of 20 images of healthy volunteers not included in this study.
A more detailed description of our morphometric data analysis can be found in our previous publication22 which applied the same approach to MR images of patients with borderline personality disorder.
Statistics
Due to the small sample size nonparametric methods were used for data analysis. All statistical tests were two-tailed with an alpha level of P = 0.05. Group comparisons were performed using Wilcoxon tests for independent samples. Spearman rank order correlations (rsp) were used to assess correlations between volumetric parameters and other variables. Due to the exploratory nature of the study, no correction for multiple testing (i.e., Bonferroni correction) was applied when analyzing group differences with respect to brain structures.
RESULTS
PI patients showed significantly disturbed subjective sleep estimates compared to the good sleepers as measured by the PSQI (sleep onset latency: 55.8 min + 42.0 min vs. 15.3 + 8.7 min, Wilcoxon test, two-tailed, P = 0.010; total sleep time: 273.8 + 116.1 vs. 423.8 + 46.6 min, Wilcoxon test, two-tailed, P <0.01; sleep efficiency: 56.8 + 26.9 % vs. 91.5 + 7.7 %, Wilcoxon test, two-tailed, P <0.01). The groups did not display any significant differences in habitual bedtimes (PI bedtime 22:47 vs. 22.58 in good sleepers, Wilcoxon test, two-tailed, P = 0.278; PI rising time 07:04 vs. 06:42 in good sleepers, Wilcoxon test, two-tailed, P = 0.526).
Table 1 displays data derived from the morphometric analysis of the MRI scans.
Table 1.
Morphometric analysis of different regions of interest in controls and insomniac patients. Volumes are expressed in cm3, normalized to mean total brain volume. Total brain volume was 1209±104 cm3 for controls and 1272±116 cm3 for insomniacs (W=22, P = 0.328).
| Region of Interest | Good sleepers (n = 8) Mean ± SD | Patients with PI (n = 8) Mean ± SD | Wilcoxon test (two-tailed) | |
|---|---|---|---|---|
| W | P | |||
| Left Hippocampus | 3.29 ± 0.45 | 2.79 ± 0.14 | 56 | 0.010 |
| Right Hippocampus | 3.64 ±0.58 | 3.08 ± 0.24 | 54 | 0.021 |
| Left Amygdala | 2.02 ± 0.34 | 1.77 ± 0.29 | 47 | 0.130 |
| Right Amygdala | 1.92 ± 0.52 | 1.76 ± 0.24 | 38 | 0.574 |
| Left anterior cingulate | 0.69 ± 0.44 | 0.43 ±0.16 | 44 | 0.234 |
| Right anterior cingulate | 0.58 ± 0.20 | 0.54 ± 0.22 | 34 | 0.878 |
| Left orbitofrontal cortex | 15.61 ±2.81 | 16.33 ±2.82 | 25 | 0.505 |
| Right orbitofrontal cortex | 17.04 ±1.75 | 17.37 ±3.28 | 29 | 0.798 |
| Left dorsolateral prefrontal cortex | 39.30 ± 5.22 | 40.38 ± 7.31 | 24 | 0.442 |
| Right dorsolateral prefrontal cortex | 39.39 ± 5.76 | 39.99 ± 7.31 | 24 | 0.442 |
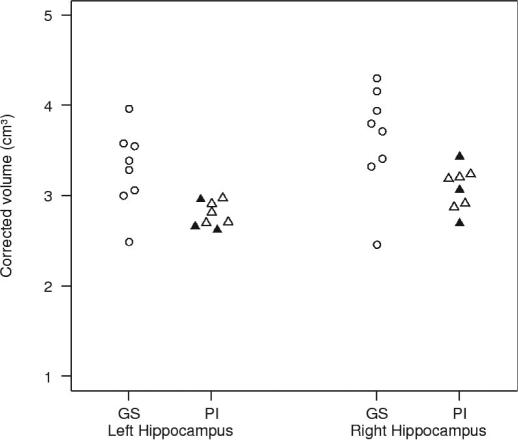
PI subjects had significantly smaller hippocampal volumes bilaterally. None of the other ROIs showed any difference between both groups. Figure 1 shows single subject values for right and left hippocampal volumes. Correction for multiple comparisons using the Bonferroni procedure rendered Pi-control differences nonsignificant.
Figure 1.
Single subject values for left and right hippocampal volumes (corrected volumes/ cm3).
GS = Good Sleepers, open circles;
PI = Patients with Primary Insomnia, triangles; 3 PI patients who had never taken hypnotic or other psychopharmacological agents are depicted by closed triangles. The other PI patients had been free of any kind of medication for at least 2 weeks.
As an alternative to formal correction for multiple testing, and given the additional consideration of significant findings bilaterally, we assessed the probability of positive findings compared to the null hypothesis. Assuming that our single rater had a very high random error of 20%, the observed difference in hippocampus volume would be observed by chance in only 2.5% of measurements for groups of 8 subjects (df=7). The probability of observing this difference simultaneously in 2 different parts of the brain is (2.5%)2 or 0.6%. If the measurement error is more realistically assumed to be around 10%, these probabilities are reduced to 10−3 and 10−6, respectively.
Correlation analyses were conducted to examine relationships between medication-free interval, insomnia duration (for the PI group only), and morphometric data. None of these correlations approached significance.
DISCUSSION
Manually derived morphometric analysis revealed significantly reduced hippocampal volumes bilaterally in patients with chronic PI compared to healthy good sleeper controls. The differences were comparable in magnitude to differences previously described in other neuropsychiatric conditions like depression, posttraumatic stress disorder, and borderline personality disorder.23
Before drawing conclusions from these data about the impact of insomnia on brain structure several methodological caveats need to be addressed. Sample size was small, including only 8 subjects in each group; replication in larger independent samples is needed. Statistical correction for multiple comparisons would have rendered the differences in hippocampal volumes nonsignificant. Due to the exploratory nature of the study a more lenient statistical level is probably acceptable, at least with respect to the heuristic value and hypothesis-generating character of the investigation. The fact that hippocampal volumes were detected bilaterally argues against a mere chance finding. Finally, when inspecting individual values of hippocampal volumes (see figure 1) the difference of data distribution between good sleepers and PI patients appears rather distinct and not resulting from extreme outliers in the insomnia group.
Insomnia diagnosis was only based on clinical reports, structured interviewing and PSQI data. Replication studies might benefit from further subtyping PI by using recently published RDC24 (Research Diagnostic Criteria), sleep diaries and polysomnography; the latter would also definitely rule out any other occult sleep disorder. This will allow correlations between morphological measures and refined measures of sleep in both insomnia and good sleeper groups.
Possible confounding variables including age, sex, body mass index, and years of school education were controlled by carefully matching groups. Comorbid medical and psychiatric disorders were unlikely to confound the findings given the stringent screening procedures in both groups. Within the insomnia group, medication-free interval and duration of insomnia were not correlated with the extent of hippocampal volume reductions, suggesting that these variables also cannot account for the observed findings.
Given the cross-sectional design of this study, we cannot infer a causal link between insomnia and hippocampal volume reduction in either direction, or whether a third unmeasured factor might account for both findings. Animal studies have shown that short-term sleep deprivation and sleep restriction have a negative impact on hippocampal neurogenesis.3–6 These data support the hypothesis that sleep itself might be necessary for neuronal regeneration in hippocampal structures. The relationships between sleep and learning have received considerable attention in recent years. Sleep fosters nocturnal memory consolidation and prolonged wakefulness prevents this consolidation. Specifically, declarative memory is assumed to be associated with the amount of slow wave sleep, whereas procedural memory consolidation is related to the amount of REM sleep.2 We and others have shown that nocturnal memory consolidation is impaired in PI compared to healthy control sleepers.8,11 These data complement the wellknown involvement of hippocampal structures in learning and memory processes12 and are consistent with the findings of the present study.
Another avenue in psychiatric research has linked changes in hippocampal structures with increased production of cortisol in other psychiatric conditions.25 Interestingly, increased cortisol secretion has also been described in some studies of primary insomnia,26 though not consistently.27 Thus, both disturbed sleep and increased rates of cortisol production could plausibly be linked to changes in hippocampal structures in PI. Future studies could further elucidate this possibility by combining morphometric analysis with neuropsychological testing, neuroendocrine studies, polysomnography and nocturnal learning paradigms and task-related fMRI paradigms in insomnia and good sleeper samples.
In summary, we tentatively conclude from our data that insomnia may either result from, or contribute to, changes in brain structure. Therefore it may be important to measure and control for insomnia and sleep disturbances in neuropsychiatric research aiming at delineating the morphological correlates or even antecedents of psychopathology. This conclusion also applies to the study of learning and memory in humans, where little attention has been paid to the sleep quality of subjects. Our data, carefully interpreted, suggest that sleep and sleep disturbances may impact on brain structures involved in these processes.
ACKNOWLEDGMENTS
This study was supported by a grant from the Ministry of Science, Research and the Arts of Baden Wurttemberg (AZ: 23-7532.22-11/1) and intramural means of the Department of Psychiatry and Psychotherapy of the University Hospital of Freiburg.
We thank Christine Paris for conducting the MRI analyses.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Reiman has received research support from Cephalon and Takeda and has participated in speaking engagements for Sanofi-Aventis and Takeda. Dr. Hornyak has received research support from Hoffman-La Roche and has participated in speaking engagements for Boehringer Ingelheim, GlaxoSmithKline, and Hoffman-LaRoche. Dr. Buysse has consulted for Actelion, Cephalon, Eli Lilly, GlaxoSmithKline, Merck, Neurocrine Biosciences, Nuerogen, Pfizer, Respironics, Sanofi-Aventis, Servier, Sepracor, and Takeda. Dr. Perlis has received research support from Cephalon and Sanofi-Aventis; has consulted for MiniMitter-Respironics, Gerson Lehman Group, and Elan-King Pharmaceuticals; has served on the speakers bureau of Sanofi-Aventis; and is the managing member of Internet Didactics Services, Ltd. Dr. van Elst has participated in speaking engagements for Eli Lilly and Pfizer. Drs. Voderholzer, Spiegelhalder, Nissen, Hennig, and Feige have indicated no financial conflicts of interest.
REFERENCES
- 1.Tononi G, Cirelli C. Gene expression during sleep and wakefulness: a review of recent findings. Neuropsychopharmacology. 2001;25:S28–S35. doi: 10.1016/S0893-133X(01)00322-0. [DOI] [PubMed] [Google Scholar]
- 2.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–8. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 3.Kopp C, Longordo F, Nicholson JR, Lüthi A. Insufficient sleep reversibly alters bidirectional synaptic plasticity and NMDA receptor function. J Neurosci. 2006;26:12456–65. doi: 10.1523/JNEUROSCI.2702-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guzman-Marin R, Suntsova N, Methippara M, Greiffenstein R, Szmusiak R, McGinty D. Sleep deprivation suppresses neurogenesis in the adult hippocampus of rats. Eur J Neurosci. 2005;22:2111–16. doi: 10.1111/j.1460-9568.2005.04376.x. [DOI] [PubMed] [Google Scholar]
- 5.Mirescu C, Peters JD, Noiman L, Gould E. Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. PNAS. 2006;103:19170–5. doi: 10.1073/pnas.0608644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hairston DS, Little MTM, Scanlon MD, et al. Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J Neurophysiol. 2005;94:4224–33. doi: 10.1152/jn.00218.2005. [DOI] [PubMed] [Google Scholar]
- 7.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation Semin Neurol. 2005;25:117–29. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 8.Backhaus J, Junghanns K, Born J, Hohaus K, Faasch F, Hohagen F. Impaired declarative memory consolidation during sleep in patients with primary insomnia: influence of sleep architecture and nocturnal cortisol release. Biol Psychiatry. 2006;60:1324–30. doi: 10.1016/j.biopsych.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 9.American Psychiatric Association. Washington DC: American Psychiatric Association; 1994. Diagnostic and statistical manual of mental disorders - DSM-IV. [Google Scholar]
- 10.Fulda S, Schulz H. Cognitive dysfunction in sleep disorders. Sleep Med Rev. 2001;5:423–45. doi: 10.1053/smrv.2001.0157. [DOI] [PubMed] [Google Scholar]
- 11.Nissen C, Kloepfer C, Nofzinger E, Feige B, Voderholzer U, Riemann D. Impaired sleep-related memory consolidation in primary insomnia-a pilot study. Sleep. 2006;29:1068–73. doi: 10.1093/sleep/29.8.1068. [DOI] [PubMed] [Google Scholar]
- 12.Kandel ER, Kupfermann I, Iversen S. Learning and memory. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of neural science. New York: McGraw-Hill; 2000. pp. 1227–1246. [Google Scholar]
- 13.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–9. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 14.Perlis ML, Giles DE, Mendelson WB. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6:179–88. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 15.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 16.Schramm E, Hohagen F, Grasshoff U, et al. Test-retest reliability and validity of a structured interview for sleep disorders according to DSM-III-R (SIS-D) Am J Psychiatry. 1993;150:867–72. doi: 10.1176/ajp.150.6.867. [DOI] [PubMed] [Google Scholar]
- 17.Wittchen HU, Pfister H. Diagnostisches Expertensystem Interview (DIA-X) Frankfurt: Swets & Zeitlinger; 1997. [Google Scholar]
- 18.Mugler JP, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE) Magn Reson Med. 1990;15:152–7. doi: 10.1002/mrm.1910150117. [DOI] [PubMed] [Google Scholar]
- 19.Watson C, Andermann F, Gloor P, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42:1743–50. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- 20.Tebartz van Elst L, Baeumer D, Lemieux L, et al. Amygdala abnormalities in psychosis of epilepsy. A magnetic resonance imaging study in patients with temporal lobe epilepsy. Brain. 2002;125:140–9. doi: 10.1093/brain/awf008. [DOI] [PubMed] [Google Scholar]
- 21.Watson C, Jack CRJ, Cendes F. Volumetric magnetic resonance imaging. Clinical applications and contributions to the understanding of temporal lobe epilepsy. Arch Neurol. 1997;54:1521–31. doi: 10.1001/archneur.1997.00550240071015. [DOI] [PubMed] [Google Scholar]
- 22.Tebartz van Elst L, Hesslinger B, Thiel T, et al. Frontolimbic brain abnormailities in patients with borderline personality disorder: a volumetric resonance imaging study. Biol Psychiat. 2003;54:163–71. doi: 10.1016/s0006-3223(02)01743-2. [DOI] [PubMed] [Google Scholar]
- 23.Geuze E, Vermetten E, Bremner JD. MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Mol Psychiatry. 2005;10:160–84. doi: 10.1038/sj.mp.4001579. [DOI] [PubMed] [Google Scholar]
- 24.Edinger J, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American academy of sleep medicine work group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 25.Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4:141–94. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Vgontzas AN, Bixler EO, Lin HM, et al. Chronic insomnia is associated with nychtemeral activation of the hypothalamo-pituitaryadrenal axis. J Clin Endocr Metab. 2001;86:3787–94. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 27.Riemann D, Klein T, Rodenbeck A, et al. Nocturnal cortisol and melatonin secretion in primary insomnia. Psychiatry Res. 2002;113:17–27. doi: 10.1016/s0165-1781(02)00249-4. [DOI] [PubMed] [Google Scholar]