Abstract
Study Objectives:
Because serotonin (5-HT) is a neurotransmitter associated with circadian rhythm regulation, we explored a possible relation among 5-HT, serotonin metabolite, 5-hydroxyindolacetic acid (5-HIAA), and the functional polymorphism of the serotonin transporter gene (SLC6A4) promoter with rotating shift work.
Design and Participants:
683 men were included in this study: 437 day workers were compared with 246 rotating shift workers.
Results:
Platelet 5-HT content differed significantly (P = 0.002) between day workers (41.28±1.99 pg/mg) and rotating shift workers (37.91±4.16 pg/mg); 5-HIAA content was also significantly (P = 0.00004) higher in day workers (11.40±0.82 pg/mg) than in rotating shift workers (9.33±1.02 pg/mg). We looked for further differences in SLC6A4 promoter (5-HTTLPR, 44 bp insertion: long (L)/deletion: short (S) alleles). We found a significant (P = 0.016) difference in genotype distribution between day workers LL: 126 (28.8%), LS: 202 (46.2%), and SS: 109 (24.9%), and rotating shift workers LL: 47 (19.1%), LS: 124 (50.4%), and SS: 75 (30.5%). When we divided the subjects between workers with less and more than 60 month rotating shift-work exposure, the difference in SLC6A4 genotypes frequency was only significant in the group with ≥60 months (P = 0.011). In addition, there was a significantly lower content of platelet 5-HIM in S allele carriers in comparison with the other genotypes (SS: 9.2±1.0 pg/mg vs. SL/LL: 11.0±0.8 pg/mg, P <0.02).
Conclusions:
Platelet 5-HT and 5-HIM contents were significantly lower in rotating shift workers than day workers, and there was a significant association between the S variant of SLC6A4 promoter and shift work. These findings may be important for targeting effective therapeutic strategies to ameliorate the associated comorbidities and behavioral problems in rotating shift workers.
Citation:
Sookoian S; Gemma C; Gianotti TF; Burgueño A; Alvarez A; Gonzalez CD; Pirola CJ. Serotonin and serotonin transporter gene variant in rotating shift workers. SLEEP 2007;30(8):1049-1053.
Keywords: Circadian rhythm, serotonin, shift work, SLC6A4, serotonin transporter, gene, promoter, 5-hydroxyindolacetic acid, desynchronization
INTRODUCTION
The major function of the circadian system is the internal cycling of physiologic and metabolic events.1 Circadian rhythms are synchronized to the 24-h day, mostly by light-dark cycles and social time cues.2 The circadian rhythm and environmental conditions can become desynchronized in rotating shift workers whose night activity is out of phase with many coupled rhythms, owing to desynchronization of the normal phase relation between biological rhythms within the circadian system.3 Such desynchronization may contribute to important public health problems whose impact may be underestimated. For instance, the effects of rotating and night shift work on the cardiovascular and metabolic systems have been reported, showing that shift work may be directly responsible for increased body fatness, higher blood pressure levels, and some features of the metabolic syndrome.4–6 Additionally, up to 20% of shift workers have clinical intolerance to shift work. This intolerance may range from minor problems to others that are so severe as to cause the worker to give up a job, often following medical advice.7
Actually, the term shift work maladaptation syndrome has been used to describe the typical constellation of signs and symptoms seen in shift work intolerant workers,8 and its etiology is not yet fully understood. Nevertheless, the research evidence supports the idea that interindividual variability exists in the ability to tolerate shift work,9,10 suggesting the involvement of genetic factors operating at the level of certain neurotransmitter systems.
Between the diverse neurochemical systems that interact to regulate wakefulness and sleep, serotonergic neurotransmission plays an important role within brain areas modulating circadian rhythm, sleep, and waking.11,12 In addition, serotonin (5-HT) is involved in both photic and non-photic synchronization of the mammalian biological clock located in the suprachiasmatic nuclei.2,13
It was previously shown that serotonergic agonists and antagonists or agents that alter levels of serotonin in the synapse can modulate many aspects of circadian rhythmicity in animal models, supporting the involvement of serotonin turnover in circadian rhythm regulation.14 In this regard, serotonin reuptake is controlled by the serotonin transporter (5-HTT) and therefore dependent on a common functional length polymorphism of the serotonin transporter gene (SLC6A4) promoter region (5-HTTLPR, 44 bp insertion: long (L)/deletion: short (S) alleles).15 Thus, 5-HTT recycles serotonin after its release, thereby determining the magnitude and duration of serotonergic responses.
The aim of the present study was to investigate the role of the serotonin, 5-hydroxyindolacetic acid, and the functional insertion/deletion polymorphism in the corresponding 5-HTTLPR in individuals under the rotating shift work schedule.
MATERIALS AND METHODS
Healthy subjects recruited from a factory in Buenos Aires metropolitan area who underwent an annual health examination during the 2005 period were included in a cross-sectional population-based study. Altogether, 683 men of self-reported European ancestry were included in this study, in which 437 day workers were compared with 246 rotating shift workers.
The job schedule type was divided into rotating shift work and daytime work. Rotating shift work was based on 2-shift and clockwise rotation in 28 days (4 work days, 3 rest days, 2 work nights, 3 rest days, 4 work nights and 3 rest days, 2 work days, 3 rest days, 4 work days). Day and night work periods started at 06:00 and 18:00 respectively. None of the subjects interchanged their job schedule.
Medical history was investigated using a self-administered questionnaire. The answers were confirmed by individual interviews conducted by occupational physicians.
The participants were asked to fast for ≥8 hours. After 5 minutes' rest in a quiet room, systolic (SABP) and diastolic (DABP) arterial blood pressure were measured on the right arm with a standard mercury sphygmomanometer with the participant in a sitting position. Health examinations included anthropometric measurements, a questionnaire on health related behaviors, and biochemical determinations. Information about the years of work, whether shift work or day work, and past medical history were also included. The day or shift work duration was defined as the total number of months during which the subject had engaged in day work or shift work.
Blood was drawn from fasting subjects who had lain in a supine resting position for at ≥30 min. Serum insulin, total cholesterol, HDL and LDL-cholesterol, triglycerides, and plasma glucose were measured by standard clinical laboratory techniques.
All the investigations performed in this study were conducted in accordance with the guidelines of The Declaration of Helsinki. Written consent from individuals had been obtained in accordance with the procedures approved by the Ethical Committee of our institution.
5-HT and 5-Hydroxyindolacetic Acid (5-HIAA) Determinations
Blood (4 ml) was drawn from cubical vein in a plastic syringe with 1 ml of acid citrate dextrose (ACD) anticoagulant between 06:00 and 09:00 after at least an 8 hour or overnight fast in all cases, regardless of the work schedule. Platelet-rich plasma was centrifuged at 10,000 rpm and the platelet pellet treated with 400 μl of 0.4 mol/L perchloric acid. After centrifugation, the supernatant was frozen at −20°C in light-protected vials for 5-HT and 5-HIAA assays. Pellets were re-suspended in 500 μl 0.1 mol/L NaHO for protein determination through a commercial reagent and according to the manufacturer's indication (Bio-Rad protein assay, Bio-Rad, Hercules, CA, USA).
In this assessment, 5-HT and 5-HIAA were separately assayed by standard isocratic electrochemical detection-high performance chromatographic methods. Briefly, a Waters Chromatographic system (Waters Corporation, Milford, MA, USA) consists of a Water 590 solvent delivery system, a 7725i 100 μl fixed-loop Rheodyne injector connected to a Lichospher 100 RP-8 (5 μ, 125×4 mm) cartridge (Merck KgaA Performance & Life Science Chemicals, Darmstadt, Germany) and a LC-4B BAS electrochemical detector with a glassy carbon electrode at 0.8 V with respect to a Ag/AgCl reference electrode (Bioanalytical Systems, West Lafayette, IN, USA). The analogical signal was processed and integrated by an A/D card inserted in a PC. For 5-HT, the mobile phase (36 mmol/L NaHPO4, 17.5 mmol/L citric acid, 0.5 mmol/L EDTA and 10 mmol/L triethyl amine, pH 3.0) was delivered at 1.5 ml/min. For 5-HIAA, the mobile phase had no triethylamine, and 10% methanol was added. Standards were daily prepared in 0.4 mol/L perchloric acid. The inter- and intra-assay variation coefficients were less than 10%. The 5-HT and 5-HIAA data were normalized by platelet protein content. All reagents were from Sigma Co (St Louis, MO, USA) unless otherwise indicated.
Genotyping
The genetic analysis was performed on genomic DNA extracted from white blood cells by a standard method as previously described.16
Genotyping for the 44 bp deletion—insertion polymorphism in the 5-HTTLPR was performed by hot-start polymerase chain reaction using Molecular Biology grade reagents and a Robocycler 96 thermal cycler (Stratagene, La Jolla, CA, USA).
The details of PCR protocol have been described previously17 but primers to detect the variant were different: 5'-CGT TGC CGC TCT GAA TGC-3' and 5'-TGG TAG GGT GCA AGG AGA ATG 3'- amplifying a 384-bp (L long allele) and a 340-bp (S short allele) fragment.
Statistical Analysis
Quantitative data were expressed as mean ± SE unless otherwise indicated. Since for most of the variables we observed a significant variance difference between groups, we chose to be conservative and to assess differences between groups by means of the Mann-Whitney test. For 5-HT and 5-HIAA mean difference between groups, we used ANCOVA with age as covariate on log transformation of the variables.
Genotypes frequencies were analyzed by means of the χ2 test. Logistic regressions were used to test multivariate association between variables, taking rotating shift work as the outcome variable and 5-HTTLPR short allele (dominant model was formed by pooling LS+SS in comparison with LL genotype as the reference group, recessive model was formed by pooling LL+LS as a reference group in comparison with SS genotype, and the additive model corresponds to the 3 genotypes separately assigning 0 to LL, 1 to LS, and 2 to SS genotype), and age, work exposure time, waist-hip ratio, DABP, and triglycerides as independent log-transformed variables in which we observed significant differences between day and shift workers. In addition, multiple regression was used to analyze the correlation between the log-transformed values of platelet 5-HT and 5-HIAA content as dependent variables and rotating shift work, work exposure time, BMI, waist circumference, SABP, DABP, total and HDL cholesterol, triglycerides, and uric acid as independent variables.
We used the CSS/ Statistica program package, StatSoft V 6.0 (Tulsa, OK, USA) to perform these analyses.
RESULTS
Clinical features, anthropometric variables and laboratory findings of the participants according to the job schedule are shown in Table 1. Rotating shift workers had most of the risk factors of the metabolic syndrome: elevated waist-hip ratio, DABP, fasting glucose and insulin, HOMA index, and triglycerides.
Table 1.
Clinical Features, Anthropometric Variables, and Laboratory Findings of the Participants According to Job Schedule
| Variables | Day workers | Shift workers | P level |
|---|---|---|---|
| Number of subjects | 437 | 246 | |
| Age (years) | 35.0±0.4 | 37.0±0.6 | 0.001 |
| Smoking habit (cigarettes/day) | 4.0±0.3 | 4.0±0.4 | 0.62 |
| Physical activity (h/week) | 2.2±0.2 | 1.1±0.15 | 0.0002 |
| Drinking habit (g/day) | 28.0±3.1 | 36.0±4.9 | 0.22 |
| BMI (Kg/m2) | 26.4±0.2 | 27.2±0.5 | 0.06 |
| Waist-hip ratio | 0.93±0.01 | 0.96±0.01 | 0.001 |
| SABP (mmHg) | 122.0±0.6 | 123.0±0.9 | 0.3 |
| DABP (mmHg) | 77.0±0.5 | 79.0±0.6 | 0.006 |
| Fasting glucose (mmol/L) | 5.00±0.03 | 5.10±0.03 | 0.018 |
| Fasting Insulin (pmol/L) | 48.8±2.1 | 57.4±3.5 | 0.011 |
| HOMA | 1.58±0.07 | 1.88±0.11 | 0.008 |
| Total Cholesterol (mmol/L) | 5.0±0.04 | 5.1±0.06 | 0.54 |
| HDL-Cholesterol (mmol/L) | 1.3±0.02 | 1.3±0.02 | 0.11 |
| LDL-Cholesterol (mmol/L) | 3.2±0.04 | 3.1±0.05 | 0.09 |
| Triglycerides (mmol/L) | 1.5±0.05 | 1.7±0.07 | 0.011 |
BMI: body mass index. SABP and DABP: systolic and diastolic arterial blood pressure. HOMA: homeostatic model assessment.
Results are expressed as mean±SE. P stands for statistical significance using Mann-Whitney test and remained significant after multiple testing adjustment by the Benjamini & Hochberg's32 false discovery rate method.
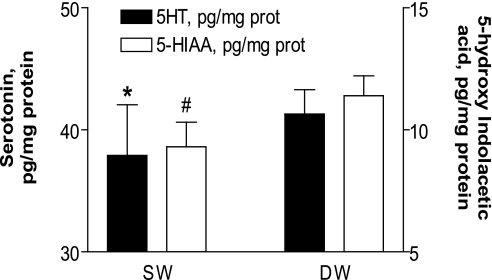
Platelet 5-HT content differed significantly (P <0.002) between day workers and rotating shift workers (Figure 1). Platelet metabolite (5-HIAA) content was also significantly different in both populations (P <0.00004), proving higher in day workers than in shift workers (Figure 1). In addition, in multiple regression analysis, the log transformed values of platelet 5-HT and 5-HIAA content were correlated with rotating shift work (5-HT, beta±SE: 0.17±0.05, P <0.002; -HIAA, beta±SE: 0.19±0.05, P <0.0003) independently of work exposure time, BMI, waist circumference, SABP, DABP, total and HDL cholesterol, triglycerides, and uric acid.
Figure 1.
Platelet serotonin (5-HT) and 5-hydroxyindolacetic acid (5-HIAA) levels in shift workers (SW) and day workers (DW) according to the job schedule. Results are expressed as mean±SE. * 5-HT P <0.002 vs. day workers. # 5-HIAA P <0.00004 vs. day workers. ANCOVA with age as covariate on log transformation of the variables.
In the day workers' population—taken as the reference population—genotypes for the SLC6A4 gene promoter were in Hardy-Weinberg equilibrium and frequencies resembled those reported in other populations (LL: 29%, LS: 46% and SS: 25%)17; the observed allele frequencies were 52% for the L and 48% for the S allele.
In univariate analysis, we found that there was no difference between 5-HTT genotypes and clinical and biochemical characteristics, such as age, systolic and diastolic arterial blood pressure, waist circumference, total cholesterol, HDL and LDL cholesterol, triglycerides, plasma fasting glucose, insulin, and HOMA index (data not shown).
The distribution of genotypes among day workers was LL: 126 (28.8%), LS: 202 (46.2%), and SS: 109 (24.9%). The distribution of genotypes among shift workers was LL: 47 (19.1%), LS: 124 (50.4%), and SS: 75 (30.5%). The group difference between allele distributions was statistically significant (chi-square = 8.3, df = 2, P = 0.016). Moreover, 71.1% and 80.9% of day workers and shift-workers had at least 1 S allele.
Consequently, as shift-work exposure time can be a selection factor, we divided the subjects between workers with less and equal or more than 60 months of rotating shift work exposure. Among those with ≥ 60 months exposure, the distribution of genotypes among day workers was LL: 85 (28.5%), LS: 144 (48.3%), and SS: 69 (23.2%). The distribution of genotypes among shift workers was LL: 31 (17.6%), LS: 88 (50.4%), and SS: 57 (30.5%), Table 2. The group difference between allele distributions was statistically significant (chi-square = 9.0, df = 2, P = 0.011). In day workers and shift workers 71.5% and 80.9%, respectively, had at least 1 S allele.
Table 2.
5-HTTLPR genotype frequencies in the population according to day work and shift work exposure time (<60 months and ≥60 months of work exposure time).
| LL | LS | SS | ||||
|---|---|---|---|---|---|---|
| Exposure time <60 months | ||||||
| Day workers N: 428 | 38 (29.2%) | 53 (40.8%) | 39 (30.0%) | |||
| Shift workers N: 244 | 16 (23.5%) | 34 (50.0%) | 18 (26.5%) | |||
| Exposure time (<60 months | ||||||
| Day workers N: 428 | 85 (28.5%) | 144 (48.3%) | 69 (23.2%) | |||
| Shift workers N: 244 | 31 (17.6%) | 88 (50.4%) | 57 (30.5%)* | |||
LL: Homozygous for the long allele. SS: Homozygous for the short allele. LS: heterozygous.
*P = 0.011 vs. day work at the same work exposure time (chi-square = 9.0, df = 2). Note that the subject number included in this analysis is slightly lower, owing to the lack of information about exposure time for a few subjects.
Among those with <60 months exposure, the distribution of genotypes among day workers was LL: 38 (29.2%), LS: 53 (40.8%), and SS: 39 (30.0%); the distribution of genotypes among shift workers was LL: 16 (23.5%), LS: 34 (50.0%), and SS 18 (26.5%), Table 2. The group difference between allele distributions was not statistically significant (chi-square = 1.6, df = 1, P = 0.45). In day workers and shift workers 70.8% and 76.5%, respectively, had at least 1 S allele.
Logistic regression analysis for the dominant model showed that shift work was associated with the presence of at least one 5-HTTLPR short allele independently of age, work exposure time, waist-hip ratio, DABP, triglycerides, and BMI (adjusted OR: 1.60, 95% CI:1.02 to 2.51, P <0.04). Otherwise, for the additive model, compared to LL homozygous subjects, S allele carriers are 1.4 per allele more likely found as shift workers than as day workers (adjusted OR: 1.42, 95% CI: 1.09 to 1.86, P <0.009), and for the recessive model, logistic regression analysis showed that shift work was associated with the presence of two 5-HTTLPR short alleles (adjusted OR: 1.62, 95%CI: 1.06 to 2.48, P <0.025) independently of age, work exposure time, waist-hip ratio, DABP, triglycerides, and BMI.
Finally, there was no difference in platelet 5-HT levels between 5-HTTLPR genotypes (SS: 38.3±3.2 pg/mg vs. SL+LL: 40.5±2.5 pg/mg, P = 0.28). However, for the platelet 5-HIAA levels, there was a significantly lower content in 5-HTTLPR short allele homozygous in comparison with other genotypes (SS: 9.2±1.0 pg/ mg vs. SL+LL: 11.0±0.8 pg/mg, P <0.02).
DISCUSSION
Biological rhythms are essential components of homoeostasis and circadian rhythms are synchronized to the 24-hour day mostly by light-dark cycles.18
Because serotonin (one of the first neurotransmitters to be associated with regulation of circadian rhythmicity) is involved in a wide range of behavioral and physiological processes,19 we looked for differences between day workers and shift workers by exploring a possible relation between 5-HT and its metabolite, 5-HIAA, and rotating shift work.
The main finding of our study was that platelet 5-HT and 5-HIAA contents were significantly lower in rotating shift workers than in day workers. As far as we know, there are no previous observations about this association, though it has been demonstrated that 5-HT is involved in photic shifting of the mammalian circadian clock, illustrating the modulatory role of the serotoninergic system in the regulation of the circadian clock.20 It has also been reported that 5-HT contributes to the phase-resetting response to a new light-dark cycle.20 Additionally, in the human adult brain, serotonergic neurons play key roles in integrating circadian and neuroendocrine functions such as food intake, sleep, and sexual behavior21 through the coordination of the activity of a number of other neurotransmitter systems.
This study should be interpreted in the light of the limitations resulting from the fact that our results are obtained from peripheral serotonergic markers; therefore a direct extrapolation of the data from peripheral to central serotonergic function should be avoided. But the dynamics (uptake, storage, and release) of platelet 5-HT resembles the corresponding processes in the central serotonergic neurons, blood platelets have been used as a limited peripheral model for the central serotonergic neurons.22
Nonetheless, we wish to note that our observations were made on the basis of a cross-sectional study, and all samples were drawn from the subjects at the same time during the morning and regardless of their work schedules. This may introduce a bias between the groups, since there could be biological circadian variation at the 5-HT and 5-HIAA levels. In this regard, it has been reported that serotonin concentration exhibits a 12-h rhythmicity23 and the serotonin uptake also shows a circadian rhythm with a 24-h period. Moreover, a seasonal variation has been reported in platelet 5-HT showing that it was significantly higher at 08:00 than at 16:00 in spring but not in autumn.24 By contrast, in another study no common circadian rhythm for platelet serotonin concentrations was observed.25
Following our first finding, we looked for further differences in the genetic variants of the 5-HTTLPR in order to explain the reduced platelet 5-HT and 5-HIAA contents in rotating shift workers since the 5-HT transport into the presynaptic neuron acts as a functional "bottle neck." In addition, 5-HTT is responsible for the active transport of serotonin not only in the brain but also in many peripheral tissues.21
Human 5-HTT is encoded by a single gene (SLC6A4) on chromosome 17q11.1−17q12 26 and a polymorphic region was described in the SLC6A4: a 44 base pair (bp) insertion/deletion in the promoter (5-HTTLPR).27 We have focused our attention on the 44 bp insertion/deletion polymorphism, since transfection studies demonstrated that long (L) and short (S) variants of the promoter differentially modulate SLC6A4 transcription rate, S variant being less efficient,27 a fact that suggests an association between 5-HTTLPR variants and an altered functional response of the serotonin system.
In this regard, we found a significant association between the S variant of the SLC6A4 promoter and rotating shift work. Besides, although we found no differences in platelet 5-HT levels between SLC6A4 genotypes, we observed a significant difference for platelet 5-HIAA levels between the SLC6A4 genotypes. Platelet 5-HIAA content was lower in homozygous SS carriers, as might be expected if less 5-HT is taken from the intracellular space and degraded to 5-HIAA by the intracellular monoamine oxidase enzyme. Accordingly, the S allele was previously associated with decreased serotonergic function, a low cerebrospinal fluid 5-HIAA concentration in the Rhesus macaque.28
In addition, we observed that the proportion of the S allele carriers in the group of rotating shift workers depended on the number of years during which the person had engaged in rotating shift work, finding significant differences only in those individuals who had worked under such conditions for more than 5 years. This may explain the interindividual difference in the adaptability to or compliance with the desynchronization of the biological rhythms imposed by the shift work. We may hypothesize that there is a sort of natural selection of the S carriers in the group of individuals with a higher duration of rotating shift work. Furthermore, we may speculate that S carriers are more prone to develop a phenotypic adaptation to a novel input in their lives. In this case, the changes imposed by shift work might be triggering the phenomenon.
The genetic influence on the rotating shift work adaptability may be associated with a locus near the serotonin transporter gene. In this regard, potential genes may be carboxypeptidase D (CPD) and neurofibromatosis type 1 (NF1) located at chromosome band 17q11.2.29 In fact, an involvement of the NF1 locus in the susceptibility to several psychiatric disorders has been suggested.30 We cannot rule out the possibility that the association observed in our study is due to another functional polymorphism in linkage disequilibrium with the 5-HTTLPR variant.
Lastly, it is known that stressful environments modify the integration of neuroendocrinological, morphological, and behavioral regulatory systems by means of an accumulation of phenotypically neutral genetic variance which, in a way, ensures the evolutionary persistence of stress-response strategies and provides a link between individual adaptability and evolutionary adaptation.31
Although we studied a relatively large population, further research is needed to confirm and extend the current findings, improving the power of the study by increasing the number of subjects. Further research is also needed to reveal the intimate mechanism by which the decreased levels in the platelet 5-HT and 5-HIAA and shift work are connected, and to explain why the carriers of the SLC6A4 S allele might adapt to the conditions imposed by rotating shift work.
Finally, rhythm disturbance is frequently manifested as sleep problems, but some major health disorders (for instance, cardiovascular disease and metabolic syndrome) are also associated with repeated rhythm interruption as found in shift workers. We observed an almost two-fold increase in metabolic risk factors (abdominal obesity, diastolic blood pressure hypertension, high triglycerides, and insulin resistance) in shift workers in comparison with day workers.
In conclusion, platelet 5-HT and 5-HIAA contents were significantly lower in rotating shift workers than day workers, and there was a significant association between the S variant of the SLC6A4 promoter and rotating shift work. These findings may be important not only to understand the mechanisms related to the circadian rhythm desyncrhonization imposed by the rotating shift work regime but also to target truly effective therapeutic strategies that may ameliorate the associated comorbidities and behavioral problems.
ACKNOWLEDGMENTS
This study was partially supported by grants B119 (Universidad de Buenos Aires) and PICT 05-08719 and 25920 (Agencia Nacional de Promotión Científica y Tecnológica) and PIP 5195 (Consejo Nacional de Investigaciones Científicas y Téecnicas). SS, CG, AB and CJP belong to Consejo Nacional de Investigaciones Científicas y Téecnicas. SS and TFG are recipients of a Health Ministry Fellowship (Beca Ramón Carrillo-Arturo Oñativia Ministerio de Salud y Ambiente de la Nación-Convocatoria 2006).
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Sookoian, Ms. Gemma, Mr. Gianotti, Ms. Burgueño, Ms. Alvarez, Dr. Gonzalez, and Dr. Pirola have indicated no financial conflicts of interest.
REFERENCES
- 1.Murphy PJ, Campbell SS. Physiology of the circadian system in animals and humans. J Clin Neurophysiol. 1996;13:2–16. doi: 10.1097/00004691-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Oster H. The genetic basis of circadian behavior. Genes Brain Behav. 2006;5(Suppl 2):73–9. doi: 10.1111/j.1601-183X.2006.00226.x. [DOI] [PubMed] [Google Scholar]
- 3.Klerman EB. Clinical aspects of human circadian rhythms. J Biol Rhythms. 2005;20:375–86. doi: 10.1177/0748730405278353. [DOI] [PubMed] [Google Scholar]
- 4.Chau NP, Mallion JM, de GR, et al. Twenty-four-hour ambulatory blood pressure in shift workers. Circulation. 1989;80:341–7. doi: 10.1161/01.cir.80.2.341. [DOI] [PubMed] [Google Scholar]
- 5.Di LL, De PG, Zocchetti C, et al. Effect of shift work on body mass index: results of a study performed in 319 glucose-tolerant men working in a Southern Italian industry. Int J Obes Relat Metab Disord. 2003;27:1353–8. doi: 10.1038/sj.ijo.0802419. [DOI] [PubMed] [Google Scholar]
- 6.Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58:747–52. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa G. Shift work and occupational medicine: an overview. Occup Med (Lond) 2003;53:83–8. doi: 10.1093/occmed/kqg045. [DOI] [PubMed] [Google Scholar]
- 8.Reinberg A, Andlauer P, Teinturier P, De PJ, Malbecq W, Dupont J. Desynchronization of the circadian rhythm of oral temperature in young human subjects with poor tolerance for night work. C R Seances Acad Sci III. 1983;296:267–70. [PubMed] [Google Scholar]
- 9.Harma M. Individual differences in tolerance to shiftwork: a review. Ergonomics. 1993;36:101–9. doi: 10.1080/00140139308967860. [DOI] [PubMed] [Google Scholar]
- 10.Nachreiner F. Individual and social determinants of shiftwork tolerance. Scand J Work Environ Health. 1998;24(Suppl 3):35–42. [PubMed] [Google Scholar]
- 11.Ursin R. Serotonin and sleep. Sleep Med Rev. 2002;6:55–69. doi: 10.1053/smrv.2001.0174. [DOI] [PubMed] [Google Scholar]
- 12.Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–62. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 13.Malek ZS, Dardente H, Pevet P, Raison S. Tissue-specific expression of tryptophan hydroxylase mRNAs in the rat midbrain: anatomical evidence and daily profiles. Eur J Neurosci. 2005;22:895–901. doi: 10.1111/j.1460-9568.2005.04264.x. [DOI] [PubMed] [Google Scholar]
- 14.Legutko R, Gannon RL. Serotonin transporter localization in the hamster suprachiasmatic nucleus. Brain Res. 2001;893:77–83. doi: 10.1016/s0006-8993(00)03290-x. [DOI] [PubMed] [Google Scholar]
- 15.Lesch KP, Balling U, Gross J, et al. Organization of the human serotonin transporter gene. J Neural Transm Gen Sect. 1994;95:157–62. doi: 10.1007/BF01276434. [DOI] [PubMed] [Google Scholar]
- 16.Kawasaki ES. Sample preparation from blood, cells, and other fluids. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR Protocols. A guide to methods and applications. San Diego, CA: Academic Press, INC; 1990. pp. 146–52. [Google Scholar]
- 17.Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 18.Merrow M, Spoelstra K, Roenneberg T. The circadian cycle: daily rhythms from behaviour to genes. EMBO Rep. 2005;6:930–5. doi: 10.1038/sj.embor.7400541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koella WP. Neurohumoral aspects of sleep control. Biol Psychiatry. 1969;1:161–77. [PubMed] [Google Scholar]
- 20.Gardani M, Blance RN, Biello SM. MDMA alters the response of the mammalian circadian clock in hamsters: effects on re-entrainment and triazolam-induced phase shifts. Brain Res. 2005;1046:105–15. doi: 10.1016/j.brainres.2005.03.056. [DOI] [PubMed] [Google Scholar]
- 21.Lesch KP, Mossner R. Genetically driven variation in serotonin uptake: is there a link to affective spectrum, neurodevelopmental, and neurodegenerative disorders? Biol Psychiatry. 1998;44:179–92. doi: 10.1016/s0006-3223(98)00121-8. [DOI] [PubMed] [Google Scholar]
- 22.Andres AH, Rao ML, Ostrowitzki S, Entzian W. Human brain cortex and platelet serotonin2 receptor binding properties and their regulation by endogenous serotonin. Life Sci. 1993;52:313–21. doi: 10.1016/0024-3205(93)90223-p. [DOI] [PubMed] [Google Scholar]
- 23.Opper C, Weiner N, Xu F, Adam W, Fruhstorfer H, Wesemann W. Daily variations of functional parameters and density distribution in human blood platelets. Chronobiol Int. 1994;11:309–19. doi: 10.3109/07420529409057247. [DOI] [PubMed] [Google Scholar]
- 24.Wirz-Justice A, Lichtsteiner M, Feer H. Diurnal and seasonal variations in human platelet serotonin in man. J Neural Transm. 1977;41:7–15. doi: 10.1007/BF01252961. [DOI] [PubMed] [Google Scholar]
- 25.Eynard N, Flachaire E, Lestra C, et al. Platelet serotonin content and free and total plasma tryptophan in healthy volunteers during 24 hours. Clin Chem. 1993;39:2337–40. [PubMed] [Google Scholar]
- 26.Lesch KP, Wolozin BL, Estler HC, Murphy DL, Riederer P. Isolation of a cDNA encoding the human brain serotonin transporter. J Neural Transm Gen Sect. 1993;91:67–72. doi: 10.1007/BF01244919. [DOI] [PubMed] [Google Scholar]
- 27.Heils A, Teufel A, Petri S, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–4. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 28.Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol Psychiatry. 2002;7:1058–63. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- 29.Shen S, Battersby S, Weaver M, Clark E, Stephens K, Harmar AJ. Refined mapping of the human serotonin transporter (SLC6A4) gene within 17q11 adjacent to the CPD and NF1 genes. Eur J Hum Genet. 2000;8:75–8. doi: 10.1038/sj.ejhg.5200400. [DOI] [PubMed] [Google Scholar]
- 30.North K. Neurofibromatosis type 1. Am J Med Genet. 2000;97:119–27. doi: 10.1002/1096-8628(200022)97:2<119::aid-ajmg3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Badyaev AV. Stress-induced variation in evolution: from behavioural plasticity to genetic assimilation. Proc Biol Sci. 2005;272:877–86. doi: 10.1098/rspb.2004.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Statist. Soc. B. 1995;57:289–300. [Google Scholar]