Abstract
Study Objectives:
To evaluate 6 months' eszopiclone treatment upon patient-reported sleep, fatigue and sleepiness, insomnia severity, quality of life, and work limitations.
Design:
Randomized, double blind, controlled clinical trial.
Setting:
54 research sites in the U.S.
Patients:
830 primary insomnia patients who reported mean nightly total sleep time (TST) ≤6.5 hours/night and/or mean nightly sleep latency (SL) >30 min.
Intervention:
Eszopiclone 3 mg or matching placebo.
Measurements:
Patient-reported sleep measures, Insomnia Severity Index, Medical Outcomes Study Short-Form Health Survey (SF-36), Work Limitations Questionnaire, and other assessments measured during baseline, treatment Months 1–6, and 2 weeks following discontinuation of treatment.
Results:
Patient-reported sleep and daytime function were improved more with eszopiclone than with placebo at all months (P <0.001). Eszopiclone reduced Insomnia Severity Index scores to below clinically meaningful levels for 50% of patients (vs 19% with placebo; P <0.05) at Month 6. SF-36 domains of Physical Functioning, Vitality, and Social Functioning were improved with eszopiclone vs placebo for the Month 1–6 average (P < 0.05). Similarly, improvements were observed for all domains of the Work Limitations Questionnaire with eszopiclone vs placebo for the Month 1–6 average (P <0.05).
Conclusions:
This is the first placebo-controlled investigation to demonstrate that long-term nightly pharmacologic treatment of primary insomnia with any hypnotic enhanced quality of life, reduced work limitations, and reduced global insomnia severity, in addition to improving patient-reported sleep variables.
www.clinicaltrials.gov Identifier #:
Citation:
Walsh JK; Krystal AD; Amato DA; Rubens R; Caron J; Wessel TC; Schaefer K; Roach J; Wallenstein G; Roth T. Nightly treatment of primary insomnia with eszopiclone for six months: Effect on sleep, quality of life, and work limitations. SLEEP 2007;30(8):959-968.
Keywords: Primary insomnia, quality of life, work limitations, eszopiclone, long-term pharmacotherapy
INTRODUCTION
DESPITE THE CHRONIC NATURE OF PRIMARY INSOMNIA AND THE REQUIREMENT FOR NIGHTTIME AND DAYTIME SYMPTOMS FOR DIAGNOSIS,1 RANDOMIZED clinical trials have predominantly evaluated short-term treatment effects on measures such as SL and TST.2 However, insomnia is known to be associated with functional impairments and reduced quality of life.3,4 The National Institutes of Health recently published a State of the Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults5 highlighting the need for (1) long-term efficacy studies for chronic insomnia and (2) assessment of quality of life, impact on work performance, and other outcome measures in addition to quantitative sleep symptom variables. Consistent with these recommendations, we compared 6 months of nightly eszopiclone treatment to placebo on measures of quality of life, work limitations, fatigue, sleepiness, insomnia severity, and patient-reported sleep and daytime function measurements in patients with primary insomnia. Eszopiclone 3 mg has previously been demonstrated to be efficacious and safe during long-term, nightly use in primary insomnia.6,7
METHODS
Patients
Men and women aged 21 to 64 years meeting DSM-IV criteria for primary insomnia1 and reporting ≤6.5 hours sleep and/or >30 minutes to fall asleep on a typical night for at least the past month were recruited via advertisements and physician referrals. Patients were compensated monetarily for their time, travel, and compliance with study procedures. Exclusion criteria included unstable medical conditions; DSM-IV Axis I or personality disorder diagnosis; difficulty in sleep initiation or maintenance associated with known medical diagnosis or any condition that may affect sleep (e.g., sleep apnea, restless leg syndrome, chronic pain, BPH); history of substance abuse or dependence; and women who were pregnant, lactating, or less than 6 months post partum. Prior use of medication for insomnia was not exclusionary, but patients had to be off these medications at the screening visit.
Study Design and Procedures
This investigation was conducted between October 2003 and October 2004 at 54 research sites in the United States using a common protocol written by the sponsor's personnel with consultation from authors and other investigators. Institutional review boards at each site approved the protocol and informed consent, and research was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
The study included a screening visit (during which the study was explained in detail, informed consent was obtained, and eligibility was assessed), a baseline visit, 6 monthly visits during double-blind treatment, and a final visit following 14 days of single-blind placebo treatment. In addition to collection of efficacy data, study visits allowed assessment of safety, treatment adherence (pill counts), and outcome measures, and medication was dispensed. An Interactive Voice Response System was used to collect patient-reported sleep and waking function information by telephone. Patients called once each night during the screening/baseline period, the last week of double-blind treatment and the discontinuation period, and once per week for 6 months during double-blind treatment. Chronic prescription medications taken at a stable dose for at least 30 days prior to screening (Visit 1) were allowed. Over the counter (OTC) medications (analgesics, topical ointments, etc.) were also allowed. Patients were instructed to avoid medications that affect sleep or are contraindicated with the use of hypnotics.
Patients were randomly assigned in a 2:1 ratio to receive either eszopiclone 3 mg or matching placebo tablets with instructions for nightly use at bedtime. All research and sponsor personnel involved in the conduct of the study or analysis of data were blind to treatment assignments until all data were collected and the study database was finalized.
Sleep Related Measures
Nine variables were reported by telephone: SL, wake after sleep onset (WASO), TST, number of awakenings, sleep quality, daytime alertness, ability to concentrate, physical well-being, and ability to function (the last 5 items were rated on an 11-point Likert scale). During nightly reporting periods, patients provided responses for the prior 24-hour period. During weekly reporting periods, patients provided responses with reference to the prior 7 days. Nightly measures were averaged to produce weekly scores, and 4 consecutive weekly values were averaged to produce monthly scores.
Other sleep related data were collected during clinic visits with: (1) the Insomnia Severity Index,8 completed at baseline, Months 1, 3, and 6, and following discontinuation, to determine the severity and impact of insomnia symptoms; (2) the Fatigue Severity Scale,9 completed at baseline and at Months 1, 3, and 6, to rate symptoms of fatigue and their impact on daily functioning; and (3) the Epworth Sleepiness Scale10 completed at baseline and monthly, to measure daytime sleepiness.
Health Outcome Instruments
The following health outcomes instruments were completed at baseline and Months 1, 3, and 6: (1) the Medical Outcomes Study Short-Form Health Survey (SF-36)11 to evaluate quality of life; and (2) the Work Limitations Questionnaire,12 to capture the degree to which chronic health problems interfere with ability to perform job roles and to address the content of the job through a demand-level methodology. Lower scores indicate less interference with work, with normal values of approximately 15% for Time Management, Physical Demands, Mental-Interpersonal Demands, and Output Demands, and about 4% for Productivity Loss (personal communication, Debra Lerner, 2006).
Safety Assessments
Urinalysis, hematology, blood chemistry, vital signs, and adverse events were monitored during each visit. Physical examinations were performed at screening, baseline, and Months 3 and 6. Twelve-lead electrocardiograms occurred at screening, baseline, and Month 6. For patients not completing 6 months of treatment, safety assessments were performed at end of participation, if possible. The Benzodiazepine Withdrawal Symptom Questionnaire13 was administered following the discontinuation period to evaluate withdrawal symptoms. Adverse events were recorded throughout the study.
Statistical Analyses
Based on our previous 6-month study,6 we expected standard deviations of 0.40 and 0.60 for the change from baseline in SL and WASO, respectively, using a natural log transformed scale. The study was powered to have at least a 90% chance of detecting treatment differences of 0.130 for SL and 0.195 for WASO, both using the log transformed scale. These differences correspond to 12.2% and 17.7% reductions from baseline, respectively. Based on these assumptions and the use of a 2:1 randomization ratio, approximately 450 completed patients would be required (300 eszopiclone, 150 placebo), using a 2-sided test at the 5% significance level. With the expectation that 58% of randomized patients would complete the 6-month treatment period, at least 780 randomized patients were required.
Analyses of data collected during the double-blind period included all patients who received at least one dose of double blind study medication. Analyses of discontinuation period data included patients who received at least one dose of placebo during discontinuation. Missing values were handled with a Last Observation Carried Forward (LOCF) approach to derive monthly values for sleep, next-day function, and health outcomes measures for the double-blind period, but LOCF was not used for discontinuation period measures.
To guard against Type 1 error, a priori primary analyses were clearly specified. Specifically, mean SL for Months 4–6 was designated as the primary endpoint, and mean TST and mean WASO for Months 4–6 were specified as key secondary endpoints. Comparison between treatments for these efficacy variables utilized an analysis of variance model with treatment and site as fixed effects. The sleep and next-day function parameter data were log-transformed prior to analysis to account for non-normal distributions. Medians are displayed in Figure 3 because they best represent central tendency of log transformed data.
Figure 3.
Insomnia Severity Index (ISI) scores at Baseline, Month 6 and End of Study (after the 2-week placebo run-out period). Overall P-values between treatment groups: Baseline P = 0.34; Month 6, P <0.001; and End of Study P = 0.005. *P-value vs placebo <0.05. ISI total score 0–7 = Insomnia not clinically significant (NCS), 8–14 = subthreshold insomnia, 15–21 = moderate insomnia and 22–28 = severe insomnia.
Supportive analyses involved monthly comparisons between groups and examined change from baseline using an analysis of covariance model (ANCOVA) with treatment and site as fixed effects and baseline as the covariate in all patient-reported sleep, next-day effects, and health outcome measures. Data for SL and WASO were log-transformed prior to analysis, prior to averaging, and prior to computing the change from baseline. For categorical outcomes, treatment comparisons were made using the Cochran-Mantel-Haenszel test for general association, controlling for site. Health-related quality of life as measured by the SF-36 scales (scored on a 0–100 scale) used ANCOVA with treatment and site as fixed effects and baseline as the covariate. Patients were classified as responders if their change scores improved by 0.5 standard deviation.14 The SF-36 burden analysis comparison to the US healthy population was made using an ANCOVA with age and gender as covariates. All scales were scored using norm-based methods to standardize the scale to a mean of 50 with a standard deviation of 10 in the general US population, with higher scores indicating better health.15,16
Relative effect sizes were computed using the absolute value of the difference between the two treatment means (eszopiclone – placebo) divided by the weighted pooled standard deviation. Magnitude of the effect size calculations are characterized below as small (0.1), moderate (0.3), or large (0.5) according to Cohen17 with the understanding that there are inherent risks in such definitions.
All adverse events were coded using the COSTART dictionary (Version 5.0, 1995). Treatment-emergent adverse events were defined as those that occurred or increased in severity and/or frequency after the first dose of study medication. Adverse events that occurred within 14 days after treatment discontinuation were considered discontinuation related.
Funding for this study was provided by Sepracor Inc. Data and statistical analyses for this study were fully available to all authors. Creation of the randomization sequence and statistical analyses were performed by the sponsor and its representatives. The sponsor placed no limitations on the data analyses, the interpretation of the results, or the writing of this manuscript. All authors were involved with the preparation of the manuscript and vouch for the completeness and accuracy of the data and analyses presented.
RESULTS
Disposition and Baseline Characteristics of Patients
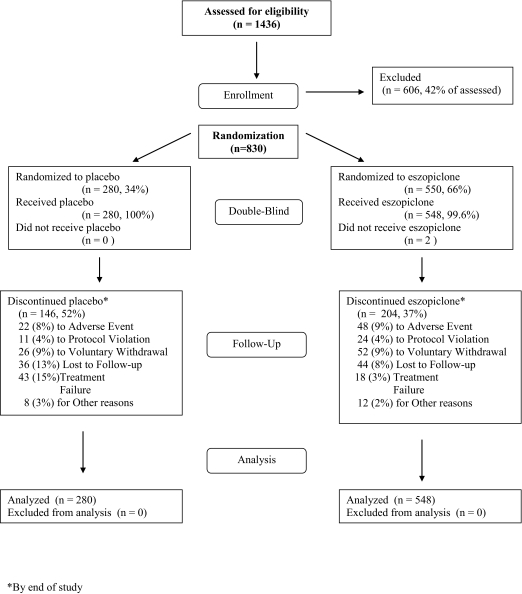
A total of 1436 individuals were screened; 550 were randomized to eszopiclone and 280 to placebo (Figure 1). Two patients discontinued prior to taking study drug, therefore, the population analyzed included 548 patients who received eszopiclone (mean age: 46.0 ± 11.8 years; 212 males, 336 females) and 280 who received placebo (mean age: 44.7 ±11.8 years; 111 males, 169 females). There were no statistically significant differences between treatment groups in age, sex, ethnicity distributions, duration of insomnia at baseline, or other dependent measures. Previous treatments for insomnia were not documented.
Figure 1.
Flow diagram to illustrate progression of patients through the trial, based upon the Consolidated Standards of Reporting Trials (CONSORT) statement. See Altman DG, Schulz KF, Moher D. et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 2001; 134: 663–694.
A higher percentage of placebo patients terminated participation prematurely (52% vs 37%, respectively, Figure 1). There were no significant differences between patients completing the trial and those discontinuing early for any primary or secondary outcome measure (data not shown), and compliance rates were similar between treatment groups. Of premature terminations, 7.5% of placebo patients discontinued due to an adverse event, compared with 8.6% of eszopiclone patients. Adverse events leading to termination by more than one patient included headache (1.1%), chest pain (0.7%), coronary artery disorder (0.7%), and somnolence (0.7%) with placebo; and somnolence (1.8%), anxiety (0.7%), and unpleasant taste (0.7%) with eszopiclone. More placebo patients discontinued due to treatment failure compared with eszopiclone patients (15% vs 3%; P <0.001). The time at which 25% of eszopiclone patients had discontinued was 90.5 days vs 36.0 days in the placebo group. The highest discontinuation rate in both treatment groups occurred within the first month (8% with eszopiclone and 20.4% with placebo).
Sleep Related Variables
The eszopiclone group reported a significantly shorter median SL during Months 4–6 than the placebo group (27.3 vs 45.0 minutes; P <0.001). Patient-reported change from baseline values for all sleep and next day function measures (except of number of awakenings, P <0.027) were significantly improved with eszopiclone relative to placebo at every monthly time point, the Months 4–6 average and the Month 1–6 average (P <0.001, Figure 2 and Table A in electronic supplement). Relative effect size analyses indicated that, relative to placebo, eszopiclone treatment was associated with large effect sizes (0.5–0.7) on all nighttime and daytime insomnia symptoms (except number of awakenings: 0.2–0.3) for Months 4–6 and for the double-blind average (Table A, supplement).
Figure 2.
Median values for key hypnotic efficacy measures at baseline and during months 1–6 of double-blind treatment for eszopiclone (solid lines) and placebo (dashed lines) groups. Panel A: sleep latency. Panel B: wake after sleep onset; Panel C: total sleep time; *Change from baseline P-value vs placebo <0.0001.
The distribution of baseline Insomnia Severity Index scores and categories was not significantly different between groups (Table 1, Figure 3), and mean ISI scores were significantly lower at Months 1, 3, and 6 with eszopiclone. At Month 6, 50% of eszopiclone patients had scores ≤7, indicating no clinically significant insomnia, compared with 19% of placebo patients (P <0.001, Figure 3) and 82% of eszopiclone patients had improved one or more categories (versus 54% for placebo, P <0.001). At Months 1 and 3, the percentage of patients with ISI scores ≤7 was also significantly higher with eszopiclone compared to placebo (42% with eszopiclone vs 13% with placebo at Month 1 and 46% vs 15% at Month 3; P values <0.0001). The relative effect of eszopiclone on the ISI for the double-blind average compared to placebo was large (0.84, Table 1).
Table 1.
Mean (SD) Scores for the Insomnia Severity Index, Fatigue Severity Scale and Epworth Sleepiness Scale for the Placebo and Eszopiclone Treatment Groups.
| Assessment | Timepoint | Placebo (N=280) | Eszopiclone (N=548) | P-value* | Effect Size∧ |
|---|---|---|---|---|---|
| Insomnia Severity Index | Baseline | 17.8 (4.1) | 17.9 (4.1) | – | – |
| Month 1 | 13.8 (5.4) | 9.2 (5.7) | <0.0001 | 0.79 | |
| Month 3 | 13.1 (5.3) | 8.6 (5.8) | <0.0001 | 0.77 | |
| Month 6 | 12.9 (5.7) | 8.3 (6.0) | <0.0001 | 0.75 | |
| Double-blind Avg | 13.2 (4.9) | 8.7 (5.3) | <0.0001 | 0.84 | |
| Epworth Sleepiness Scale | Baseline | 7.8 (5.0) | 7.7 (5.2) | – | – |
| Month 1 | 6.8 (4.5) | 5.8 (4.1) | 0.003 | 0.23 | |
| Month 2 | 6.5 (4.2) | 5.6 (4.0) | 0.003 | 0.22 | |
| Month 3 | 6.6 (4.5) | 5.3 (4.1) | <0.001 | 0.34 | |
| Month 4 | 6.3 (4.4) | 5.1 (3.9) | <0.001 | 0.30 | |
| Month 5 | 6.4 (4.5) | 5.1 (4.0) | <0.001 | 0.32 | |
| Month 6 | 6.3 (4.4) | 5.2 (4.2) | <0.001 | 0.27 | |
| Months 4–6 | 6.5 (4.1) | 5.3 (3.7) | <0.001 | 0.31 | |
| Double-blind Avg | 6.3 (4.3) | 5.1 (3.9) | < 0.001 | 0.31 | |
| Fatigue Severity Scale | Baseline | 4.1 (1.3) | 4.3 (1.4) | – | – |
| Month 1 | 3.7 (1.4) | 3.4 (1.4) | 0.003 | 0.34 | |
| Month 3 | 3.7 (1.3) | 3.3 (1.4) | 0.011 | 0.32 | |
| Month 6 | 3.7 (1.4) | 3.3 (1.4) | 0.003 | 0.35 | |
| Double-blind Avg | 3.7 (1.3) | 3.3 (1.3) | <0.001 | 0.36 |
P-values are based on change from baseline vs placebo
Effect sizes based upon change from baseline scores.
Lower mean scores on the Fatigue Severity Scale and the Epworth Sleepiness Scale were observed in the eszopiclone group relative to placebo for each month and the Month 1–6 average (P <0.05; Table 1), and relative effect sizes were small to moderate for each scale (0.3, Table 1).
Health Outcome Measures
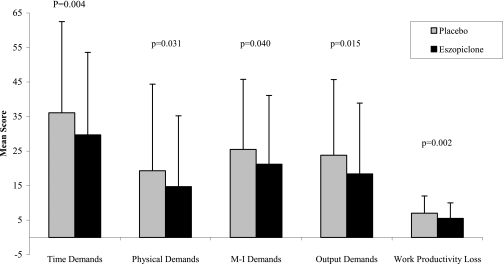
Mean Work Limitations Questionnaire scores were similar in the eszopiclone and placebo groups at baseline for all 5 domains (Table 2). Averaged over the Month 1–6 period, the eszopiclone group had significantly lower average scores relative to placebo on all domains of the Work Limitations Questionnaire (P < 0.05; Figure 4). Additionally, there were significant improvements with eszopiclone relative to placebo at Months 1, 3, and 6 for the Work Productivity Loss domain, Months 1 and 6 for Time Demand, Months 1 and 3 for Physical Demands, and Month 3 for the Output domain (P-values <0.05 vs placebo). There was a small effect of eszopiclone relative to placebo for the double-blind average in the Work Productivity Loss domain (0.16) and the Time Demands domain (0.21; see Table 2).
Table 2.
Mean (SD) Work Limitations Questionnaire (WLQ) Scores for Placebo and Eszopiclone Treatment Groups
| Assessment | Timepoint | Placebo (N=280) | Eszopiclone (N=548) | P-value* | Effect Size∧ |
|---|---|---|---|---|---|
| Time Demands | Baseline | 41.0 (28.1) | 41.2 (26.3) | – | – |
| Month 1 | 36.7 (28.7) | 30.3 (27.9) | 0.014 | 0.21 | |
| Month 3 | 31.8 (27.9) | 27.7 (29.2) | 0.21 | 0.12 | |
| Month 6 | 34.1 (29.2) | 27.0 (30.3) | 0.009 | 0.32 | |
| Double-blind Avg | 36.1 (26.4) | 29.7 (23.9) | 0.004 | 0.21 | |
| Physical Demands | Baseline | 22.8 (29.9) | 23.4 (29.5) | – | – |
| Month 1 | 18.3 (27.0) | 14.1 (22.9) | 0.049 | 0.08 | |
| Month 3 | 21.8 (30.8) | 15.2 (25.7) | 0.017 | 0.15 | |
| Month 6 | 17.2 (24.7) | 13.5 (24.0) | 0.44 | 0.04 | |
| Double-blind Avg | 19.3 (25.1) | 14.7 (20.5) | 0.031 | 0.07 | |
| Mental-Interpersonal Demands | Baseline | 34.5 (23.8) | 31.4 (22.7) | – | – |
| Month 1 | 26.5 (23.5) | 22.5 (22.9) | 0.15 | 0.04 | |
| Month 3 | 23.6 (23.1) | 19.4 (23.6) | 0.063 | 0.09 | |
| Month 6 | 24.4 (24.8) | 18.8 (24.3) | 0.12 | 0.05 | |
| Double-blind Avg | 25.5 (20.3) | 21.2 (19.9) | 0.040 | 0.06 | |
| Output Demands | Baseline | 32.9 (27.3) | 29.8 (25.9) | – | – |
| Month 1 | 23.5 (24.0) | 18.8 (23.7) | 0.10 | 0.02 | |
| Month 3 | 23.6 (25.5) | 17.6 (24.0) | 0.025 | 0.14 | |
| Month 6 | 22.3 (25.3) | 17.1 (25.6) | 0.14 | 0.10 | |
| Double-blind Avg | 23.8 (21.9) | 18.4 (20.5) | 0.015 | 0.06 | |
| Work Productivity Loss | Baseline | 8.9 (5.5) | 8.4 (5.3) | – | – |
| Month 1 | 6.9 (5.1) | 5.7 (4.9) | 0.011 | 0.13 | |
| Month 3 | 6.6 (5.6) | 5.2 (5.3) | 0.013 | 0.18 | |
| Month 6 | 6.8 (5.9) | 5.1 (5.6) | 0.008 | 0.26 | |
| Double-blind Avg | 7.0 (5.0) | 5.5 (4.5) | 0.002 | 0.16 |
SD=Standard Deviation; Avg=Average; Lower scores indicate less interference with work.
P-values are based on change from baseline vs placebo.
Effect sizes based upon change from baseline scores.
Figure 4.
Mean scores for the 5 Work Limitations Questionnaire averaged across Months 1–6. Placebo group = gray bars; eszopiclone group= black bars. Error bars indicate standard deviation of the mean.
Baseline norm-based adjusted SF-36 values in the study population were significantly worse than normative values for the US healthy population18 for the domains of Vitality (44.1 in this study vs 49.8 in healthy population; P <0.05), Social Functioning (48.0 vs 49.9; P <0.05), and the Mental Component Scale score (47.7 vs 49.7; P <0.05). Eszopiclone patients had significantly higher scores than placebo patients (P <0.05, Table B, supplement) in Vitality and Social Functioning (Months 1, 3, and 6, and Month 1–6 average), Physical Functioning (Months 1 and 6, and Month 1–6 average), Bodily Pain (Month 6), and the Mental Component Scale (Month 6). At Month 6, a greater percent of eszopiclone patients showed an increase of ≥0.5 SD compared with placebo on the Physical and Mental Component summary scales and on 5 individual scales: Role Physical, Bodily Pain, General Health, Vitality, and Social Functioning (P <0.05). The largest difference was observed for the Vitality scale, on which 45.3% of eszopiclone-treated patients improved by ≥0.5 SD, compared with 25.7% of placebo-treated patients (Chi-square = 29.84, P <0.001). The effect size for the Vitality scale for the double-blind average with eszopiclone relative to placebo was 0.31 (Table B, supplement), whereas relative effect sizes in the other scales were smaller (0.00–0.21).
Discontinuation Effects
There was no evidence of rebound insomnia after discontinuation of eszopiclone, as SL, WASO, and TST remained significantly improved from baseline values (all P-values <0.001; Figure 5). There were no between-treatment differences observed during the discontinuation period except for a significantly greater SL on Night 1 with eszopiclone vs placebo (45 min vs 30 min; P = 0.015). After 2 weeks of single-blind placebo, 28% of eszopiclone patients were classified as having no clinically significant insomnia with an Insomnia Severity Index score ≤7, compared with 18% of placebo patients (P <0.05, Figure 3), but 43% of both placebo and eszopiclone patients had moderate to severe insomnia. No significant group differences were observed in mean Benzodiazepine Withdrawal Symptom Questionnaire scores (3.0 with eszopiclone and 2.3 with placebo, P = 0.12), or adverse event rates (15.2% for eszopiclone and 11.1% for placebo).
Figure 5.
Median values for key hypnotic efficacy measures at baseline, month 6, and each night during the discontinuation period for eszopiclone (solid lines) and placebo (dashed lines) groups. Panel A: sleep latency; Panel B: wake after sleep onset; Panel C: total sleep time. Both placebo and eszopiclone groups remained significantly improved from baseline (P <0.001) during the discontinuation period. *P <0.05 vs placebo
Safety Assessments
No patient had clinically relevant changes in hematology, blood chemistry, urinalysis, vital signs, electrocardiograms, and physical examination findings during the study period. Overall 6-month adverse event rates were 75.7% in the eszopiclone group compared with 58.9% in the placebo group (P <0.05). The majority of events were rated mild or moderate in severity; 10.7% and 14.1% were rated as severe in the eszopiclone and placebo groups, respectively. Significantly more reports of unpleasant taste (19.7% vs 1.1%, P <0.001), somnolence (8.8% vs 3.2%, P <0.003), and myalgia (6.0% vs 2.9%, P <0.05) occurred in the eszopiclone group compared with the placebo group (Table 3). Thirteen patients (1.5%) reported an adverse event judged to be serious during the double-blind period, with 8 considered unrelated to treatment and 5 determined to be potentially related to treatment (3 in placebo group: 2 chest pain and 1 abdominal pain; and 2 in eszopiclone group: worsening of degenerative joint disease and cerebrovascular accident). Of those patients having a serious adverse event, treatment was discontinued for 2 placebo patients (chest pain, abdominal pain) and 1 eszopiclone patient (cerebrovascular accident).
Table 3.
Adverse Events*
| Adverse events with incidence rate of >5% during double-blind period | Placebo N=280 Number (%) | Eszopiclone N=548 Number (%) | P-value |
|---|---|---|---|
| Unpleasant Taste | 3 (1.1) | 108 (19.7) | < 0.001 |
| Infection | 34 (12.1) | 91 (16.6) | 0.090 |
| Headache | 42 (15.0) | 83 (15.1) | 0.96 |
| Pain | 29 (10.4) | 48 (8.8) | 0.45 |
| Somnolence | 9 (3.2) | 48 (8.8) | 0.0029 |
| Myalgia | 8 (2.9) | 33 (6.0) | 0.047 |
| Pharyngitis | 11 (3.9) | 33 (6.0) | 0.20 |
| Dyspepsia | 15 (5.4) | 34 (6.2) | 0.63 |
| Back Pain | 20 (7.1) | 29 (5.3) | 0.29 |
| Accidental Injury | 17 (6.1) | 27 (4.9) | 0.49 |
| Adverse events with incidence rate of >1% placebo discontinuation period | Placebo N=135 Number (%) | Eszopiclone N=343 Number (%) | P-value |
| Headache | 1 (0.7) | 9 (2.6) | 0.20 |
| Abdominal Pain | 0 | 5 (1.5) | 0.16 |
| Accidental Injury | 2 (1.5) | 2 (0.6) | 0.33 |
| Infection | 0 | 6 (1.7) | 0.12 |
Events = the total number of events occurring within the given treatment where a single patient may experience multiple events.
DISCUSSION
The results of this study support the prior demonstration of sustained hypnotic efficacy over 6 months with eszopiclone.6 Although significant benefits of eszopiclone treatment were documented on all quantitative sleep measures, it is possible that the more clinically relevant findings are the changes in the Insomnia Severity Index. At the end of treatment, 81% of eszopiclone-treated patients improved by one or more categories, and 50% had no clinically significant insomnia, 2.5-fold more than those who received placebo. Analysis of rebound and withdrawal effects after abrupt discontinuation of 6 months of nightly eszopiclone use demonstrated no evidence of rebound insomnia, benzodiazepine withdrawal symptoms, or other negative effects. Interestingly, 2 weeks after eszopiclone discontinuation, 28% of patients had no clinically meaningful insomnia based on Insomnia Severity Index criteria, versus 18% in the placebo group (P <0.05). Thus, a small proportion of pharmacotherapy patients may have at least shortlived benefit that persisted after discontinuation of medication. Whether the improvement would persist for a clinically relevant period of time after treatment cessation requires both replication and a longer discontinuation period. Moreover, whether the difference between groups in the percent of patients falling in the no clinically meaningful insomnia range at the end of the 2-week discontinuation period is attributable to direct drug effects or indirect effects (e.g., more regular sleep schedules in the eszopiclone group) cannot be determined. As with most clinical trials, between-group statistical analyses compare the effects of random assignment to study condition, not solely the drug itself. Adherence to protocol-prohibited behaviors, how often study drug is taken, dropout rates, and other potential indirect effects of drug or placebo, cannot be disentangled from pure drug effects. At the end of the discontinuation phase of this study random assignment to the eszopiclone group was more likely to result in an ISI value consistent with no clinically meaningful insomnia than was assignment to placebo. Because an observed case analysis was used to compare groups during the discontinuation phase, it is possible that the groups may not be balanced in all respects by the initial randomization process, which might introduce some bias into the observed discontinuation results.
Reduced quality of life, increased work absenteeism, patientreported fatigue and/or sleepiness, and increased risk of developing depression are documented correlates of chronic insomnia.19–26 Treatment efficacy studies, however, have largely been limited to quantitative nighttime sleep measurements, ignoring more syndromal aspects of chronic insomnia. The recent NIH state-of-the-science conference emphasized the need for a greater range of outcome measures in insomnia research, including quality-of-life, job-related disability and performance, and other measures that would provide additional clinical relevance beyond classic quantitative sleep measures.5 Although such outcomes have been evaluated in a few studies of cognitive behavioral therapy for insomnia,27 to our knowledge, this is the first pharmacotherapy clinical trial in patients with chronic primary insomnia to demonstrate enhanced quality of life, reduced work limitations, and less severe global insomnia ratings, in addition to improvements in quantitative sleep and next day function measures. Although the size of the treatment effect on the SF-36 and Work Limitations Scale was small in most instances, according to the descriptors of Cohen, one interpretation of the effects sizes observed on scales which differed between groups is that roughly 15% to 20% of the eszopiclone group distribution did not overlap with the placebo group distribution on these measures. Given all the factors that may influence quality of life and work performance, any separation of distributions attributable to treatment is not without clinical relevance. Importantly, the most comprehensive measure of insomnia used, the ISI, showed a large effect of treatment throughout the study.
Perspective on the burden of chronic insomnia can be gained by comparing baseline values of our study sample to normative samples. Pre-treatment SF-36 values from the present study indicate that Vitality, Social Functioning, and the Mental Component Summary score were significantly worse than normative values for the US healthy population. Similarly, patients in both treatment groups had higher baseline Fatigue Severity Scores than healthy individuals and were similar to those noted in multiple sclerosis (4.8 ± 1.3) and systemic lupus erythematosus (4.7 ± 1.5).9 In our study, the mean FSS score with eszopiclone at Month 6 (3.3 ± 1.4) was significantly improved compared to placebo, and final values were close to normal (2.3 ± 0.7).9 Pre-treatment Work Limitations ratings also indicated substantial work-related limitations in patients with insomnia. Normal ranges for all categories are 15 (except work productivity loss, which is 4), whereas baseline levels in this study population were between 23 and 41 (8.5 for work productivity loss). These baseline impairments were greater than those observed in patients with migraine headache (mean scores 15–22, work productivity loss 4.1)28 and similar to those in patients with clinical depression (mean scores 19–37, work productivity loss 9.1; Debra Lerner personal communication, October 31, 2005). Mean Work Limitation Questionnaire scores for the Month 1–6 average were reduced to 15–30 (5 for work productivity loss) with eszopiclone treatment, with significant improvements relative to placebo in all domains.
The large number of statistical comparisons reported herein may lead to concern about Type 1 error; however, the primary and key secondary endpoints were established a priori. Group differences on those measures (and other measures) were found to be highly significant with relative effect sizes indicating that roughly 35%–40% of the distributions of the 2 treatment groups did not overlap. Moreover, a large number of comparisons are inherent in a long-term randomized clinical trial that includes examination of both efficacy and health outcomes; the fact that all sleep-related and next-day function endpoints showed highly statistically significant differences at all time points and replicated the findings of Krystal et al6 should moderate these concerns.
Most investigations utilizing patient-reported sleep variables collect data each night and average nightly values over 1- or 2-week periods. In the current study, patient reports were generally collected weekly, except for nightly estimates during the last week of double-blind treatment and during the discontinuation period. In order to compare daily versus weekly patient reports, we first demonstrated that there were significant correlations between successive weekly estimates for LPS, TST, and WASO. For each sleep endpoint, the last weekly assessment (Month 6, Week 3) was significantly and strongly correlated (r = 0.59 to 0.79; all P <0.0001) with the previous weekly assessment (Month 6, Week 2). We then calculated correlations for nightly (Month 6, Week 3) and weekly (Month 6, Week 4) estimates of SL, TST, and WASO. There were strong correlations (r = 0.64 to 0.84, all P <0.0001) between nightly and weekly reports of the 3 sleep endpoints. In addition, median values were similar when collected nightly and weekly (WASO 16 min nightly vs 20 min weekly for placebo, and 5 min nightly vs 8 min weekly for eszopiclone; SL 44 vs 30 and 23 vs 20; TST 351 vs 360 and 398 vs 410). Since correlations between nightly and weekly assessments were high and similar to correlations between the 2 weekly assessments, we believe that weekly and nightly patient reports provide similar information.
While the main purpose of this study was to evaluate the effects of insomnia and insomnia treatment on patient-reported sleep, the lack of polysomnography and objective measures of daytime functioning may be seen as a methodological limitation, as objective assessments may be less prone to patient bias in the event of “unblinding” due to side effects or treatment failure. The consistent results across endpoints suggest that treatment of insomnia with eszopiclone had a positive effect on quality of life, work productivity, fatigue, and daytime functioning. Conclusions drawn from the present study should be limited to patients with primary insomnia, and it would be beneficial to determine whether similar benefits would be observed in patients with insomnia comorbid with other conditions. For example, in a recent study of depressed patients with insomnia, eszopiclone and fluoxetine cotherapy significantly reduced insomnia symptoms and improved ratings of depression as compared with fluoxetine alone.29
Higher dropout rates were observed with placebo due to insomnia treatment failure, particularly at Month 1, but overall discontinuation rates in both groups were consistent with those seen previously in a 6-month clinical trial.6 The Last Observation Carried Forward method used in this study attempts to adjust for differential dropout by retaining data from patients who discontinued for any reason, on the assumption that the value at dropout would have remained the same until the end of the trial. Results using Observed Case data were consistent with those using the LOCF method.
As is seen in the majority of randomized clinical trials, some improvement was observed in the placebo group, with the greatest improvement noted between baseline and the first month of double-blind treatment. As described by McCall,30 nonspecific factors of participating in clinical trials appear to account for these observations more than placebo pill ingestion per se.
To our knowledge this investigation is the first to demonstrate that long-term treatment of primary insomnia with eszopiclone 3 mg, or any hypnotic, enhanced quality of life, reduced work limitations, and reduced global insomnia severity, in addition to improving quantitative, patient-reported sleep variables. Although group effect sizes on quality of life and work limitations were modest, given the multitude of social, economic, personal, and health factors which influence quality of life and work performance any benefit of treatment is notable. Additionally common clinical concerns related to long-term, nightly hypnotic use, such as tolerance and discontinuation effects, were not observed.
ACKNOWLEDGMENTS
The authors wish to acknowledge Kimberly Pfleeger of Sepracor Inc. for her assistance in the preparation of this manuscript, and Phebe Wilson of Sepracor Inc. for her assistance with the statistical analysis.
Footnotes
Disclosure Statement
Support for this study provided by Sepracor Inc. Dr. Walsh's institution has received research support from Pfizer, Merck, Neurocrine Biosciences, Somaxon, Evotec Neurosciences, and Cephalon. He has provided consulting services for Pfizer, Sanofi-Aventis, Cephalon, Organon, Neurocrine Biosciences, Takeda, Actelion, Sepracor, Elan, Guilford, Respironics, Merck KgaA-Darmstadt, Eli Lilly, Evotec Neurosciences, and Merck. Dr. Krystal has consulted for, received research support from and has had advisory board relationships with NIH, Sanofi-Aventis, GlaxoSmithKline, King Pharmaceuticals, Merck, Cephalon, Eli Lilly, Neurocrine Biosciences, Sepracor, Pfizer, Johnson & Johnson, Organon, Takeda, Respironics, Neuronetics, Cyberonics, Mecta Corporation, Transoral, Neurogen, Somaxon, and Astellas. Dr. Wallenstein is the Director of Consulting Services for Qualitymetric Incorporated and received financial support for analysis of portions of the data reported in this study and for contribution to the writing of this manuscript. Dr. Roth is a consultant for Abbott, Accadia, Acoglix, Actelion, Alchemers, Alza, Ancil, Arena, AstraZeneca, Aventis, BMS, Cephalon, Cypress, Dove, Elan, Eli Lilly, Evotech, Forest, GlaxoSmithKline, Hypnion, Johnson & Johnson, King, Ludbeck, McNeil, MediciNova, Merck, Neurim, Neurocrine, Neurogen, Novartis, Orexo, Organon, Orginer, Prestwick, Proctor and Gamble, Pfizer, Purdue, Resteva, Roche, Sanofi, Schering-Plough, Sepracor, Servier, Somaxon, Syrex, Takeda, Transoral, Vanda, Vivometric, Wyeth, Yamanuchi, and Xenoport; has received research support from Aventis, Cephalon, GlaxoSmithKline, Neurocrine, Pfizer, Sanofi, Schering- Plough, Sepracor, Somaxon, Syrex, Takeda, TransOral, Wyeth, and Xenoport; and has participated in speaking engagements supported by Sanofi and Takeda. Drs. Amato, Rubens, Caron, Wessel, Roach, and Kendyl Schaefer are employees of Sepracor.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Publishing; 1994. Sleep disorders; pp. 597–661. [Google Scholar]
- 2.Morin MM. Measuring outcomes in randomized clinical trials of insomnia treatments. Sleep Med Rev. 2003;4:263–79. doi: 10.1053/smrv.2002.0274. [DOI] [PubMed] [Google Scholar]
- 3.Katz DA, McHorney CA. The relationship between insomnia and health-related quality of life in patients with chronic illness. J Fam Pract. 2002;51:229–35. [PubMed] [Google Scholar]
- 4.Leger D, Scheurmaier K, Philip P, Paillard M, Guilleminault C. SF-36: evaluation of quality of life in severe and mild insomniac compared with good sleepers. Psychosom Med. 2001;63:49–55. doi: 10.1097/00006842-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 5.National Institutes of Health. National Institutes of Health State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults, June 13-15, 2005. Sleep. 2005;28:1049–57. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- 6.Krystal AD, Walsh JK, Laska E, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep. 2003;26:793–9. doi: 10.1093/sleep/26.7.793. [DOI] [PubMed] [Google Scholar]
- 7.Roth T, Walsh JK, Krystal A, Wessel T, Roehrs TA. An evaluation of the efficacy and safety of eszopiclone over 12 months in patients with chronic primary insomnia. Sleep Med. 2005;6:487–95. doi: 10.1016/j.sleep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 9.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 10.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 11.Ware JE, Jr, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey manual and interpretation guide. Boston, MA: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 12.Lerner D, Amick BC, 3rd, Rogers WH, Malspeis S, Bungay K, Cynn D. The Work Limitations Questionnaire. Med Care. 2001;39:72–85. doi: 10.1097/00005650-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Tyrer P, Murphy S, Riley P. The Benzodiazepine Withdrawal Symptom Questionnaire. J Affect Disord. 1990;19:53–61. doi: 10.1016/0165-0327(90)90009-w. [DOI] [PubMed] [Google Scholar]
- 14.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–92. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 15.Ware JE, Jr, Harris WJ, Gandek B, Rogers BW, Reese PR. MAP-R for Windows: multitrait/multi-item analysis program-revised user's guide. Boston, MA: Health Assessment Lab; 1997. [Google Scholar]
- 16.Ware JE, Jr, Kosinski M, Dewey J. How to score version two of the SF-36 Health Survey. Lincoln, RI: QualityMetric Inc; 2000. [Google Scholar]
- 17.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 18.National Survey of Functional Health Status. 1998.
- 19.Simon G, VonKorff M. Prevalence, burden, and treatment of insomnia in primary care. Am J Psychiatry. 1997;154:1417–23. doi: 10.1176/ajp.154.10.1417. [DOI] [PubMed] [Google Scholar]
- 20.Weissman MM, Greenwald S, Nino-Murcia G, Dement WC. The morbidity of insomnia uncomplicated by psychiatric disorders. Gen Hospital Psych. 1997;19:247–50. doi: 10.1016/s0163-8343(97)00056-x. [DOI] [PubMed] [Google Scholar]
- 21.Godet-Cayre V, Pelletier-Fleury N, Le Vaillant M, Dinet J, Massuel MA, Leger D. Insomnia and absenteeism at work. Who pays the cost? Sleep. 2006;29:179–84. doi: 10.1093/sleep/29.2.179. [DOI] [PubMed] [Google Scholar]
- 22.Leger D, Massuel MA, Metlaine A SISYPHE Study Group. Professional correlates of insomnia. Sleep. 2006;29:171–8. [PubMed] [Google Scholar]
- 23.Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbances and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–8. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 24.Chang PP, Ford DE, Mead LA, Cooper-Patrick L, Klag MJ. Insomnia in young men and subsequent depression: The Johns Hopkins Precursors Study. Am J of Epidemiol. 1997;146:104–14. doi: 10.1093/oxfordjournals.aje.a009241. [DOI] [PubMed] [Google Scholar]
- 25.Dryman A, Eaton WW. Affective symptoms associated with the onset of major depression in the community: Findings from the US National Institute of Mental Health Epidemiologic Catchment Area Program. Acta Psychiatr Scand. 1991;84:1–5. doi: 10.1111/j.1600-0447.1991.tb01410.x. [DOI] [PubMed] [Google Scholar]
- 26.Vollrath M, Wicki W, Angst J. The Zurich study: VIII. Insomnia: Association with depression, anxiety, somatic syndromes, and course of insomnia. Eur Arch Psychiatry Neurol Sci. 1989;239:113–24. doi: 10.1007/BF01759584. [DOI] [PubMed] [Google Scholar]
- 27.Morgan K, Dixon S, Mathers N, Thompson J, Tomeny M. Psychological treatment for insomnia in the regulation of long-term hypnotic drug use. Health Technol Assess. 2004;8:1–68. doi: 10.3310/hta8080. [DOI] [PubMed] [Google Scholar]
- 28.Lerner D, Rogers WH, Bungay K, Chang H. National survey of work and health. Boston, MA: The Health Institute; 2004. [Google Scholar]
- 29.Fava M, McCall WV, Krystal A, et al. Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol Psychiatry. 2006;59:1052–60. doi: 10.1016/j.biopsych.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 30.McCall VW, Perlis ML, Tu X, Groman AE, Krystal A, Walsh JK. A comparison of placebo and no-treatment during a hypnotic clinical trial. Int J Clin Pharmacol Ther. 2005;43:355–9. doi: 10.5414/cpp43355. [DOI] [PubMed] [Google Scholar]