Abstract
Study Objective:
To examine whether the level of sleep efficiency of children diagnosed with ADHD moderates their performance on the Continuous Performance Test (CPT) while receiving a placebo and while receiving methylphenidate (MPH).
Design:
Nightly sleep actigraphic assessment during a double-blind, placebo-controlled, crossover clinical study (1 week of 0.5 mg/kg MPH; 1 week of placebo) were obtained on 37 children between 6 and 12 years of age with a DSM-IV diagnosis of ADHD. Subjects were divided into 2 groups based on the mean sleep efficiency score during the placebo condition, with subjects above and below the mean placed in the Poor Sleep Group (PSG) and Good Sleep Group (GSG), respectively.
Setting:
Vigilance testing was conducted in the laboratory; sleep was assessed in the home.
Measurements:
Sleep was monitored using actigraphy for 2 weeks. In addition, parents were asked to complete nightly sleep logs and a sleep questionnaire. The Conners' Continuous Performance Test (CPT) was used to assess vigilance.
Results:
Significant interaction of Sleep Group with Medication was found on 1 CPT factor.
Conclusions:
The findings of the present study support the hypothesis that sleep moderates performance on CPT in children with ADHD while receiving placebo or MPH.
Citation:
Gruber R; Grizenko N; Schwartz G; Bellingham J; Guzman R; Joober R. Performance on the continuous performance test in children with ADHD is associated with sleep efficiency. SLEEP 2007;30(8):1003–1009.
Keywords: ADHD, Continuous Performance Test, sleep efficiency, vigilance
ATTENTION DEFICIT HYPERACTIVITY DISORDER (ADHD), ONE OF THE MOST PREVALENT CONDITIONS IN CHILD PSYCHIATRY, MANIFESTS AS AN UNUSUALLY high and chronic level of inattention and/or impulsivity/hyperactivity. ADHD is estimated to occur in 3% to 7.5% of school-aged children and often persists into adolescence and adulthood.1 If ADHD is left untreated, affected individuals struggle with impairments in crucial areas of life.2
Several models have suggested that children with ADHD suffer from underarousal in the cortex or other CNS systems.3–5 The Continuous Performance Test (CPT) is a neuropsychological task that has repeatedly been shown to differentiate ADHD from normal groups.6 Children with ADHD show deficits in the d' parameter,7 a consensus index of arousal in the CPT.8 Additional empirical support to the hypoarousal hypothesis comes from electroencephalography (EEG) studies. These studies have shown that children with ADHD are prone to daytime hypoarousal characterized by increased theta activity (primarily in the frontal areas), decreased alpha and beta activity, and increased theta/alpha and theta/beta ratios9 compared to normal children. Sustained wakefulness and sleep deprivation causes similarly increased theta and decreased alpha activities in normal participants suggesting that insufficient sleep is associated with daytime hypoarousal. Objective studies designed to assess fatigue/alertness revealed that children with ADHD exhibited significantly more daytime sleepiness than controls.10–12
ADHD is most commonly treated with stimulant medications, such as methylphenidate (MPH). It has been suggested that psychostimulants enhance the levels of arousal in the central nervous system (CNS) and autonomic nervous system (ANS) of these individuals. Treatment with MPH and other stimulants (i.e., amphetamines) has been shown to normalize EEG patterns and to improve CPT performance13–16 in children with ADHD. These findings indicate that such medications stimulate the underaroused cortex16,17 and at least partially normalize low arousal levels,17 providing further support for the hypothesis that children with ADHD suffer from hypoarousal.
Sleep problems, particularly difficulties in initiating and maintaining sleep, have been reported in an estimated 25% to 50% of children and adolescents with ADHD.18 Indeed, restless and disturbed sleep was initially included in the DSM diagnostic criteria for ADHD, though it was later excluded as being a nonspecific symptom. Parental reports indicate a 2- to 3-fold higher prevalence of sleep problems in children with ADHD compared with controls, including difficulty falling asleep, night awakenings, and restless sleep.18 Actigraphic studies have suggested that activity during sleep is higher in children with ADHD, and that these children tend to have unstable sleep patterns.18 Sleep apnea19 and restless leg syndrome (RLS)/periodic leg movement disorder (PLMD)20 have also been associated with hyperactivity and inattention. In addition, MPH and amphetamine have been found to be associated with insomnia.21
A few studies conducted with normal adult subjects have shown that stimulants enhanced performance only in fatigued individuals,22 and the impact of the medication depended on the individual's basal level of alertness or sleepiness.23–25 Several studies have examined the impact of MPH on the sleep of children with ADHD. No previous study, however, has examined whether the impact of MPH on vigilance in children with ADHD is related to the efficiency of their sleep. Given the high prevalence of sleep complaints in children with ADHD and the overlap of some ADHD symptoms with the consequences of disrupted sleep, it is important to examine whether methylphenidate increases vigilance and reverses attentional problems to different degrees in ADHD children having poor versus good sleep efficiency.
In the present study, we sought to examine whether the sleep efficiency of children diagnosed with ADHD moderates their performance on the CPT and whether this is influenced by treatment with methylphenidate. We used a double-blind, placebo-controlled, within-subject (crossover) design to assess the performance of children with ADHD with different sleep efficiency, both while they were on medication and while they were on placebo. We hypothesized that the performance of children with low, but not of those with high, sleep efficiency would improve following the administration of MPH. To our knowledge, this is the first study in which the MPH response in children with ADHD has compared poor and good sleepers using an objective neuropsychological test and a validated clinical scale as the outcome measures.
METHODS
Subjects
Children recruited from the Disruptive Behavior Disorders Program and the outpatient department of the Douglas Mental Health University Institute in Montreal were assessed for ADHD using criteria from the Diagnostic and Statistical Manual-4th edition.26 The Diagnostic Interview Schedule for Children (DISC-IV),27 which generates DSM-IV diagnoses, was administered to parents. ADHD diagnoses were confirmed by multidisciplinary consensus after clinicians and researchers reviewed data from parent and teacher interviews, results from psychological and psychiatric testing, and behavior rating scales obtained from teachers and parents, including the Child Behavior Checklist (CBCL)28 and the Conners' Parents and Teachers Rating Scales.29,30 A total of 37 children (31 boys and 6 girls) between 6 and 12 years of age (mean age = 9.2 years, SD = 1.8) with a DSM-IV diagnosis of ADHD were included in the study. Of these, 4 met the criteria for Inattentive, 7 for Hyperactive-Impulsive, and 26 for Combined subtypes. Most of the children were Caucasian (94%); number of children in the family ranged from 1 to 4 (M=1.38, SD=0.91); parents' ages at child's birth ranged from 18 to 40 (age of fathers; M=29.6 years, SD=6.2; age of mothers: M=26.3 years, SD=6.0).
Subjects were excluded if they scored less than 80 on the Weschler Intelligence Scale for Children-3rd edition (WISC-III)31 or if they had been diagnosed with psychosis,▁Tourette syndrome (TS), or a pervasive developmental disorder. Children who were taking any medication other than MPH or had shown previous intolerance or allergic reactions to any psychostimulant were also excluded. The study was approved by the Research Ethics Board of Douglas Mental Health University Institute; informed consent was obtained from parents, and all children assented to participation in the study.
Study Design
A double-blind, placebo-controlled, within subject (crossover) design was used to assess sleep and CPT performance in participants who were taking either a placebo or 0.5 mg/kg MPH. These were given in 2 equal doses (morning and noon) for 7 days each. The order of administration (MPH or placebo) was determined by random assignment. MPH and placebo were prepared in identical colored gelatin capsules by the hospital's clinical pharmacist, who was not involved in the study in any other way. Capsules were sealed in individual, daily-dose envelopes to help control accurate administration. Parents were instructed to maintain the child's regular sleep schedule and routine. All children were monitored during regular school days (i.e., excluding weekends and holidays) for 2 weeks. Parents were instructed to attach a miniature actigraph (AW-64 series, Mini Mitter Co, Bend, OR) to the child's nondominant wrist each evening when preparing for sleep and to remove the apparatus each morning. In addition, parents were asked to document the child's sleep schedule in nightly sleep logs. On the third day of each condition, vigilance was assessed using the CPT, and, in addition, the behavioral response to MPH or placebo was evaluated using the Clinical Global Impression Scale (CGI).32 The clinicians making CGI ratings were blind to both sleep group and medication status.
Sleep Assessment
Miniature actigraphs (AW-64 series) were used to assess the children's sleep patterns in their natural home environments. Actigraphy has been widely used to assess sleep in both clinical trials and in studies requiring multiple measurements. It has been validated against polysomnography with agreement rates for minute-by-minute sleep-wake identification higher than 90%.33 A recent study tested the validity of AW-64 actigraphy and found it to be a satisfactory objective measure of sleep when using the scoring algorithm utilized by the software Actiware Sleep v. 3.3.34
Actigraphic data were analyzed during each sleep episode based on 1-minute epochs. The reported bedtime and wake time were used as the start and end times for these analyses. For each 1-minute epoch, the total sum of activity count was computed. If the sum exceeded a threshold sensitivity value (calculated as the mean score during an active period/45), the epoch was categorized as awake. If the sum fell below the threshold value, the epoch was categorized as asleep. Actigraphic sleep measures included the following parameters: (a) Sleep Time-sleep period; (b) Wake Time-amount of time spent awake during the night; (c) Sleep Efficiency-index (%) of the amount of time in bed that is actually spent sleeping; (d) Motionless Sleep Time-summation of the time in which the subject does not move, (e) Motionless Sleep Percent-motionless time during sleep period; (f) Movement and Fragmentation Index-an index of restlessness derived from the percentage of time in which changes from sleep to wake state occurred.
Daily Sleep Logs
The daily sleep logs, which were completed by the parents, included information about children's bedtimes and waking times.
Reported Sleep Problems
To supplement the daily information on daytime sleepiness with a general overview of the child's sleep habits based on the Child Behavior Checklist (CBCL)29-each mother completed a questionnaire that included a 3-point Likert-type scale with items regarding her child's sleep habits. At the screening stage of the study, each mother was asked to indicate whether their child never (0), sometimes (1), or often (2) slept less than most children, had nightmares, and/or had trouble sleeping. In addition, parents were asked to indicate whether the child "sleeps more than most kids during the day" and "is generally overtired." The interrater reliability of the questionnaire is 0.95 and the internal consistency value of the questionnaire is 0.70.
Continuous Performance Test
The Continuous Performance Test (CPT)35 is a standardized computer-administered test in which single letters are presented on a computer screen at 2 different rates: once per second, once every 2 s, or once every 4 s. Over the course of the test (14.5 minutes), the participant is asked to press a button in response to every signal except the target signal. The utilized CPT measures included the total number of omissions (missed targets), total number of commissions (false hits), reaction time (RT), RT variability, RT standard error, risk taking, and signal detectability (d').
Clinical Global Impression (CGI) Scale
The CGI scale is used to assess treatment response in psychiatric patients. In the present study, we included the Severity of Illness and the Global Improvement components of the CGI scale. The Severity of Illness item, which requires the clinician to rate the severity of the patient's illness at the time of assessment, relative to the clinician's past experience with patients having the same diagnosis, is rated on a 7-point scale in which 1 = normal and 7 = extremely ill. The Global Improvement item, which requires the clinician to rate how much the patient's symptoms have improved or worsened relative to a baseline state, is rated on a 7-point scale with 1 = significantly improved to 7 = significantly worse.
Data Analysis
Subjects were divided into 2 groups based on the mean sleep efficiency score during the week of the placebo, with subjects above and below the mean (M=80%) placed in the Poor Sleep Group (PS) and Good Sleep Group (GS), respectively. Demographic, intellectual, and psychiatric characteristics were considered as dependent variables and were compared across these groups using either one-way analysis of variance (ANOVA) or chi-square analysis, depending on the nature of the data. Actigraphic and questionnaire measures of sleep quality, duration, and fragmentation were compared between PS and the GS on the placebo and MPH weeks.
It is important to note that due to the number of analyses and the relatively small sample size there is a potential concern regarding Type I and Type II errors. In order to reduce the probability of making Type I error, multivariate analyses of covariance (MANCOVAs) were computed with the Sleep Group (PS or GS) taken as the between-subject independent factor, the sleep change scores taken as the dependent variables, and child's age taken as a covariate. In addition, principal component analyses were used to reduce the number of variables and aggregate the CPT measures into reliable indices reflecting the integrity of attention-related dimensions. Additional MANCOVAs were used to analyze the association between level of sleep efficiency and improvement on objective measures of attention and behavior following administration of MPH, with the Sleep Group (PS or GS) taken as the between-subjects independent factor, the medication (MPH or placebo) used as the repeated, within-subject independent factor, the CPT Factors as the dependent variables, and the child's age and sex as covariates. Significant interactions of Sleep Group with Medication would indicate that the effects of MPH were moderated by sleep efficiency. Finally, to analyze the association between initial level of sleep efficiency and improvement on clinical measures, MANOVA was used to compare improvement on clinical measures, with Sleep Group (PS or GS) as the between-subject independent factor, the medication (MPH or Placebo) as the repeated, within-subject, independent factor, and the change in CGI score (severity or improvement) as the dependent variable. All analyses were performed using SPSS 12.0 for Windows; P values <0.05 were considered to indicate statistical significance.
RESULTS
Characterization of Sleep Groups
Tables 1 and 2 present the demographic and clinical characteristics (means and standard deviations) of the enrolled children with ADHD, divided into the PS and GS groups. The only significant differences between the groups were observed on the sleep measures. Compared to the GS, children in the PS spent significantly less time sleeping during the night and significantly more time being awake; they spent less of the night in immobile sleep and their fragmentation index was higher regardless of medication or placebo.
Table 1.
Demographic and Clinical Characteristics of Children with ADHD by Sleep Group
| PS (N=18) | GS (N=19) | ||
|---|---|---|---|
| Sex (M/F) | 17/1 | 14/5 | χ2= 2.9, df =1 P = 0.09 |
| Age | 9.1 (1.74) | 9.3 (1.9) | F1,35 = 0.1, P = 0.75 |
| IQ | 98.0 (15.3) | 95.5 (17.9) | F1,35 = 0.18, P = 0.67 |
| CBCL (total score) | 73.0 (11.35) | 70.5 (10.02) | F1,35 = 0.03, P = 0.96 |
| CBCL, Internalizing | 65.3 (11.2) | 63.6 (11.4) | F1,35 =0.22, P = 0.64 |
| CBCL, Externalizing | 71.8 (0.9) | 69.5 (11.9) | F1,35 =0.37, P = 0.55 |
| Conners-Total Parents | 71.6 (13.9) | 68.8 (12.4) | F1,35 = 0.35, P = 0.56 |
| Conners-Total Teachers | 76.7 (10.3) | 75.6 (12.74) | F1,35= 0.38, P = 0.78 |
| DISC-IV Inattention Items | 7.5 (1.3) | 7.6 (1.6) | F1,35 = 0.02, P = 0.87 |
| DISC-IV Hyperactivity Items | 6.9 (2.2) | 6.6 (2.11) | F1,35 = 0.19, P = 0.67 |
| DISC-IV Total items | 14.4 (2.5) | 14.2 (2.75) | F1,35 = 0.07, P =0.79 |
| Inattentive Subtype | 3/18 | 4/19 | X2 = 1.27, df = 1, P = 0.53 |
| Hyperactive-Impulsive Subtype | 1/18 | 3/19 | X2 = 1.32, df = 1, P = 0.51 |
| Combined Subtype | 14/18 | 12/19 | X2 = 1.21, df = 1, P = 0.27 |
| Comorbid ODD | 5/18 | 6/19 | X2 = 1.33, df = 1, P = 0.25 |
| Comorbid CD | 10/18 | 7/19 | X2 = 1.7, df = 1, P = 0.32 |
| Comorbid Major Depression | 1/18 | 2/19 | X2 = 0.05, df = 1, P = 0.83 |
| Comorbid General Anxiety Disorder | 0/18 | 1/19 | X2 = 0.60, df = 1, P = 0.44 |
PS=Poor Sleep Group; GS=Good Sleep Group; CBCL=Child Behavioral Checklist,
DISC-IV=Diagnostic Interview Schedule for Children fourth edition;
ODD=Opposition Defiant Disorder, CD=Conduct Disorder, LD= Learning Disability Values are mean (SD).
Table 2.
Means (SD) for Sleep Variables by Sleep Group
2.1 Sleep Questionnaires
| Sleep Group | |||||
|---|---|---|---|---|---|
| PSG | GSG | F | P | ||
| Less daytime sleep than most children | M | 0.88 | 0.50 | 2.6 | |
| SD | 0.96 | 0.86 | |||
| Nightmares | M | 0.48 | 0.68 | 1.34 | |
| SD | 0.60 | 0.71 | |||
| Has trouble sleeping | M | 1.06 | 0.61 | 3.5 | + |
| SD | 1.00 | 0.78 | |||
| Overtired | M | 0.53 | 0.51 | 0.68 | |
| SD | 0.67 | 0.84 | |||
| More daytime sleep than most children | M | 0.13 | 0.28 | 1.5 | |
| SD | 0.34 | 0.67 | |||
2.2 Actigraphic Sleep Measures
| Sleep Group | Sleep Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PSP | GSG | F | P | PSP | GSG | F | P | ||
| Sleep time (hrs) | M | 7.42 | 8.35 | 17 | * | 7.47 | 8.15 | 5.3 | * |
| SD | 0.49 | 0.26 | 0.35 | 0.37 | |||||
| Wake time (hrs) | M | 1.49 | 1.13 | 12 | * | 1.49 | 1.18 | 10.9 | * |
| SD | 0.40 | 0.17 | 0.36 | 0.17 | |||||
| Sleep time (%) | M | 81.17 | 87.5 | 17.3 | * | 81.1 | 86.49 | 13.8 | * |
| SD | 6.08 | 2.55 | 5.60 | 2.75 | |||||
| Immobile time (%) | M | 80.32 | 85.0 | 10.3 | * | 80.5 | 83.85 | 5.8 | * |
| SD | 5.66 | 2.80 | 4.86 | 3.46 | |||||
| Fragmentation Index | M | 36.7 | 28.1 | 18.6** | 34.5 | 29.73 | 3.6 | + | |
| SD | 9.79 | 5.00 | 9.39 | 7.19 | |||||
PS =Poor Sleep Group; GS=Good Sleep Group; M=Mean; SD=Standard Deviation
*P<.05;
+P<.07
Factor Analyses
Principal component analyses with varimax rotation produced similar 3-factor solutions for CPT measures obtained while the children were on placebo or MPH. Based on component loadings of 0.6, three factors accounted for 46%, 22.4%, and 17.6%, respectively, of the variance (see Table 3). The first factor, which yielded eigenvalues of 3.72 and 3.31 for children on placebo and MPH, respectively, was weighted by scores from the omissions (missed targets), RT variability, RT standard error variability, and beta. The second factor, yielding eigenvalues of 1.57 and 1.11 in children receiving placebo or MPH, respectively, was weighted by scores of total commissions (false hits) and d'. The third factor, yielding eigenvalues of 1.2 and 1.21 in children receiving placebo or MPH, respectively, was weighted by scores of reaction time. A score for each factor was calculated for each child by weighting the items according to the factor loadings, as shown in Table 3.
Table 3.
Means (SD) for CPT Performance Measures by Sleep Group
| Placebo | MPH | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sleep Group | Factor Loading | Sleep Group | Factor Loading | ||||||||
| PS | GS | 1 | 2 | 3 | PS | GS | 1 | 2 | 3 | ||
| Omissions Errors (%) | M | 13.7 | 13.1 | 0.85 | −0.12 | 0.15 | 10.8 | 13.2 | 0.86 | −0.01 | 0.15 |
| SD | 9.0 | 15.5 | 12.6 | 14.6 | |||||||
| Commission Errors (%) | M | 66.7 | 56.2 | −0.18 | 0.93 | 0.13 | 71.2 | 59.3 | −0.11 | 0.87 | 0.21 |
| SD | 19.8 | 25.3 | 26.1 | 25.5 | |||||||
| RT | M | 426.4 | 482.8 | 0.17 | 0.02 | 0.94 | 402.2 | 454.7 | 0.04 | 0.05 | 0.91 |
| SD | 84.8 | 163.9 | 129.9 | 116.4 | |||||||
| RT (SE) | M | 23.6 | 20.8 | 0.95 | 0.04 | −0.1 | 18.4 | 20.9 | 0.94 | 0.01 | −0.14 |
| SD | 9.6 | 16.4 | 13.2 | 15.5 | |||||||
| VSE | M | 50.2 | 40.5 | 0.89 | 0.15 | 0.11 | 42.5 | 42.1 | 0.92 | 0.05 | 0.01 |
| SD | 23.2 | 32.7 | 43.3 | 34.6 | |||||||
| d' | M | 0.73 | 1.20 | 0.42 | 0.8 | −0.27 | 0.88 | 0.96 | 0.24 | 0.64 | −0.4 |
| SD | 0.79 | 1.00 | 1.12 | 1.12 | |||||||
| B | M | 0.66 | 0.54 | 0.67 | 0.19 | −0.57 | 0.74 | 0.62 | 0.63 | 0.08 | −0.41 |
| SD | 0.28 | 0.30 | 0.55 | 0.35 | |||||||
| Factor Scores | |||||||||||
| 1* | M | 0.66 | 0.08 | 0.18 | 0.46 | ||||||
| SD | 0.59 | 0.91 | 1.07 | 0.81 | |||||||
| 2 | M | 0.22 | −0.17 | −0.71 | −0.78 | ||||||
| SD | 1.01 | 1.20 | 0.66 | 1.02 | |||||||
| 3 | M | −0.33 | −0.65 | 0.07 | −0.23 | ||||||
| SD | 0.99 | 0.75 | 1.08 | 0.96 | |||||||
M=Mean; SD-Standard Deviation; SE= Standard Error; RT =Reaction Time;
VSE =Variability of Standard Error; PS =Poor Sleep Group; GS=Good Sleep Group; d'=detectability; β= Response Style Indicator
*Lower score indicates better performance on Factor 1
Comparison of Medication-Associated Changes in Sleep Between Sleep Groups
In Table 2, we present the means and the standard deviations of the actigraphic and questionnaire sleep measures divided into groups according to their sleep efficiency level MANCOVA was used to compare changes in sleep following the administration of MPH and Placebo in the 2 sleep groups. There were no significant differences in sleep between the condition in which the children were receiving MPH and the condition in which they were receiving placebo in the PS and in the GS groups.
Comparison of Medication-Associated Changes in CPT Performance Between Sleep Groups
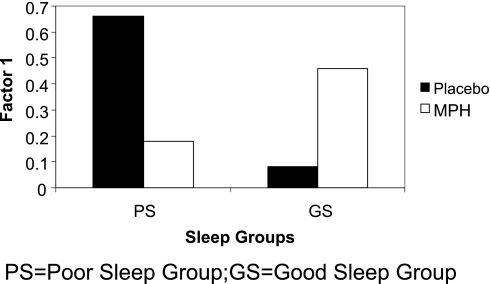
In Table 3, we present the means and the standard deviations on the CPT divided into groups according to their sleep efficiency levels. When we compared the CPT performance of children in the GS and PS groups receiving MPH versus placebo, MANCOVA revealed a significant main effect for medication [F3,33 = 11.8, P <0.05]. Post hoc univariate analyses revealed significant medication-related differences on the 2nd and 3rd factors [F1,36 = 10.9, P <0.05; F1,36 = 20, P <0.05, respectively]. In addition, there was a Sleep Group by Medication interaction [F1,36 = 9.4; P <0.05]. Univariate analysis revealed a significant on the first CPT factor [F2,28 = 4.05 P <0.05], indicating that the score of children in the PS significantly improved when they received MPH compared to placebo, whereas it deteriorated for children in the GS when they received MPH compared to placebo (Figure 1).
Figure 1.
Performance of ADHD children in the poor sleep group (black) and good sleep group (white) on a CPT task while receiving placebo and while receiving medication. Graph represents task performance as measured by the tasks that were included in Factor 1: Omissions (missed targets), Reaction Time variability, Reaction Time Standard Error Variability and Beta.
Comparison of Medication-Associated Changes in CGI Scale Between Sleep Groups
In Table 4, we present the means and the standard deviations on the CGI divided into groups according to their sleep efficiency levels. When we compared the scores on the clinical global impression scales of children in the PS group versus those in the GS group when receiving MPH or placebo, MANCOVA revealed a marginal difference [F2,32 = 2.96 P <0.07]. Post hoc univariate analysis revealed a marginal difference on the Improvement subscale [F1,33 = 3.5 P <0.07], indicating that the clinical symptoms of children in the PS group, but not of children in the GS group, were marginally better when they received MPH compared to placebo.
Table 4.
Means (SD) for Clinical Global Improvement Scale by Sleep Group
| Placebo | MPH | ||||
|---|---|---|---|---|---|
| Clinical Global | Sleep Group | Sleep Group | |||
| Improvement Scale | PS | GS | PS | GS | |
| Severity of Illness Score | M | 4.67 | 4.05 | 4.44 | 4.11 |
| SD | 1.08 | 0.97 | 1.25 | 1.05 | |
| Global Improvement Score | M | 4.22 | 4.37 | 3.06 | 4.05 |
| SD | 0.94 | 0.96 | 1.24 | 1.27 | |
PS =Poor Sleep Group; GS=Good Sleep Group; M=Mean; SD=Standard Deviation
DISCUSSION
We report that children with ADHD experienced significant improvement on some measures of vigilance performance when given MPH if their sleep efficiency was poor, but not if their sleep efficiency was good. These findings agree with previous reports that the performance-enhancing effects of amphetamine were dependent on prior sleep deprivation in normal adults.24,36,37 Thus, children with low sleep efficiency might improve performance following the administration of MPH as it increases their arousal level to a moderate level, which is presumed to facilitate vigilance performance.
Successive increases of arousal beyond the optimal arousal level could lead to impaired performance. Future studies looking at the association between the impact of MPH, basal characteristics of sleep, and the efficiency of different attentional systems in children with ADHD are needed to further examine the association between sleep and neurobehavioral functioning in ADHD.
An alternative explanation should also be considered. Because good vs. poor sleep determination was made during the placebo stage, the association does not determine what came first or which events caused other events.
The factors underlying the differences of sleep quality in children with ADHD are not yet fully understood. Because of the mixed reports that associate sleep problems with the clinical presentations of the disorder and with the commencement of the pharmacological intervention, it is not clear whether sleep problems in children with ADHD are caused by intrinsic (e.g., cholinergic, dopaminergic, and noradrenergic mechanisms) or extrinsic (e.g., MPH) factors.38 In the present study, we did not observe more sleep disturbances in the MPH week versus the placebo week in the good or poor sleep groups, and thus this does not explain the group-specific differences in CPT performance following administration of MPH.
ADHD has long constituted the largest single source of referrals in the mental health, educational, and medical settings.39 However, despite the prevalence of this disorder, there is limited consensus concerning the exact methods and tests that should be combined for accurate diagnosis. In recent years, evaluators have increasingly included the CPT in the basic clinical battery administered for evaluation of ADHD in children. The finding that sleep differences may underlie the ADHD-like symptoms and can affect CPT performance suggests that it may be useful to include objective sleep assessments (e.g., actigraphy) in the clinical assessment of ADHD, to accompany the clinical use of the CPT.
Our finding that MPH response is associated with sleep efficiency in children with ADHD suggests that a child's sleep efficiency may affect the performance-enhancing effects of MPH in that individual. Thus, researchers should stringently control for variation in sleep efficiency during clinical trials examining the effectiveness of MPH and its impact on tasks that require vigilance and sustained attention.
Limitations and Future Research
The findings concerning the differential impact of MPH must be qualified based upon several limitations of the current study: 1) small sample size and subsequent potential for Type II errors (although a change has been detected despite the relatively small sample size). The results should be considered preliminary and require replication by future studies; 2) Multiple analyses and subsequent potential for Type I errors (although MANOVA and principal component analyses have been used to decrease risk and number of outcome measures to be compared; 3) The lack of objective measures of daytime sleepiness or alertness such as the multiple sleep latency test40 or the maintenance of wakefulness test.40 Such measures, as well as information regarding the children's daytime activity, could indicate whether higher daytime hypoarousal (e.g., increased daytime sleepiness or reduced vigilance) is associated with greater improvement in CPT performance in children receiving MPH versus placebo. 4) Due to the small number of patients and the fact that they were mainly boys, these results should be considered preliminary and require replication by future studies that include boys and girls, and will take into consideration children's age andpubertal status; 4) Lastly, in future research, the findings of this study should be replicated with children from a general pediatrician's office to explore the ability to generalize the findings to children referred to a less specialty-focused clinic and "real world" outcomes should be included in order to estimate the direct clinical implications of the finding.
Although actigraphy is a valuable method for reliable, continuous assessment of a child's sleep in his or her home environment, actigraphic measurements do not reflect the structure of the child's sleep. Future studies would benefit from the use of both polysomnography and actigraphy to investigate the impact of MPH on performance in children who vary in terms of their sleep quality and architecture. Lastly, it would seem useful to explore the effect of different treatment regimens (e.g., long-acting MPH or thricedaily_regimens of short-acting MPH) and varied MPH doses on subjective and objective measures of vigilance and behavior in ADHD children from the poor and good sleep groups.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Gruber, Dr. Grizenko, Mr. Schwartz, Ms. Bellingham; Ms. Guzman, and Dr. Joober have indicated no financial conflicts of interest.
REFERENCES
- 1.Fischer M, Barkley R, Smallish L, Fletcher K. Executive functioning in hyperactive children as young adults: attention, inhibition, response perseveration, and the impact of comorbidity. Dev Neuropsychol. 2005;27:107–33. doi: 10.1207/s15326942dn2701_5. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention; National Center on Birth Defects and Developmental Disabilities, editor. Atlanta, GA: NCBDDD publication; 2001. Attention deficit/hyperactivity disorder - A public health perspective. 01-0602. [Google Scholar]
- 3.Satterfield J, Cantwell D. Proceedings: CNS function and response to methylphenidate in hyperactive children. Psychopharmacol Bull. 1974;10:36–7. [PubMed] [Google Scholar]
- 4.Sergeant J. Modeling Attention-Deficit/Hyperactivity Disorder: a critical appraisal of the cognitive-energetic model. Biol Psychiatry. 2004;57:1248–55. doi: 10.1016/j.biopsych.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Zentall S, Zentall T. Optimal stimulation: a model of disordered activity and performance in normal and deviant children. Psychol Bull. 1983;94:446–471. [PubMed] [Google Scholar]
- 6.Epstein JN, Erkanli A, Conners CK, Klaric J, Costello JE, Angold A. Relations between Continuous Performance Test performance measures and ADHD behaviors. J Abnorm Child Psychol. 2003;31:543–54. doi: 10.1023/a:1025405216339. [DOI] [PubMed] [Google Scholar]
- 7.Nigg JT. Neuropsychological theory and findings in attention-deficit/hyperactivity disorder: the state of the field and salient challenges for the coming decade. Biol Psychiatry. 2005;57:1424–35. doi: 10.1016/j.biopsych.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Losier BJ, McGrath PJ, Klein RM. Error patterns on the continuous performance test in non-medicated and medicated samples of children with and without ADHD: A meta-analytic review. J Child Psychol Psychiatry. 1996;37:971–88. doi: 10.1111/j.1469-7610.1996.tb01494.x. [DOI] [PubMed] [Google Scholar]
- 9.Barry R, Clarke A, Johnstone S. A review of electrophysiology in Attention-Deficit/Hyperactivity Disorder: I. qualitative and quantitative electroencephalography. Clin Neurophysiol. 2003;114:171–83. doi: 10.1016/s1388-2457(02)00362-0. [DOI] [PubMed] [Google Scholar]
- 10.Golan N, Shahar E, Ravid S, Pillar G. Sleep disorders and daytime sleepiness in children with Attention-Deficit/Hyperactive Disorder. Sleep. 2004;27:261–6. doi: 10.1093/sleep/27.2.261. [DOI] [PubMed] [Google Scholar]
- 11.Lecendreux M, Konofal E, Bouvard M, Falissard B, Mouren-Simeoni M. Sleep and alertness in children with ADHD. J Child Psychol Psychiatry. 2000;41:803–12. [PubMed] [Google Scholar]
- 12.Palm L, Persson E, Bjerre I, Elmqvist D, Blennow G. Sleep and wakefulness in preadolescent children with deficits in attention, motor control and perception. Acta Paediatr. 1992;81:618–24. doi: 10.1111/j.1651-2227.1992.tb12313.x. [DOI] [PubMed] [Google Scholar]
- 13.Clarke A, Barry A, Bond D, McCarthy R, Selikowitz M. Effects of stimulant medications on the EEG of children with attention-deficit/hyperactivity disorder. Psychopharmacology. 2000;164:277–84. doi: 10.1007/s00213-002-1205-0. [DOI] [PubMed] [Google Scholar]
- 14.Hermens D, Williams L, Clarke S, Kohn M, Cooper N, Gordon E. (2005). Responses to methylphenidate in adolescent AD/HD: Evidence from concurrently recorded autonomic (EDA) and central (EEG and ERP) measures. Int J Psychophysiol. 2005;58:21–33. doi: 10.1016/j.ijpsycho.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Swartwood M, Swartwood J, Lubar J, Timmermann D, Zimmerman A, Muenchen R. Methylphenidate effects on EEG, behavior, and performance in boys with ADHD. Pediatr Neurol. 1998;18:244–50. doi: 10.1016/s0887-8994(97)00205-1. [DOI] [PubMed] [Google Scholar]
- 16.Lazzaro I, Gordon E, Whitmont S, et al. Quantitative EEG activity in adolescent Attention-Deficit/Hyperactivity Disorder. Clin Electroencephalogr. 1998;29:37–42. doi: 10.1177/155005949802900111. [DOI] [PubMed] [Google Scholar]
- 17.Oades R, Sadile A, Sagvolden T, et al. The control of responsiveness in ADHD by catecholamines: evidence for dopaminergic, noradrenergic and interactive roles. Dev Sci. 2005;8:122–31. doi: 10.1111/j.1467-7687.2005.00399.x. [DOI] [PubMed] [Google Scholar]
- 18.Owens J. The ADHD and sleep conundrum: a review. J Dev Behav Pediatr. 2005;26:312–22. doi: 10.1097/00004703-200508000-00011. [DOI] [PubMed] [Google Scholar]
- 19.O'Brien LM, Holbrood CR, Mervis CB, et al. Sleep and neurobehavioral characteristics of 5 to 7 year old children with parentally reported symptoms of attention-deficit/hyperactive disorder. Pediatrics. 2003;111:554–63. doi: 10.1542/peds.111.3.554. [DOI] [PubMed] [Google Scholar]
- 20.Picchietti DL, Underwood DJ, Farris WA. Further studies on periodic limb movement disorder and restless legs syndrome in children with Attention-Deficit Hyperactivity Disorder. Mov Disord. 1999;14:1000–7. doi: 10.1002/1531-8257(199911)14:6<1000::aid-mds1014>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 1.Ballas CA, Evans DL, Dinges DF. Psychostimulants in psychiatry: amphetamine, methylphenidate, and modafinil. In: Schatzberg AF, Nemeroff CB, editors. Textbook of psychopharmacology. 3rd ed. Arlington American Psychiatric Publishing, Inc; 2004. pp. 671–84. [Google Scholar]
- 22.Koelega HS. Stimulant drugs and vigilance performance: a review. Psychopharmacology. 1993;111:1–16. doi: 10.1007/BF02257400. [DOI] [PubMed] [Google Scholar]
- 23.Bishop C, Roehrs T, Rosenthal L, Roth T. Alerting effects of methylphenidate under basal and sleep-deprived conditions. Exp Clin Psychopharmacol. 1997;5:344–52. doi: 10.1037//1064-1297.5.4.344. [DOI] [PubMed] [Google Scholar]
- 24.Roehrs T, Papineau K, Rosenthal L, Roth T. Sleepiness and the reinforcing and subjective effects of methylphenidate. Exp Clin Psychopharmacol. 1999;7:145–50. doi: 10.1037//1064-1297.7.2.145. [DOI] [PubMed] [Google Scholar]
- 25.Roehrs T, Johanson CE, Meixner R, Turner L, Roth T. Reinforcing and subjective effects of methylphenidate: dose and time in bed. Exp Clin Psychopharmacol. 2004;12:180–9. doi: 10.1037/1064-1297.12.3.180. [DOI] [PubMed] [Google Scholar]
- 26.American Psychiatric Association. 4th ed. Washington, DC: American Psychiatric Association; 1994. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 27.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child and Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Achenbach, T . Manual for the Child Behavioral Checklist/4-18 and 1991 Profile. Burlington: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- 29.Conners C, Sitarenios G, Parker J, Epstein J. The revised Comers' Parent Rating Scale (CPRS-R): Factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26:257–68. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- 30.Conners C, Sitarenios G, Parker J, Epstein J. Revision and restandardization of the Conners Teacher Rating Scale (CTRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26:279–91. doi: 10.1023/a:1022606501530. [DOI] [PubMed] [Google Scholar]
- 31.Weschler D. San Antonio: Psychological Corporation; 1991. Manual for the Wechsler Intelligence Scale for Children-Third Edition. [Google Scholar]
- 32.Rapoport J. Rating scales and assessment for use in pediatric psychopharmacology research: Clinical Global Impression. Psychopharmacol Bull. 1985;21:839–41. [PubMed] [Google Scholar]
- 33.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 34.Lichstein KL, Stone KC, Donaldson J, et al. Actigraphy validation with insomnia. Sleep. 2006;29:232–9. [PubMed] [Google Scholar]
- 35.Conners CK. Toronto, Canada: Multi-Health Systems, Inc; 1994. (1994). The Conners Continuous Performance Test. [Google Scholar]
- 36.Bishop C, Roehrs T, Rosenthal L, Roth T. Alerting effects of methylphenidate under basal and sleep-deprived conditions. Exp Clin Psychopharmacol. 1997;5:344–52. doi: 10.1037//1064-1297.5.4.344. [DOI] [PubMed] [Google Scholar]
- 37.Newhouse PA, Belenky G, Thomas M, Thorne D, Sing HC, Fertig J. The effects of d-amphetamine on arousal, cognition, and mood after prolonged total sleep deprivation. Neuropsychopharmacology. 1989;2:153–64. doi: 10.1016/0893-133x(89)90018-3. [DOI] [PubMed] [Google Scholar]
- 38.Cohen-Zion M, Ancoli-Israel S. Sleep in children with attention-deficit hyperactivity disorder (ADHD): a review of naturalistic and stimulant intervention studies. Sleep Med Rev. 2004;8:379–402. doi: 10.1016/j.smrv.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Garland AF, Hough RL, McCabe KM, Yeh M, Wood PA, Aarons GA. Prevalence of psychiatric disorders in youths across five sectors of care. J Am Acad Child Adolesc Psychiatry. 2001;40:409–18. doi: 10.1097/00004583-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Littner M, Kushida C, Wise M, et al. Standards of Practice Committee of the American Academy of Sleep Medicine: Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–21. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]