Abstract
Context:
Sleep disturbances, pain, and inflammation co-occur in various medical conditions, but their interrelationships are poorly understood.
Objective:
We investigated the effects of reduced sleep duration (by approximately 50%) to 4 h/night across 10 days, on peripherally circulating inflammatory mediators. In addition, we tested the prediction that degree of inflammation is quantitatively related to the extent to which pain is increased in response to prolonged sleep restriction.
Design:
Randomized, 16 day controlled in-laboratory study conducted in GCRC.
Methods:
Eighteen volunteers were randomly assigned to either 12 days of sleeping 8 h/night or 4 h/night. Participants rated mood and pain symptoms throughout experimental days. Urine was collected and blood was drawn frequently on the baseline day and after the 10th experimental day for 25 hours.
Outcome Measures:
Levels of plasma interleukin (IL)-6, serum C-reactive protein (CRP), plasma soluble tumor necrosis factor receptor p55 (sTNF-R p55), urinary levels of prostaglandin (PG) metabolites D2 and E2, subjective assessment of pain and tiredness-fatigue.
Results:
IL-6 levels were elevated in the 4-h sleep condition over the 8-h sleep condition (P <0.05). CRP levels showed the same trend as IL-6, but did not differ significantly between groups (P = 0.11). Levels of sTNF-R p55 were unchanged in both groups. PG E2 and 11β-F2α metabolite increased in 4-h sleepers, but did not differ significantly from the 8-h sleepers. Elevated IL-6 levels were strongly associated with increased pain ratings in response to sleep restriction (r = 0.67, P <0.01), and this association could not be explained by elevations in tiredness-fatigue.
Conclusion:
Insufficient sleep quantity may facilitate and/or exacerbate pain through elevations of IL-6. In disorders where sleep disturbances are common, insufficient sleep quantity itself may establish and maintain its co-occurrence with pain and increased inflammation.
Citation:
Haack M; Sanchez E; Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. SLEEP 2007;30(9):1145-1152.
Keywords: Sleep deprivation, inflammation, IL-6, TNF receptor p55, prostaglandin, pain, fatigue
INTRODUCTION
SLEEP DISTURBANCES, PAIN AND INFLAMMATORY PROCESSES ARE FREQUENTLY OBSERVED IN A VARIETY OF MEDICAL DISORDERS AND CONDITIONS, SUCH as depression1 chronic fatigue syndrome,2 fibromyalgia,3 rheumatoid diseases,4 and surgery.5 However, their interrelationships are poorly understood, due to a lack of studies assessing sleep, pain, and inflammation simultaneously.
Recent studies have documented that within the triad of sleep, pain, and inflammation, insufficient sleep quantity itself can cause an increase in inflammation as well as an increase in pain. Several, but not all studies have documented a pain-modulatory effect of total sleep deprivation over a single night 6–8 of selective sleep stage deprivation,9 and recently of sleep restriction10 or fragmentation over several days.11–14
Several studies have addressed changes in cytokine levels in response to sleep loss. Total, but not partial sleep deprivation over 3 nights increased TNF-R p55,15 which is known to modulate the bioavailability of TNF-α and its somnogenic actions.16 Acute sleep loss of up to 3 nights15,17,18 as well as more commonly experienced forms of sleep loss, i.e., sleep reduced by 25%-50% across consecutive days,18,19 have been shown to induce an increase of interleukin-6 (IL-6) and C-reactive Protein (CRP) levels. In addition, increased levels of IL-6 have been found in patients suffering from primary insomnia.20,21
Prostaglandins (PG) mediate some of the cardinal features of inflammation22 and are elevated in the rats' cerebrospinal fluid in response to sleep deprivation.23 In humans, some indirect evidence supports an influence of sleep loss on PG production. Higher PGE2 production from stimulated whole blood has been found in patients with insomnia compared to healthy controls.24 In addition, higher serum levels of PG D synthase, an enzyme responsible for the synthesis of PGD2 in the brain, have been reported to be associated with a higher degree of excessive daytime sleepiness in narcoleptic patients.25 Pain is a hallmark of inflammatory processes. Prostaglandins, in particular PGE2, are classical pain mediators, but in the last decade a variety of novel pain modulators have been identified. For example, proinflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α) have been found to be potent pain-inducing and pain-facilitating agents, able to sensitize primary sensory neurons.26 We recently showed that 12 days of sleep restriction to 50% of the habitual sleep time led to an increase in subjective pain ratings when compared to subjects sleeping a regular amount of time.10 In sum, prostaglandins and proinflammatory cytokines are both influenced by the quantity of sleep and are involved in the experience of pain.
In the present study we examined whether prolonged sleep restriction elevates urinary levels of prostaglandins and plasma IL-6, plasma sTNF-R p55, and serum CRP levels, and whether the degree of inflammation is quantitatively related to the extent to which pain is reported in response to prolonged sleep restriction.
METHODS
Subjects
The study was approved by the Institutional Review Board for the Protection of Human Subjects at the Beth Israel Deaconess Medical Center. Subjects were recruited by advertisement in newspapers and websites covering the Greater Boston area. Following informed consent, the subjects underwent comprehensive screening and were included if they had no current or past history of psychiatric, neurological, immune, cardiovascular, or sleep disorders; no history of drug dependence/abuse. Subjects were between the ages 21 and 40 years, were nonsmokers and had normal blood chemistry (complete and differential blood counts, T-cell subsets, glucose, creatinine, sodium, potassium, thyroid stimulating hormone) and negative blood and urine toxicology; no shift work and time zone changes during the last 3 months; body mass index (BMI) (kg/m2) <29; average sleep duration between 6.5 and 9 hours as evaluated by a sleep log period of ≥10 days, and no current signs of sleep disorders as evaluated by polysomnography, recorded during the adaptation night. Participants received monetary compensation for participation in the study.
The current study is part of a larger study (N = 40) on the effects of sleep loss on host response in a model of endotoxin challenge. In the current study, only those participants receiving placebo were included (N = 18).
Study Protocol
Subjects stayed for 16 days and 15 nights in a private room in the General Clinical Research Center (GCRC). After an adaptation and baseline day with an 8-h sleep opportunity per night (23:00 to 07:00), subjects were randomized (block design) to an 8-h sleep opportunity per night (23:00 to 07:00) or a 4-h sleep opportunity per night (23:00 to 03:00) for the following 12 days. In the 4-h sleep condition, subjects stayed awake in bed from 03:00 to 07:00 in a semi-supine position under dimmed light conditions (<40 lux). In both sleep conditions, subjects were accompanied by a trained person during all scheduled waking periods to ensure maintenance of wakefulness. The subjects' usual activities during their stay in the GCRC were talking, reading, writing, watching movies, and playing board games. Subjects were encouraged to maintain their daily pre-study activity levels by going to the hospital fitness center, except on the blood drawing days. They were able to maintain social relationships by having access to telephone and e-mail and being allowed visitors except on blood drawing days. Caloric intake was controlled and caloric- and electrolyte-balanced meals were served at 08:00, 12:00, 18:00, and 21:30 (breakfast, lunch, dinner, and light snack). In addition, a very light snack consisting of one arrowroot cookie and 120 mL of apple juice (80 kcal; 2.1–5.6% of daily caloric intake) was served at 05:00 in the morning in the 4-h group. Every 2 hours during the waking periods of the study, except the blood drawing days, subjects had a 20-minute computerized test battery for the assessment of mood, physical symptoms, and performance.
On the baseline day (day 0) and after 10 days of sleeping either 4 h/night or 8 h/night (experimental day 11), blood, urine, and physiological data were collected throughout a 24-h period. At 09:00 on both physiological recording days (day 0 and day 11), an intravenous 18-gauge catheter was positioned into a superficial forearm vein and kept patent with a heparinized saline drip (30 mL/h).
On the baseline and the 11th experimental day, less than 100 mL and approximately 300 mL, respectively, was drawn at varying frequency, depending on the planned analyte (usually in 15- to 60-min intervals), transferred into K3-EDTA tubes or Corvac tubes for plasma or serum extraction, respectively, immediately set on ice before centrifuging at 4 °C at 2600 g for 10 min, and stored at −80 °C until assaying.
The total amount of each urine void was measured, and a portion transferred to a sterile 80 mL container and stored at −20 °C. Urine was combined to 24-h collections across baseline day and day 11, respectively. Therefore, a proportional amount of all urine collects from either baseline day or day 11 was transferred into a new sterile container, pipetted into ten 4 mL tubes after thoroughly mixing and again stored at −20 °C. Accordingly, all PGs were measured in one-time thawed urine samples.
On day 11, at 23:00, 40 participants were randomized to an endotoxin or placebo challenge. The current paper only includes subjects who received placebo (N = 18).
To estimate total sleep time, subjects wore an actigraph (Mini-Mitter, Bend, OR) on their nondominant wrist throughout the study period. Due to technical problems, data were not available for one subject in the 4-h sleep condition.
Assessment of Subjective Pain and Tiredness-Fatigue
Subjects rated their current level of emotional and physical well-being on 108 computerized visual analog scales (VAS) presented every 2 hours during the waking periods of the protocol, except the blood drawing periods. Subjects used the arrow keys to slide the cross hatch on the scale either to the right or the left, and the rating was stored on the computer in measurement units between 1 and 100. Data were further processed with factor analysis, and could be optimally described by 4 factors. Adjectives were assigned to thematic groupings when they demonstrated a unique loading pattern (≥0.30 difference from other loadings), with a weighting of ≥0.50. These thematic grouping factors were used for subsequent statistical analysis (for details, see Haack & Mullington, 2005). From the 4 factors extracted by factor-analysis, those labeled as bodily discomfort and tiredness-fatigue were used for correlational analyses in the present paper. Bodily discomfort compiled all pain-related items, e.g., headache, back pain, muscle pain, joint pain, stomach pain, and generalized body pain. Tiredness-fatigue compiled items such as drowsy, sluggish, worn out, mentally or physically tired, exhausted, and fatigued. Ratings of bodily discomfort and tiredness-fatigue were averaged across the daytime period (11:20 to 21:20, 6 tests total) after the last baseline blood draw, and across the same time period after 12 days of having a sleep opportunity of either 4 h/night or 8 h/night.
Assays
IL-6 was measured in plasma with a high sensitivity ELISA (Quantikine HS, R&D Systems, Minneapolis, MN). Our intra- and inter-assay coefficient of variations (CVs) were 5.5%±4.3% and 5.0%±0.5%, respectively. Sensitivity was 0.039 pg/mL. Samples were 2-fold diluted to capture values up to 20.0 pg/mL, and were run in duplicates. To control for IL-6 increases induced by catheter changes,27 values one hour before and after catheter change were excluded from statistical analysis. In the 8-h sleep condition, one catheter change occurred on the baseline day, and 2 changes on day 11. No catheter changes occurred in the 4-h sleep condition. This excluded 3 out of 216 IL-6 samples from statistical analysis, equaling 1.4% of data points.
CRP was measured in serum with the high sensitivity assay (Immulite, DPC, Los Angeles, CA). Sensitivity was <0.02 mg/dL. Within-run and total assay precision were both ≤8.7%, at concentrations between 0.023 and 0.32 mg/dL. IL-6 and CRP were measured every 4 hours (10:35, 14:35, 18:35, 22:35, 02:35, 06:35) on baseline and after 10 days with a sleep opportunity of either 4 h or 8 h/night.
Soluble tumor necrosis factor receptor p55 (sTNF-R p55) was measured in plasma with an ELISA (Quantikine ELISA, R&D Systems, Minneapolis, MN). Our intra- and inter-assay CVs were 5.0%±3.7% and 6.9%±18.2%. Sensitivity was 0.77 pg/mL. Samples were assayed in duplicates.
Prostaglandin E2 (PGE2) metabolite and 11α-PGF2α (a primary and active metabolite of PGD2 in vivo, in the following text referred to as PGD2 metabolite) were measured in 24-h urine collection with an Enzyme Immunoassay (EIA, Cayman Chemical, Ann Arbor, MI). For PGE2 and PGF2α, a 20- and 10-fold dilution, respectively, was found to be optimal. Our intra- and inter-assay CVs were 5.7%±3.6% and 12.5%±6.6% for PGE2, respectively, and 6.5%±4.8% and 12.8%±31.4% for PGF2α, respectively. Detection limits were 2 pg/mL and 5.5 pg/mL for PGE2 and PGF2α, respectively. All PG samples were run in triplicates. PGE2 and F2α metabolite were measured in one-time thawed samples.
Creatinine was determined in urine by the hospital lab using the spectrophotometric, kinetic Jaffe reaction. Sensitivity was 0.2 mg/dL, and inter- and intra-assay CVs were less than 2%. Levels of PGE2 and D2 metabolite were expressed as amount per mg creatinine.
Statistics
General linear model (GLM) for repeated measures was used for cytokine analysis with time (10:35, 14:35, 18:35, 22:35, 02:35, 06:35 of difference values [day 11 minus baseline]) as within-subject factor and sleep condition (4 h of sleep/night vs. 8 h of sleep/night) as between-subject factor. Values were averaged across days when the time by condition interaction effect was nonsignificant. For 24-h PG values and ratings of pain and fatigue, difference data (day 11 minus baseline) were computed and processed with univariate GLM. Pearson correlation coefficient and partial correlation were used for examining strength of associations between variables. Effect sizes (ES) were calculated using Cohen's formula.28
Due to positive skewed distribution, IL-6, sTNF-R p55, CRP, and subjective rating data were each normalized using log-transformation before entering into models.
Data in text are presented as mean±SD; data in graphs are presented as mean±SEM.
Alpha level of rejection was set to P ≤0.05.
RESULTS
Eighteen subjects (6 females, 12 males) between the ages 21 and 40 (27.3±5.8 years) with BMI 23.1±3.3 were included in the study. Subjects were randomly assigned to the 4-h sleep condition (N = 10) and to the 8-h sleep condition (N = 8). The 4-h and the 8-h sleep groups did not differ in terms of female/male ratio (4/6 vs. 2/6, respectively, P = 0.44), age (26.4±5.2 vs. 28.4±6.6 yr; P = 0.49), BMI (23.3±3.6 vs. 22.9±2.9 kg/m2; P = 0.80), and average sleep duration (based on sleep logs kept for ≥10 days) before entering the study (8.0±0.5 vs. 8.0±0.4 h; P = 0.80).
Actigraphic estimation of total sleep time during the baseline sleep period (Day 0) was 07:10h±27 min and 7:18±27 min for the 4-h group and 8-h group, respectively, and 3:39± 11 min and 6:42±29 min averaged across the following 12 days in the 4-h group and 8-h group, respectively.
Motor activity averaged across experimental overall days did not differ between the 4-h group and 8-h group (F1,15 = 0.19, n.s.). As expected, nighttime (23:00–07:00) motor activity was higher in the 4-h group than the 8-h group (F1,15 = 10.38, P <0.01).
Caloric intake averaged across experimental days did not differ between the 4-h and 8-h group (2433±265 vs. 2515±447 kCal in the 4-h vs 8-h group, respectively; F1,16 = 0.24, n.s.). There was not difference between the 4-h and 8-h group in carbohydrate, fat, or protein intake (all F1,16<0.50, n.s.).
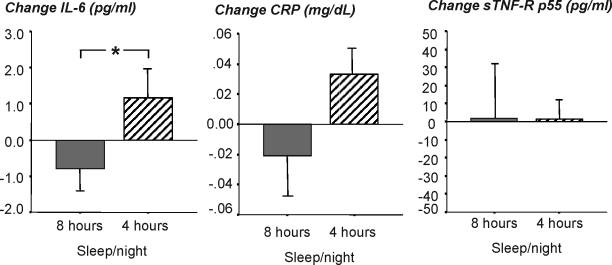
Change of IL-6 plasma levels from baseline to the 11th experimental day of sleeping 4 h/night or 8 h/night showed a significant sleep condition effect (4 h/night vs. 8 h/night; F1,16 = 4.98, P = 0.04; ES = 0.92), but no time effect (timepoints 10:35, 14:35, 18:35, 22:35, 02:35, and 06:35; F5,80 = 1.16, P = 0.34) or time by sleep condition interaction effect (F5,80 = 1.63, P = 0.2) was detected. Therefore, IL-6 values were averaged across 24-h periods, and as can be seen in figure 2, the sleep condition effect was due to an increase of IL-6 levels from 1.88±0.85 pg/mL on baseline to 3.04±2.83 pg/mL on Day 11 in the 4-h sleep condition (F1,9 = 2.54, P = 0.15), and a decrease of IL-6 levels from 3.15±1.33 pg/mL on baseline to 2.36±1.36 pg/mL on Day 11 in the 8-h sleep condition (F1,7 = 2.42, P = 0.16).
Figure 2.
Change of plasma IL-6, serum CRP, and plasma sTNF-R p55 levels from baseline to the 11th day of sleeping either 8 h/night (grey bar, N=8) or 4 h/night (hatched bar, N=10 for IL-6, N=9 for sTNF-R p55). IL-6, CRP, and sTNF-R p55 were measured every 4 h and averaged across a 24-h period. Original values are presented, and statistics were based on log-transformed values. Asterisk indicates significant difference between sleep conditions.
Change of CRP serum levels from baseline to the 11th experimental day of sleeping 4h/night or 8h/night showed a pattern similar to change of IL-6, but effects did not reach significance (F1,16 = 2.84, P = 0.11 for sleep condition effect; F5,80 = 0.64, P = 0.6 for interaction effect. ES = 0.80). As shown in figure 2, CRP levels in the 4-h sleepers nonsignificantly increased from 0.034±0.027 mg/dL at baseline to 0.069±0.076 mg/dL on Day 11 (F1,9 = 3.01, P = 0.12), and decreased in the 8-h sleepers from 0.082±0.087 on baseline to 0.061±0.054 mg/dL on Day 11 (F1,7 = 0.60, P = 0.47).
Soluble TNF-R p55 levels could not be analyzed in one subject in the 4h-sleep condition due to insufficient amounts of plasma at relevant time points. Change of sTNF-R p55 plasma levels from baseline to the 11th experimental day of sleeping 4h/night or 8h/night showed no significant effects of sleep condition (F1,15 = 0.00, n.s.), time (F5,75 = 1.27, n.s.), or time by condition interaction (F5,75 = 0.14, n.s.). In the 4-h sleep condition, sTNF-R p55 values averaged across 24-h periods were 587±225 pg/mL at baseline and 588±237 pg/mL on day 11. In the 8-h sleep condition, sTNF-R p55 values averaged across 24-h periods were 641±151 pg/mL at baseline and 642±138 pg/mL on day 11 (see figure 2).
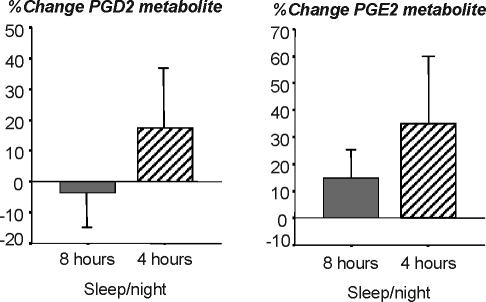
Urinary Prostaglandins: Large inter-individual differences were found for basal 24h-levels of PGD2 metabolite (range: 229–2937 pg/mg creatinine; mean±SD: 846±635 pg/mg creatinine) and PGE2 metabolite (range 230–1242 pg/mg creatinine; mean ± SD: 638±344 pg/mg creatinine). PGD2 metabolite increased from 729±405 to 796±540 pg/mL creatinine in the 4h-sleepers, and decreased from 994±851 to 837±501 pg/mL creatinine in the 8-h sleepers from baseline to day 11. PGE2 metabolite increased from 639±381 to 767±428 pg/mL creatinine in the 4-h sleepers, and increased from 639±320 to 757±515 pg/mL creatinine in the 8-h sleepers from baseline to day 11. Due to the large inter-individual differences, values were computed as percent change from baseline. As shown in figure 1, PGD2 metabolite levels increased nonsignificantly by 17.3%±64.2% in the 4-h sleep condition and slightly decreased by 3.5%±33.0% in the 8-h sleep condition (F1,16 = 0.68), n.s. for protocol effect). PGE2 metabolite levels increased nonsignificantly by 35.0%±80.5% in the 4-h sleep condition and by 15.0%±33.4% in the 8-h sleep condition (F1,16 = 0.43), n.s.).
Figure 1.
Percent change of urinary levels of PG E2 metabolite and the D2 metabolite 11β-PGF2α from baseline to the 11th day of sleeping either 8 h/night (grey bar, N=8) or 4 h/night (hatched bar, N=10). PGs were measured in 24-h urine collection. Values are presented and at percent change from baseline due to large inter-individual variability (see text for original values).
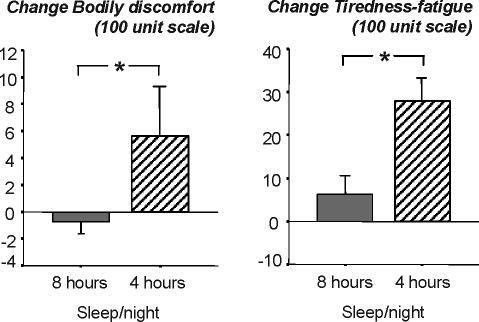
Bodily discomfort increased from baseline to the 12th day by 5.6%±12.0% in the 4-h sleep condition, and slightly decreased by −0.8%±2.0% in the 8-h sleep condition (F1,16 = 4.44, P = 0.05 for interaction effect between day and sleep condition; ES = 0.91, see figure 3).
Figure 3.
Change of subjective reports of bodily discomfort and tiredness-fatigue from baseline to the 11th day of sleeping either 8 h/night (grey bar, N=8) or 4 h/night (hatched bar, N=10). Bodily discomfort and tiredness-fatigue were assessed every 2 h throughout the waking periods of the protocol and averages across a 24-h period. Original values are presented, and statistics were based on log-transformed values. Asterisk indicates significant difference between sleep conditions.
Tiredness-fatigue increased from baseline to the 12th experimental day by 27.9%±18.5% in the 4-h sleep condition, and by 6.3%±12.3% in the 8-h sleep condition by (F1,16 = 7.97, P = 0.01 for interaction effect between day and sleep condition; ES = 1.40; see figure 3).
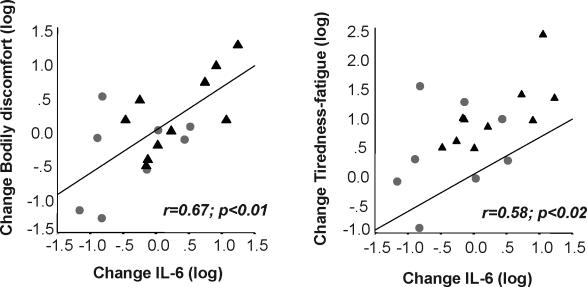
Change in IL-6 levels from baseline to the 11th day was significantly correlated with change in bodily discomfort (r = 0.67, P = 0.003; ES = 1.8) and change in tiredness-fatigue (r = 0.58, P = 0.01, ES = 1.4, see Figure 4). This indicates that increased IL-6 levels were associated with increased ratings of bodily discomfort as well as tiredness-fatigue. Change in bodily discomfort was significantly associated with change in tiredness-fatigue (r = 0.55, P = 0.02; ES = 1.3). Change in IL-6 levels remained significantly correlated with change in bodily discomfort after controlling for change in tiredness-fatigue (r = 0.51, df = 15, P = 0.04, ES = 1.3). This indicates that the IL-6 – pain association was not merely due to change in tiredness-fatigue. However, change in IL-6 levels was not significantly correlated with change in tiredness-fatigue anymore after controlling for change in bodily discomfort (r = 0.34, df = 15, P = 0.2, ES = 1.2). This result indicates that bodily discomfort partially accounts for the positive relationship between IL-6 and tiredness-fatigue.
Figure 4.
Correlation between change in IL-6 and change in bodily discomfort (left) and tiredness-fatigue (right) from baseline to the 11th day of sleep 4 h/night (▴) or 8 h/night ( ). Log-transformed data are presented.
). Log-transformed data are presented.
Changes in CRP, sTNF-R p55, PGD2, and PGE2 metabolite levels did not significantly correlate with change in bodily discomfort, change in tiredness-fatigue, or change in IL-6 levels (all r <0.24, n.s.).
DISCUSSION
The current study shows that sleep restriction to 50% of the habitual time over 10 days induces significant increases of IL-6 plasma levels. Importantly, the degree of IL-6 elevation was associated with the extent to which bodily discomfort increased in response to sleep restriction. We also found nonsignificant elevations of serum CRP levels as well as urinary PGE2 metabolite 11β-PGF2α, one of the major D2 metabolites.
Up-regulation of proinflammatory cytokines has been suggested as one of the most important factors linked to the development of chronic pain.29 Based on animal studies of inflammation, exogenous administration of IL-6 and other proinflammatory cytokines can produce pain and hyperalgesia, and their neutralization and antagonism reduces pain and hyperalgesia.30 The role of proinflammatory cytokines, in particular IL-6, in the physiology of human pain has been less well investigated. In pain-related disorders, such as rheumatoid arthritis, cytokine levels of IL-1, TNF-α and IL-6 have been shown to be upregulated in the synovial fluid and serum, and their blockade using monoclonal antibodies or receptor fusion proteins holds therapeutic promise.31 A quantitative relationship between pain complaints and cytokine levels has been recently reported in patients suffering from naturally occurring infections such as Q-fever. In these patients, the degree of headache and myalgia was positively correlated with IL-6 levels spontaneously released from PBMCs.32 The present results show that the connection between cytokines and pain may exist beyond the context of infectious and inflammatory diseases, and suggest that in healthy, pain-free volunteers, small peripheral changes of IL-6 levels of approximately 1 pg/mL may be involved in the onset or facilitation of pain during prolonged phases of insufficient sleep.
Similar to IL-6, which participates in controlling the production of CRP, CRP serum levels were elevated after sleeping after sleeping 4 h/night for 10 days. CRP production was reported to increase in a study where subjects slept 4 h/night for 10 nights,18 and the results reported here show a trend to replicate, for if a one-tailed test was used, the P-value for the daily CRP difference between 8 and 4 hours of sleep per night would have been P = 0.055. Differences in the magnitude of CRP elevations between a previous18 and the current study may be due to individual differences or to placement of the sleep period, e.g., beginning (23:00–03:00) or middle (01:00–05:00) of the night
Ten days of partial sleep deprivation did not affect levels of sTNF-R p55 in the current study. This is in accordance with Shearer et al,15 who showed that sTNF-R p55 levels did not alter across 3 days of partial sleep deprivation, but increased with a greater accumulation of a sleep deficit, e.g., 3 nights of total sleep deprivation. Although sTNF-R p55 may influence central pain processing mechanisms in patients suffering from pain-related disorders,33 the present results failed to find sTNF-R p55 elevations in response to sleep restriction. Considering various reports on the connection between the TNF system and sleep in animals, one may ask whether TNF-α, rather than its soluble receptors, may have responded to prolonged sleep restriction in the current study. We did a cross-sectional analysis of a single time point measure by using a high-sensitivity TNF-α assay in a subset of subjects, but did not find a difference between those sleeping 4 h or 8 h for 10 consecutive days. This is consistent with another report that failed to find a significant sleep deprivation effect of TNF-α in humans.15
The presence of painful, physical symptoms is generally associated with the presence of fatigue.34 Accordingly, a significant association between ratings of fatigue-tiredness and bodily discomfort has been found in the present study. In the context of an immune challenge with endotoxin in humans, higher IL-6 levels have been found to be associated with lower amounts of NREM sleep.35,36 In the context of sleep loss, fatigue has been suggested to result from cytokine elevation,17,19 and the present study adds further support to this hypothesis by showing a quantitative relationship between increases of IL-6 and increases in reports of fatigue-tiredness in response to prolonged sleep restriction. Based on the interrelationships between IL-6 levels, fatigue-tiredness, and bodily discomfort, it is reasonable to assume that tiredness-fatigue is a potential mediator of the relation between IL-6 levels and bodily discomfort. However, the IL-6-bodily discomfort association remained significant after controlling for the influence of tiredness-fatigue, suggesting a relationship between IL-6 and pain that is not primarily mediated by changes in tiredness-fatigue.
Ten days of sleep restriction to 50% of the habitual time led to an IL-6 increase of 1.16 pg/mL in the current study. Sleep restriction to approximately 25% of usual sleep time over one week has been found to lead to a slightly smaller IL-6 increase of 0.75 pg/mL compared to the present result.19 This may suggest a dose-response relationship between chronicity/severity of sleep restriction and elevation of IL-6 levels, and warrants further investigation. There is ample evidence coming from animal studies that cytokines produced in the periphery can gain access to the brain via different routes,37 and more importantly, it seems that even slight changes in peripheral cytokine production are able to affect brain functions in humans.38
While IL-6 and CRP levels increase across 10 days of sleeping 4 h/night, both parameters slightly decrease in the control condition of 8 h sleep/night. This decrease in inflammatory markers in the control condition may be due to general study requirements, including regularity of food intake and sleep-wake schedule, and/or to the in-hospital environment that eliminates confrontation with daily stressors and may also go along with a more sedentary life style, although participants in the current study were requested to maintain their pre-study workout frequency while in the hospital. It has been shown that hospitalization has a blood pressure (BP) lowering effect39 and in participants permitted 4 h sleep/night in the current study showed elevated BP over participants permitted 8 h sleep/night.40
Small, but prolonged upregulation of inflammatory markers may have negative health consequences. Elevations of IL-6 levels in the range of 1 pg/mL have been shown to be related to increased risk of cardiovascular disease,41 cancer,42 diabetes,43 and may be associated with the onset and facilitation of pain, as suggested by the present and other findings (reviewed in 44). Small elevations of IL-6 levels have been also repeatedly reported in depressive disorders.45,46 The current findings suggest that elevated IL-6 levels may contribute to the high prevalence of pain complaints in depressive disorders,47 and that both increased inflammation and pain may result from sleep disturbances, which frequently occur in depressed patients.1 Indeed, an association between severity of sleep disturbance and IL-6 levels has been recently demonstrated in major depressive disorder, suggesting a contributing role of sleep disturbances in IL-6 elevations.48
Unexpectedly, the increase we found in urinary levels of PGE2 metabolite and of the D2 metabolite 11β-PGF2α was not significant after 10 days of sleep restriction. Several prostaglandins have been shown to be critically involved in sleep-wake regulation,49 but evidence is coming almost exclusively from animal studies. In response to acute sleep deprivation, production of PGD2 as well as E2 and F2α has been found upregulated in different brain areas of the rat.23 A recent human study showed that during a single night of sleep deprivation serum lipocalin-type PGD synthase, which is responsible for the biosynthesis of PGD2 in the brain, was suppressed compared to participants with an 8-h sleep opportunity, but values appeared to be increased throughout the vigil, but this was not analyzed.50 The current study is the first that measured urinary prostaglandin levels in response to prolonged sleep restriction. Prostaglandin E2 and D2 can be produced by various cells, including macrophages, in response to various physiological and inflammatory stimuli, such as cytokines.51 They are rapidly metabolized and their metabolites can be found in urine in much higher quantities than in plasma. However, urinary prostaglandin measures reflect a combination of PGs coming from the blood stream as well as PGs originated in the kidney.52 The nonsignificant findings of urinary PG levels in the current study may be related to a high and constant PG production in the kidney, which may override potential PG changes coming from the bloodstream. More importantly, this study was not powered on PG changes in response to sleep restriction. Post hoc analysis revealed power of less than 30% to detect an effect. Accordingly, studies with adequate sample size are needed to properly examine whether PG levels change in response to prolonged sleep restriction.
Taken together, the current findings suggest that small increases in cytokines, e.g., IL-6, may provide a pathway by which insufficient sleep may facilitate and/or exacerbate pain. Thus, in disorders where sleep disturbances are common, such as depression and immune-related diseases, insufficient sleep quantity itself may play a substantial role in establishing and maintaining the co-occurrence with pain and increased inflammatory markers.
ACkNOWLEDGMENTS
This work was supported by the National Institutes of Health to J.M.M. (MH60641), and in part to the General Clinical Research Center of the Beth Israel Deaconess Medical Hospital (RR01032).
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Mullington has participated in a speaking engagement for Takeda; has participated in an educational meeting for Sanofi-Aventis; and has participated in a board to create an educational fellowship for Jazz Pharmaceuticals. Dr. Haack and Ms. Sanchez have indicated no financial conflicts of interest.
REFERENCES
- 1.Benca RM, Okawa M, Uchiyama M, et al. Sleep and mood disorders. Sleep Med Rev. 1997;1:45–56. doi: 10.1016/s1087-0792(97)90005-8. [DOI] [PubMed] [Google Scholar]
- 2.Jason LA, Richman JA, Rademaker AW, et al. A community-based study of chronic fatigue syndrome. Arch Intern Med. 1999;159:2129–37. doi: 10.1001/archinte.159.18.2129. [DOI] [PubMed] [Google Scholar]
- 3.Menefee LA, Cohen MJ, Anderson WR, Doghramji K, Frank ED, Lee H. Sleep disturbance and nonmalignant chronic pain: a comprehensive review of the literature. Pain Med. 2000;1:156–72. doi: 10.1046/j.1526-4637.2000.00022.x. [DOI] [PubMed] [Google Scholar]
- 4.Bloom BJ, Owens JA, McGuinn M, Nobile C, Schaeffer L, Alario AJ. Sleep and its relationship to pain, dysfunction, and disease activity in juvenile rheumatoid arthritis. J Rheumatol. 2002;29:169–73. [PubMed] [Google Scholar]
- 5.Raymond I, Ancoli-Israel S, Choiniere M. Sleep disturbances, pain and analgesia in adults hospitalized for burn injuries. Sleep Med. 2004;5:551–9. doi: 10.1016/j.sleep.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Cooperman NR, Mullin FJ, Kleitman N. Studies on the physiology of sleep. XI. Further observations on the effects of prolonged sleeplessness. Am J Physiol. 1934;107:589–93. [Google Scholar]
- 7.Kundermann B, Spernal J, Huber MT, Krieg JC, Lautenbacher S. Sleep deprivation affects thermal pain thresholds but not somatosensory thresholds in healthy volunteers. Psychosom Med. 2004;66:932–7. doi: 10.1097/01.psy.0000145912.24553.c0. [DOI] [PubMed] [Google Scholar]
- 8.Onen SH, Alloui A, Gross A, Eschallier A, Dubray C. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2001;10:35–42. doi: 10.1046/j.1365-2869.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- 9.Moldofsky H, Scarisbrick P, England R, Smythe H. Musculosketal symptoms and non-REM sleep disturbance in patients with “fibrositis syndrome” and healthy subjects. Psychosom Med. 1975;37:341–51. doi: 10.1097/00006842-197507000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain. 2005;119:56–64. doi: 10.1016/j.pain.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30:494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 12.Moldofsky H, Scarisbrick P. Induction of neurasthenic musculoskeletal pain syndrome by selective sleep stage deprivation. Psychosom Med. 1976;38:35–44. doi: 10.1097/00006842-197601000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Older SA, Battafarano DF, Danning CL, et al. The effects of delta wave sleep interruption on pain thresholds and fibromyalgia-like symptoms in healthy subjects; correlations with insulin-like growth factor I. J Rheumatol. 1998;25:1180–6. [PubMed] [Google Scholar]
- 14.Lentz MJ, Landis CA, Rothermel J, Shaver JL. Effects of selective slow wave sleep disruption on musculoskeletal pain and fatigue in middle aged women. J Rheumatol. 1999;26:1586–92. [PubMed] [Google Scholar]
- 15.Shearer WT, Reuben JM, Mullington JM, et al. Soluble TNF-α receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–70. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- 16.Aderka D. The potential biological and clinical significance of the soluble tumor necrosis factor receptors. Cytokine Growth Factor Rev. 1996;7:231–40. doi: 10.1016/s1359-6101(96)00026-3. [DOI] [PubMed] [Google Scholar]
- 17.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab. 1999;84:2603–7. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- 18.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 19.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–26. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 20.Vgontzas AN, Zoumakis M, Papanicolaou DA, et al. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism. 2002;51:887–92. doi: 10.1053/meta.2002.33357. [DOI] [PubMed] [Google Scholar]
- 21.Burgos I, Richter L, Klein T, et al. Increased nocturnal interleukin-6 excretion in patients with primary insomnia: a pilot study. Brain Behav Immun. 2006;20:246–53. doi: 10.1016/j.bbi.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Griffith R. Prostaglandins and inflammation. In: Gallin J, Synderman R, editors. Inflammation: basic principles and clinical correlates. Philadelphia: Lippincott Williams and Wilkins; 1999. pp. 349–60. [Google Scholar]
- 23.Ram A, Pandey HP, Matsumura H, et al. CSF levels of prostaglandins, especially the level of prostaglandin D2, are correlated with increasing propensity towards sleep in rats. Brain Res. 1997;751:81–9. doi: 10.1016/s0006-8993(96)01401-1. [DOI] [PubMed] [Google Scholar]
- 24.Song C, Lin AH, Bonaccorso S, et al. The inflammatory response system and the availability of plasma tryptophan in patients with primary sleep disorders and major depression. J Affect Disord. 1998;49:211–9. doi: 10.1016/s0165-0327(98)00025-1. [DOI] [PubMed] [Google Scholar]
- 25.Jordan W, Tumani H, Cohrs S, et al. Narcolepsy - Increased L-PGDS (beta-trace) levels correlate with excessive daytime sleepiness but not with cataplexy. J Neurol. 2005;252:1372–8. doi: 10.1007/s00415-005-0870-4. [DOI] [PubMed] [Google Scholar]
- 26.McMahon SB, Cafferty WB, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol. 2005;192:444–62. doi: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Haack M, Kraus T, Schuld A, Dalal M, Koethe D, Pollmächer T. Diurnal variations of interleukin-6 plasma levels are confounded by blood drawing procedures. Psychoneuroendocrinology. 2002;27:921–31. doi: 10.1016/s0306-4530(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 28.Cohen J. Statistical power analysis for the behavioral sciences. Revised edition. New York: Academic Press; 1977. [Google Scholar]
- 29.Poole S, Woolf CJ. Cytokine-nerve growth facor interactions in inflammatory hyperalgesia. In: Watkins LR, Maier SF, editors. Cytokines and pain. Basel: Birkhäuser; 1999. pp. 89–132. [Google Scholar]
- 30.McMahon SB, Bennett DLH, Bevan S. Inflammatory mediators and modulators of pain. In: McMahon SB, Koltzenburg M, editors. Textbook of pain. London: Elsevier; 2005. [Google Scholar]
- 31.Feldmann M, Maini RN. Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19:163–96. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- 32.Vollmer-Conna U, Fazou C, Cameron B, et al. Production of pro-inflammatory cytokines correlates with the symptoms of acute sickness behaviour in humans. Psychol Med. 2004;34:1289–97. doi: 10.1017/s0033291704001953. [DOI] [PubMed] [Google Scholar]
- 33.Maihöfner C, Handwerker HO, Neundorfer B, Birklein F. Mechanical hyperalgesia in complex regional pain syndrome: a role for TNF-alpha? Neurology. 2005;65:311–3. doi: 10.1212/01.wnl.0000168866.62086.8f. [DOI] [PubMed] [Google Scholar]
- 34.Reyes-Gibby CC, Mendoza TR, Wang S, Anderson KO, Cleeland CS. Pain and fatigue in community-dwelling adults. Pain Med. 2003;4:231–7. doi: 10.1046/j.1526-4637.2003.03033.x. [DOI] [PubMed] [Google Scholar]
- 35.Korth C, Mullington J, Schreiber W, Pollmächer T. Influence of endotoxin on daytime sleep in humans. Infect Immun. 1996;64:1110–5. doi: 10.1128/iai.64.4.1110-1115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hermann DM, Mullington J, Hinze-Selch D, Schreiber W, Galanos C, Pollmächer T. Endotoxin-induced changes in sleep and sleepiness during the day. Psychoneuroendocrinology. 1998;23:427–37. doi: 10.1016/s0306-4530(98)00030-4. [DOI] [PubMed] [Google Scholar]
- 37.Krueger JM, Majde JA. Humoral links between sleep and the immune system: research issues. Ann N Y Acad Sci. 2003;992:9–20. doi: 10.1111/j.1749-6632.2003.tb03133.x. [DOI] [PubMed] [Google Scholar]
- 38.Pollmächer T, Haack M, Schuld A, Reichenberg A, Yirmiya R. Low levels of circulating inflammatory cytokines - Do they affect human brain functions? Brain Behav Immun. 2002;16:525–32. doi: 10.1016/s0889-1591(02)00004-1. [DOI] [PubMed] [Google Scholar]
- 39.Fotherby MD, Critchley D, Potter JF. Effect of hospitalization on conventional and 24-hour blood pressure. Age Ageing. 1995;24:25–9. doi: 10.1093/ageing/24.1.25. [DOI] [PubMed] [Google Scholar]
- 40.Broussard J, Haack M, Serrador J, Mullington J. Effects of sleep restriction on blood pressure. Sleep. 2004;27(Suppl):A171. [Google Scholar]
- 41.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–72. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 42.Il'yasova D, Colbert LH, Harris TB, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2413–8. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- 43.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 44.Wieseler-Frank J, Maier SF, Watkins LR. Central proinflammatory cytokines and pain enhancement. Neurosignals. 2005;14:166–74. doi: 10.1159/000087655. [DOI] [PubMed] [Google Scholar]
- 45.Maes M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- 46.Alesci S, Martinez PE, Kelkar S, et al. Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: clinical implications. J Clin Endocrinol Metab. 2005;90:2522–30. doi: 10.1210/jc.2004-1667. [DOI] [PubMed] [Google Scholar]
- 47.Ohayon MM, Schatzberg AF. Using chronic pain to predict depressive morbidity in the general population. Arch Gen Psychiatry. 2003;60:39–47. doi: 10.1001/archpsyc.60.1.39. [DOI] [PubMed] [Google Scholar]
- 48.Motivala SJ, Sarfatti A, Olmos L, Irwin MR. Inflammatory markers and sleep disturbance in major depression. Psychosom Med. 2005;67:187–94. doi: 10.1097/01.psy.0000149259.72488.09. [DOI] [PubMed] [Google Scholar]
- 49.Hayaishi O, Urade Y. Prostaglandin D2 in sleep-wake regulation: recent progress and perspectives. Neuroscientist. 2002;8:12–5. doi: 10.1177/107385840200800105. [DOI] [PubMed] [Google Scholar]
- 50.Jordan W, Tumani H, Cohrs S, et al. Prostaglandin D synthase (beta-trace) in healthy human sleep. Sleep. 2004;27:867–74. doi: 10.1093/sleep/27.5.867. [DOI] [PubMed] [Google Scholar]
- 51.Scholz H. Prostaglandins. Am J Physiol Regul Integr Comp Physiol. 2003;285:R512–4. doi: 10.1152/ajpregu.00298.2003. [DOI] [PubMed] [Google Scholar]
- 52.Frölich JC, Wilson TW, Sweetman BJ, et al. Urinary prostaglandins. Identification and origin. J Clin Invest. 1975;55:763–70. doi: 10.1172/JCI107987. [DOI] [PMC free article] [PubMed] [Google Scholar]