Abstract
Objective:
The aim of the study was to investigate the associations between longitudinal sleep duration patterns and behavioral/cognitive functioning at school entry.
Design, Setting, and Participants:
Hyperactivity-impulsivity (HI), inattention, and daytime sleepiness scores were measured by questionnaire at 6 years of age in a sample of births from 1997 to 1998 in a Canadian province (N=1492). The Peabody Picture Vocabulary Test - Revised (PPVT-R) was administered at 5 years of age and the Block Design subtest (WISC-III) was administered at 6 years of age. Sleep duration was reported yearly by the children's mothers from age 2.5 to 6 years. A group-based semiparametric mixture model was used to estimate developmental patterns of sleep duration. The relationships between sleep duration patterns and both behavioral items and neurodevelopmental tasks were tested using weighted multivariate logistic regression models to control for potentially confounding psychosocial factors.
Results:
Four sleep duration patterns were identified: short persistent (6.0%), short increasing (4.8%),10-hour persistent (50.3%), and 11-hour persistent (38.9%). The association of short sleep duration patterns with high HI scores (P=0.001), low PPVT-R performance (P=0.002), and low Block Design subtest performance (P=0.004) remained significant after adjusting for potentially confounding variables.
Conclusions:
Shortened sleep duration, especially before the age of 41 months, is associated with externalizing problems such as HI and lower cognitive performance on neurodevelopmental tests. Results highlight the importance of giving a child the opportunity to sleep at least 10 hours per night throughout early childhood.
Citation:
Touchette E; Petit D; Séguin JR; Boivin M; Tremblay RE; Montplaisir JY. Associations between sleep duration patterns and behavioral/cognitive functioning at school entry. SLEEP 2007;30(9):1213-1219.
Keywords: Sleep duration patterns, hyperactivity, impulsivity, inattention, daytime sleepiness, cognitive functioning
INTRODUCTION
SLEEP IS IMPORTANT FOR BEHAVIORAL AND COGNITIVE DEVELOPMENT IN THE EARLY YEARS OF LIFE.1 DAHL2 DEFINES SUFFICIENT SLEEP AS THE AMOUNT necessary to permit optimal daytime functioning. Large-scale epidemiological surveys have reported that children from 2 to 6 years of age sleep an average of 10 to 11 hours a night.3,4 A comparison of 3 birth cohorts (1974, 1979, and 1986) of the same Swiss population revealed that time spent in bed shortened (~40 minutes) across cohorts.5 This raises an important question for clinicians and parents alike: how much sleep do children really need for optimal behavioral and cognitive functioning?
It is well-known that short sleep duration induces sleepiness in children and adolescents.6 However, the manifestations of sleepiness are varied in children and range from the classical signs of sleepiness (e.g., yawning) to externalizing behaviors (e.g., hyperactivity).7 Dahl reports that “many toddlers and elementary school children respond to insufficient sleep with irritability, crankiness, low frustration tolerance, and short attention span.”8 In addition, numerous authors have noted a potential link between insufficient sleep and attention deficit hyperactivity disorder (ADHD) in children.9,10
Shortened sleep may also result in observable neurocognitive performance deficits.8 Sleep deprivation is known to impact cognitive functions in adults, as revealed by a meta-analysis11 of 56 studies on sleep deprivation. However, only a few studies12–15 investigating different degrees of sleep deprivation in children have been conducted, generally on a single night, and their conclusions were not consistent.
Moreover, to our knowledge, the long-term effects of shortened sleep have never been studied in children. The aim of this study was to identify whether sleep duration is an independent risk factor for behavioral and cognitive functioning after adjusting for potentially confounding factors. We predicted that a short sleep duration pattern would significantly increase the risk for daytime externalizing problems and lower cognitive performance on neurodevelopmental tests.
METHODS
Setting
This study was conducted as part of the Quebec Longitudinal Study of Child Development (Canada) initiated by the Quebec Institute of Statistics. All children were recruited from the Quebec Master Birth Registry managed by the Ministry of Health and Social Services. The main procedures and instruments of this study have been previously described.16
Participants
Initially, 2,223 families participated in the study when the children were approximately 5 months old (4.5±0.6 months). The children were seen yearly thereafter until they started elementary school. In all, 1492 families (67.1%) participated in the study until the children were 6 years old (73.8±3.1 months). All families received detailed information by mail on the aims and procedures of the research program and signed a consent form. The protocol was approved by the Sacre-Coeur Hospital ethics committee and the Quebec Institute of Statistics.
Outcome Variables
SLEEP MEASURES
Nocturnal sleep duration was measured at 2.5, 3.5, 4, 5, and 6 years of age by an open question on the Self-Administered Questionnaire for the Mother (SAQM): “Indicate how long in total your child sleeps during the night (on average). Do not count the hours that your child is awake.” Daytime sleep was measured at 29 months of age to assess a potential between-trajectory difference. In addition, at 6 years of age, time in bed was measured for both weekdays and weekends to assess an eventual sleep duration difference indicative of a needed compensation.
BEHAVIORAL MEASURES
Hyperactivity-impulsivity (HI), inattention, and daytime sleepiness were assessed by the mothers through an Interviewer Completed Computerized Questionnaire. Children's HI was measured by a set of 6 questions on 1,442 children: “In the past three months, how often would you say your child: 1) couldn't sit still, was restless or hyperactive, 2) couldn't stop fidgeting, 3) was impulsive or acted without thinking, 4) had difficulty waiting for his/her turn at games, 5) couldn't settle down to do anything for more than a few moments, and 6) couldn't wait when you promised something.” The inattention variable comprised 5 questions and was assessed on 1442 children: “In the past three months, how often would you say your child: 1) was easily distracted, 2) had trouble to sticking to any activity, 3) was unable to concentrate, 4) could not pay attention for long, and 5) was inattentive.” Answers were categorized as “never,” “sometimes,” or “often.” The Quebec Institute of Statistics standardized all behavioral variables on a scale of 0 (“not at all”) to 10 (“exactly”). To select children with high HI or inattention scores, cutoff was set at over 1 standard deviation (SD) above the mean. Daytime sleepiness was measured on 1,267 children by the SAQM: “In general, is your child sleepy during the day?” This question was dichotomized into absence (“never”) versus presence (“sometimes”/”often”/”always”) of daytime sleepiness.
COGNITIVE TASKS
Verbal and nonverbal cognitive abilities were measured by 2 neurodevelopmental assessments. First, 948 children were tested with a Canadian translation of the Peabody Picture Vocabulary test – Revised (PPVT-R)17 at 5.1±0.3 years of age to measure receptive vocabulary. The children were asked to point out one picture among 4 that best represented a word pronounced by the interviewer. Second, the Block Design subtest of the Wechsler Intelligence Scale for Children (WISC-III)18 was administered to 1,124 children at 6.2±0.3 years of age to evaluate nonverbal intellectual skills. For both tests, low performance was defined as a score lower than 1 SD below the mean and high performance corresponded to a score higher than 1 SD above the mean.
POTENTIALLY CONFOUNDING PSYCHOSOCIAL VARIABLES
The following potentially confounding factors that were found to be associated with behavioral or cognitive functioning were also assessed:
Sex of the child – boy / girl19
Low birth weight – yes (<2.5 kg) / no (≥2.5 kg)20
No breastfeeding – never / yes (previously breastfed)21
Maternal immigrant status when the child was 5 months of age – yes / no (non-immigrant)24
Young maternal age when the child was 5 months of age – <20 years of age / no (≥20 years of age)25
Low parental education when the child was 6 years of age – both parents without a college diploma / high parental education – one or both parents with a college diploma26
Inadequate income status when the child was 5 months of age / sufficient based on 3 indicators: family income, family size, and regional area based on national census data27
Napping at 29 months of age – no nap or nap ≤ 45 minutes / ≥1h14
HI at 17 months of age – >1 SD over the mean / ≤ 1 SD28
Inattention at 17 months of age – >1 SD above the mean / ≤ 1 SD29
Statistical Analyses
Although sleep duration throughout childhood is generally stable at approximately 10 to 11 hours per night,3,4 this pattern may not be typical for all children. Rather than assume that all children follow the same developmental pattern of sleep duration over time, a semiparametric model was used to identify subgroups of children who followed different developmental trajectories.30 Briefly, trajectory methodology uses all available developmental data points and assigns individuals to trajectories based on a posterior probability rule. The identified groups represent approximations of an underlying continuous process. For each trajectory group, this probability measures the likelihood of an individual belonging to that group based on observations across assessments. In other words, 100% classification accuracy is neither assumed nor required. Participants are assigned to the trajectory group to which they show the highest probability of belonging and analyses are weighted by posterior probabilities. A censored normal model was used; this model is considered appropriate for continuous data that are normally distributed, such as sleep duration. Trajectory models with 2 to 5 trajectories and varied shapes (e.g., intercept, linear, quadratic, or cubic) were compared by PROC TRAJ,31 an SAS procedure (SAS Institute Inc., Cary, NC). To choose the best model, the maximum Bayesian information criterion (BIC) was used to determine the optimal number of groups with shapes that best fit the data. This procedure allows the inclusion of cases with some missing data. Models with either zero, 1 or 2 missing data points for a given subject (over the 5 data points) were tested; the same results were obtained regardless of missing status. Therefore, we chose the model which permitted the inclusion of the greater number of subjects (N=1829 with up to 2 missing data points).
Two types of analyses were conducted to measure the associations between sleep duration trajectories and the behavioral/cognitive variables using SPSS for Windows (version 14; SPSS Inc, Chicago, IL). Statistical significance was set at P <0.05. First, chi-squared tests were used to measure the associations between sleep patterns and behavioral/cognitive scores (±1 SD). Second, weighted multivariate logistic regressions were performed to estimate the relative risk of sleep duration for low behavioral/cognitive scores without (unadjusted model) and while (adjusted model) controlling for a variety of potentially confounding psychosocial factors, using a stepwise selection approach based on likelihood ratio.
RESULTS
The sample of children at 6 years of age was comprised of 91.6% (n=1,492) having a Canadian (non-immigrant) mother and 8.4% having a first-generation immigrant mother. The majority of the sample was Caucasian (93.8%). Black Africans, Native Amerindians, Arabs, and Asians represented 1.7%, 0.2%, 1.0%, and 1.5% of the sample, respectively. Most mothers were interviewed in French (89.1%); the remainder were interviewed in English (10.9%).
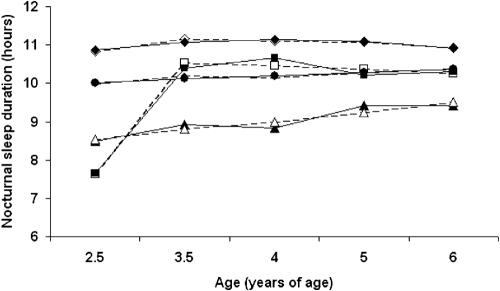
We obtained 4 developmental sleep duration patterns, as illustrated in Figure 1: a short persistent pattern (6.0%, n=109) composed of children sleeping less than 10 hours per night until age 6; a short increasing pattern (4.8%, n=88) composed of children who slept fewer hours in early childhood but whose sleep duration increased around 41 months of age, a 10-hour persistent pattern (50.3%, n=920) composed of children who slept persistently approximately 10 hours per night; and an 11-hour persistent pattern (38.9%, n=712) composed of children who slept persistently around 11 hours per night. Mean probability estimates (of being classified into the correct trajectory) were 0.85 for the short persistent, 0.79 for the short increasing pattern and 0.85 for both 10-hour and 11-hour persistent patterns. This indicates good fit of the model.
Figure 1.
Patterns of sleep duration at 2.5, 3.5, 4, 5 and 6 years of age:: short persistent sleepers (n=109; 6.0%),: short increasing sleepers (n=88; 4.8%),: 10-hour persistent sleepers (n=920; 50.3%), and: 11-hour persistent sleepers (n=712; 38.9%). Predicted (dashed lines) versus observed (solid lines) trajectories. Data courtesy of the Quebec Institute of Statistics.
The short increasing trajectory did not show a longer daytime sleep duration at 29 months (97±47 min) than that of the 3 other trajectories (118±37 min, 109±41 min, 99±50 min for the short persistent, 10-hour persistent and 11-hour persistent sleepers, respectively). There was no between-trajectory difference either on sleep duration variation between weekdays and weekends at 6 years of age (P = 0.41). All children tended to sleep a little more on weekdays (10 minutes on average) in all 4 trajectories. In light of previous findings,16 the percentages of children who were not sleeping 6 consecutive hours at night at both 5 and 17 months were calculated for the 4 trajectories and the highest percentages were found in the short increasing (5 months: 35.3%, 17: months: 19.5%), then in the short persistent trajectories (31.5%, 14.3%) compared to both the 10-hour (19.6%, 5.9%) and 11-hour (16.8%, 4.3%) persistent trajectories.
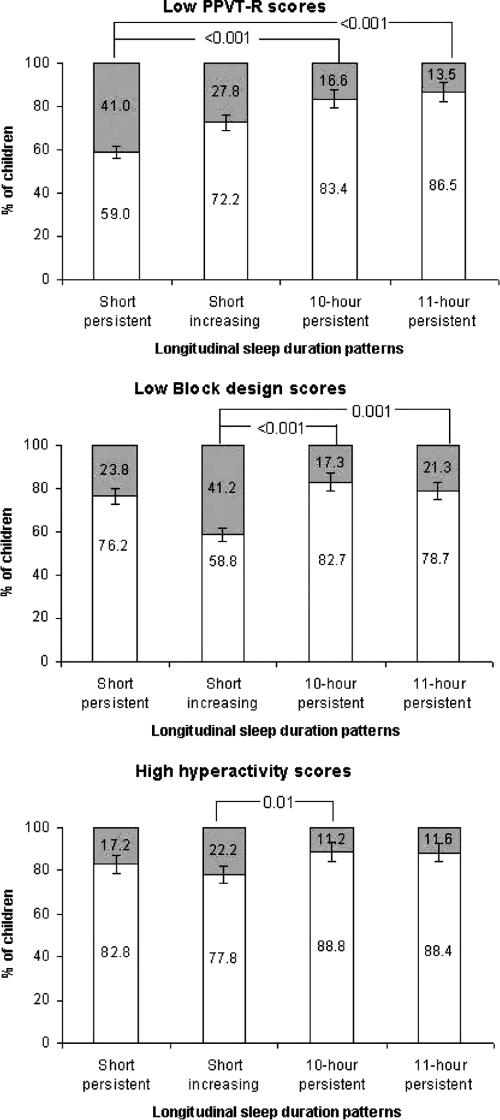
Figure 2 shows significant differences between the distributions of low performance on the PPVT-R (P <0.001), the Block design subtest (P = 0.001) and high HI scores (P = 0.04) as a function of sleep duration pattern. More precisely, more children with lower PPVT-R performance were assigned to the short persistent group (41.0%) compared to both the 10-hour (16.6%; P <0.001) and 11-hour persistent groups (13.5%; P <0.001). In addition, more children with lower Block design performance were assigned to the short increasing group (41.2%) than either the 10-hour (17.3%; P <0.001) or 11-hour persistent (21.3%; P = 0.001) groups. Finally, we observed more HI in the short increasing group compared with the 10-hour persistent group (22.2% versus 11.2%; P = 0.01). No differences in the distributions of inattention scores or daytime sleepiness as a function of sleep duration pattern were found.
Figure 2.
Grey areas indicate percentage of children who had low performances on the PPVT-R, on the Block design or high hyperactivity ratings as a function of sleep duration patterns. Chi-square tests (4 X 2) were used to measure the distribution of low performance on the PPVT-R (P<0.001) and on the Block design subtest (P=0.001) and high hyperactivity ratings (P=0.04) with different sleep duration patterns. Data courtesy of the Quebec Institute of Statistics.
Weighted logistic regression models for behavioral measures are presented in Table 1. No significant effect of sleep duration pattern was found on inattention or daytime sleepiness. The effect of sleep duration pattern on HI remained significant after adjusting for potentially confounding variables. Risk for HI was almost 3.2 times higher for short increasing sleepers compared with 11-hour persistent sleepers (P = 0.001). No significant risk for HI was found for other sleep patterns.
Table 1.
Multivariate Weighted Odds Ratios (OR) and 95% Confidence Interval (95% CI) for Behavioral Measures Based on Sleep Duration (Unadjusted Model) and After Controlling for Potentially Confounding Variables (Adjusted Model) in the Final Logistic Regression Model*
| Risk factors | Hyperactivity-impulsivity High (n=173†/n=129‡) |
Inattention High (n=124†/n=124‡) |
Daytime sleepiness Yes (n=266†/n=265‡) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | ||
| Unadjusted model† | ||||||||||
| Sleep duration patterns§ | ||||||||||
| Short persistent duration | 1.6 | (0.8 – 3.0) | 0.16 | 1.2 | (0.6 – 2.5) | 0.68 | 1.3 | (0.7 – 2.4) | 0.41 | |
| Short increasing duration | 2.4 | (1.3 – 4.4) | 0.008 | 0.7 | (0.3 −2.0) | 0.56 | 1.1 | (0.6 – 2.2) | 0.75 | |
| 10-h persistent duration | 1.1 | (0.7 – 1.5) | 0.73 | 0.9 | (0.6 – 1.3) | 0.59 | 1.0 | (0.7 – 1.3) | 0.75 | |
| n=1,391 | n=1,391 | n=1,258 | ||||||||
| Adjusted model‡ | ||||||||||
| Sleep duration patterns§ | ||||||||||
| Short persistent duration | 1.5 | (0.7 – 3.2) | 0.25 | 1.1 | (0.5 – 2.4) | 0.76 | 1.2 | (0.7 – 2.2) | 0.44 | |
| Short increasing duration | 3.2 | (1.6 −6.6) | 0.001 | 0.7 | (0.3 – 2.0) | 0.55 | 1.0 | (0.5 – 2.0) | 0.96 | |
| 10-h persistent duration | 1.2 | (0.8 – 1.8) | 0.46 | 0.9 | (0.6 – 1.3) | 0.47 | 0.9 | (0.7 – 1.2) | 0.57 | |
| n=1,169 | n=1,386 | n=1,251 | ||||||||
Data courtesy of the Quebec Institute of Statistics.
Data are expressed as weighted odds ratio (95% confidence interval).
The reference for sleep duration patterns in the logistic regression was children who slept 11-hour persistently.
Unadjusted odds ratio (95% CI) without controlling for potentially confounding factors.
Adjusted odds ratio (95% CI) while controlling for sex of the child, low birth weight, breastfeeding status, maternal immigrant status, low parental education, young mother age, inadequate income, naptime at 29 months of age, hyperactivity-impulsivity and inattention at 17 months of age.
Other independent variables were significantly associated with high HI scores. Risk for HI at 6 years of age was almost 3 times higher for children who were also HI at 17 months of age (Odds Ratio [OR]: 3.2; 95% confidence interval [CI]: 2.1 – 4.8; P <0.001). Risk for HI was almost 1.6 times higher for boys compared to girls (OR: 1.6; CI: 1.1 – 2.3; P = 0.02) and 1.9 times higher for children of parents without a college diploma (OR: 1.9; CI: 1.2 – 2.9; P = 0.004).
Other independent variables were significantly associated with high inattention scores. Risk for inattention was almost 2.1 times higher for boys than girls (OR: 2.1; CI: 1.4 – 3.1; P <0.001). In addition, risk for inattention at 6 years was almost 1.7 times higher for children who showed inattention at 17 months of age (OR: 1.7; CI: 1.1 – 2.6; P = 0.02).
Weighted logistic regression models for neurocognitive tests are shown in Table 2. No effect of sleep duration pattern was found for high PPVT-R or Block Design subtest performance. The effect of short sleep duration pattern on low PPVT-R and Block Design subtest performance remained significant after adjusting for potentially confounding variables. Risk for low PPVT-R performance was almost 3.1 times higher for short persistent sleepers compared to 11-hour persistent sleepers (P = 0.002). No effect was found for other sleep patterns with low PPVT-R performance. Risk for low Block Design subtest performance was almost 2.4 times higher for short increasing sleepers compared with 11-hour persistent sleepers (P = 0.004). No effect was found for other sleep patterns with low Block Design subtest performance.
Table 2.
Multivariate Weighted Odds Ratios (OR) and 95% Confidence Interval (95% CI) for Cognitive Tasks Based on Patterns of Sleep Duration (Unadjusted Model) and After Controlling for Potentially Confounding Variables (Adjusted Model) in the Final Logistic Regression Model*
| Risk factors | PPVT-R |
Block Design |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (n=155† / n=136‡) |
High (n=138† / n=123‡) |
Low (n=225† / n=224‡) |
High (n=191† / n=166‡) |
|||||||||
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Unadjusted model§ | ||||||||||||
| Sleep duration patterns§ | ||||||||||||
| Short persistent duration | 3.6 | (1.9 – 6.7) | <0.001 | 0.4 | (0.1 – 1.1) | 0.08 | 0.9 | (0.5 – 1.8) | 0.84 | 0.9 | (0.4 – 1.9) | 0.75 |
| Short increasing duration | 2.2 | (1.0 – 6.7) | 0.04 | 0.7 | (0.2 – 1.9) | 0.45 | 2.5 | (1.4 – 4.5) | 0.003 | 0.7 | (0.3 – 1.7) | 0.48 |
| 10-h persistent duration | 1.2 | (0.8 – 1.7) | 0.45 | 1.0 | (0.7 – 1.4) | 0.90 | 0.8 | (0.6 – 1.1) | 0.19 | 1.1 | (0.8 – 1.5) | 0.76 |
| n=915 | n=915 | n=1099 | n=1099 | |||||||||
| Adjusted model‡ | ||||||||||||
| Sleep duration patterns§ | ||||||||||||
| Short persistent duration | 3.1 | (1.5 – 6.3) | 0.002 | 0.4 | (0.1 −1.3) | 0.11 | 0.9 | (0.5 – 1.8) | 0.77 | 0.6 | (0.3 – 1.5) | 0.31 |
| Short increasing duration | 1.6 | (0.7 – 3.9) | 0.31 | 0.8 | (0.3 – 2.5) | 0.68 | 2.4 | (1.3 – 4.4) | 0.004 | 0.7 | (0.3 – 1.8) | 0.43 |
| 10-h persistent duration | 1.2 | (0.8 – 1.9) | 0.42 | 1.0 | (0.7 – 1.5) | 0.92 | 0.8 | (0.6 – 1.1) | 0.20 | 0.9 | (0.7 – 1.3) | 0.74 |
| n=800 | n=807 | n=1,098 | n=924 | |||||||||
Data courtesy of the Quebec Institute of Statistics.
Data are expressed as weighted odds ratio (95% confidence interval).
The reference for sleep duration patterns in the logistic regression was children who slept 11-hour persistently.
Unadjusted odds ratio (95% CI) without controlling for potentially confounding factors.
Adjusted odds ratio (95% CI) while controlling for sex of the child, maternal immigrant status, low parental education, young mother age, inadequate income, low birth weight, breastfeeding status, naptime at 29 months of age, hyperactivity-impulsivity and inattention at 17 months of age.
Other factors were significantly associated with low PPVT-R performance: immigrant status of the mother (OR: 3.6; CI: 2.0 – 6.6; P<0.001), insufficient income (OR: 2.1; CI: 1.3 – 3.5; P = 0.003), and low parental education (OR: 2.1; CI: 1.3 – 3.4; P = 0.002). We found 2 significant factors for low Block Design subtest performance: immigrant status of the mother (OR: 1.8; CI: 1.1 – 3.2; P = 0.03) and young mother (OR: 2.9; CI: 1.3 – 6.2; P = 0.006).
Low parental education reduced almost twofold the probability of high PPVT-R performance (OR: 0.5; CI: 0.2 – 0.9; P = 0.02) and high Block Design subtest performance (OR: 0.4; CI: 0.2 – 0.7; P = 0.001). Finally, probability of high PPVT-R performance was almost 5 times lower for children whose mothers were immigrants compared to children with nonimmigrant mothers (OR: 0.2; CI: 0.1 – 0.9; P = 0.03).
COMMENT
This study represents the first investigation of developmental sleep duration patterns throughout childhood. The majority of the children studied (50.3%) slept persistently for 10 hours a night on average, whereas 38.9% slept persistently for 11 hours a night over the period studied, demonstrating that nighttime sleep duration is fairly stable from 2.5 years of age to 6 years of age. These sleep duration values are in good agreement with those reported elsewhere.3–5 Also, there was no difference in sleep duration between weekdays and weekends indicating that children were not compensating on the weekend for sleep loss occurring during the week, even in the group of short persistent sleepers. Short increasing sleepers, who had evidence of a nocturnal sleep consolidation problem before the age of 41 months, did not compensate their short nighttime sleep duration by more daytime sleep at 29 months.
Sleep Duration and Cognitive Outcomes
More importantly, our results indicate that a modest but chronic reduction of just 1 hour of sleep nightly in early childhood can be associated with the child's cognitive performance at school entry. Short sleep duration multiplied by 3.1 the risk of low performance on the PPVT-R. This suggests that language acquisition and the consolidation of new words into memory could be significantly impeded by chronically shortened sleep duration throughout childhood. Lower performance on the Block design subtest was also observed in the short increasing sleep group. This means that, although sleep duration improved at 3 years of age, the risk for scoring low on the Block design subtest at 6 years of age remained more than 2.4 times higher, independently of potentially confounding factors. This finding points to an early critical period for cognitive development that may be jeopardized by short sleep duration. Randazzo and colleagues17 reported that a duration of 5 hours in bed results in significantly lower performance in verbal creativity and abstract thinking, compared with 11 hours of sleep. Moreover, studies on cumulative sleep restriction have shown negative effects on cognitive performance.32,33 Sadeh and his team33 reported that children who extended their sleep by 1 hour for 3 consecutive nights showed significantly improved digit forward memory test scores and reaction times on a performance test.
We could posit other potential variables to explain the associations between sleep duration pattern and cognitive measures. For example, the PPVT-R Instruction Manual17 reports that recent immigration, low parental education, and low socioeconomic status can influence PPVT-R results, as we found in this study. Likewise, immigrant status, and young age of the mother were associated with low performance on the Block design subtest of the WISC-III.34,35 However, none of the other factors studied were able to explain the association observed between sleep pattern and cognitive performance.
Sleep Duration and Behavioral Outcomes
Our results also demonstrate a significant relationship between high HI scores at 6 years of age and a short increasing sleep duration pattern. Although sleep duration improved at 3 years of age, the risk for high HI scores at 6 years of age remained 3.2 times higher independent of potentially confounding factors. We believe that there is a critical period in early childhood where the lack of sleep is particularly detrimental to various aspects of development even if sleep duration normalizes later on. There was an inclination for children in the short persistent pattern to also show high HI scores at 6 years of age. However, the sleep duration in the critical period was even shorter (by 51 minutes) in the short increasing than in the short persistent pattern. Lavigne and colleagues10 also associated less nocturnal sleep (<10 hours) in preschool children with increased daytime behavior problems, according to the Child Behavior Checklist. No association was found in the present study between daytime sleepiness and sleep patterns, whereas other studies on older children have reported that sleep-deprived children were sleepier than non–sleep-deprived children.12–15,36 Our results support the notion that 6-year-old children are more likely to exhibit externalizing behaviors such as HI instead of the classical signs of sleepiness when they do not get sufficient nocturnal sleep.7,8 These results also highlight the importance of giving a child the opportunity to sleep at least 10 hours a night throughout childhood, especially before the age of 3.5 years. No association was found between inattention and sleep duration pattern.
It might be argued that other variables might explain the association observed between sleep duration and behavioral outcomes. It is well-known in the literature that boys score higher on HI and inattention than girls.37 The present study also shows that sex of the child is an independent risk factor for higher HI and inattention scores. High HI and inattention scores at 17 months increase the risks for HI and inattention, respectively, at 6 years of age. High behavioral scores in early childhood appear to persist over time.28,29 However, despite these associations, short sleep duration was independently associated with high HI scores. Future studies could investigate the relationship between short sleep duration in early childhood and the predominantly hyperactive-impulsive subtype of ADHD.
Limitations
Our study is limited by reliance on a subjective measure of sleep quantity, which may slightly overestimate the real sleep time. Fortunately, the parental reports and actigraphy measures show generally high agreement for sleep duration.38–40 Further controlled studies are needed to better quantify the long-term effects of shortened sleep on cognitive tasks in young children, although verbal skills (e.g., PPVT-R, as used in this study) have been found to better predict school achievement than other types of cognitive tests. In future studies, it would also be interesting to use tests that involve neurocognitive executive functions, which are known to be under the control of the frontal lobe and thus affected by sleep reduction.41 Furthermore, the odds ratio of logistic regression assesses risk but cannot be used to infer causality. Finally, since the participants were generally nonimmigrant French-speaking Caucasians, the results might not be generalizable to other populations. Nevertheless, this paper highlights the importance to give the child the opportunity to sleep at least 10 hours a night in early childhood, as supported by the findings of the National Sleep Foundation Poll.42 It would be interesting to determine more precisely whether 10 hours (on average) represents a threshold for minimum required sleep duration in early childhood. One should keep in mind that the required amount of sleep shows some inter-individual variation (short- and long-sleepers). Finally, future studies should look at the emergence of chronotypes (eveningness vs morningness) in relation to childhood sleep duration.
ACKNOWLEDGMENTS
We are thankful to Miguel Chagnon, PhD, for his valuable suggestions and statistical expertise. We also thank the children and families whose ongoing participation made this study possible. We acknowledge the considerable contribution of the coordinators of the Quebec Longitudinal Study of Child Development and the Quebec Institute of Statistics, and the tireless work of all the interviewers who assessed the mothers and children during the course of this study.
Author Contributions:
Study concept and design: Touchette, Petit, Séguin, Boivin, Tremblay, Montplaisir.
Data acquisition: Quebec Institute of Statistics.
Data analysis and interpretation: Touchette, Séguin, Petit.
Manuscript drafting: Touchette.
Critical manuscript revision for important intellectual content: Petit, Séguin, Boivin, Tremblay, Montplaisir.
Statistical expertise: Touchette.
Fundraising: Touchette, Boivin, Tremblay, Montplaisir.
Administrative, technical, and material support: Public Health Network and the Quebec Institute of Statistics.
Study supervision: Quebec Institute of Statistics.
Funding/Support:
This study was funded by the Ministry of Health and Social Services (Quebec City, Quebec, Canada); the Canadian Institutes of Health Research (Ottawa, Ontario, Canada); the Social Sciences and Humanities Research Council of Canada (Ottawa, Ontario, Canada); the Quebec Fund for Research on Society and Culture (Quebec City, Quebec, Canada); the Quebec Fund for Research on Nature and Technology (Quebec City, Quebec, Canada); the Health Research Fund of Quebec (Quebec City, Quebec), the Molson Foundation (Montreal, Quebec, Canada); the Ministry of Research, Science and Technology (Quebec City, Quebec, Canada); Human Resources Development Canada (Ottawa, Ontario, Canada); the Canadian Institute for Advanced Research (Toronto, Ontario, Canada); Health Canada (Ottawa, Ontario, Canada); the National Science Foundation (Arlington, VA, USA); University of Montreal (Montreal, Quebec, Canada); Laval University (Quebec City, Quebec, Canada); and McGill University (Montreal, Quebec, Canada).
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Petit has received research support from Orphan Medical/Jazz Pharmaceuticals, GlaxoSmithKline, Sanofi-Aventis, and Aventis. Dr. Montplaisir has received research support from Sanofi Synthelabo, GlaxoSmithKline, Aventis, Orphan Medical, and Pharmacacia/Pfizer; has been a consultant to Boehringer Ingelheim, Servier, Shire BioChem, and Aventis; and has participated in speaking engagements for Boehringer Ingelheim, Shire, and GlaxoSmithKline. Drs. Séguin, Boivin, Tremblay, and Ms. Touchette have indicated no financial conflicts of interest.
REFERENCES
- 1.Dahl RE. The regulation of sleep and arousal: Development and psychopathology. Dev Psychopathol. 1996;8:3–27. [Google Scholar]
- 2.Dahl RE. The consequences of insufficient sleep for adolescents. Phi Delta Kappan. 1999:354–9. [Google Scholar]
- 3.Thorleifsdottir B, Björnsson JK, Benediktsdottir B, Gislason Th, Kristbjarnarson H. Sleep and sleep habits from childhood to young adulthood over a 10-year period. J Psychosom Res. 2002;53:529–37. doi: 10.1016/s0022-3999(02)00444-0. [DOI] [PubMed] [Google Scholar]
- 4.Spruyt K, O'Brien LM, Cluydts R, Verleye GB, Ferri R. Odds, prevalence and predictors of sleep problems in school-age normal children. J Sleep Res. 2005;14:163–76. doi: 10.1111/j.1365-2869.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- 5.Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111:302–7. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- 6.Fallone G, Owens J, Deane J. Sleepiness in children and adolescents: clinical implications. Sleep Med Rev. 2002;6:287–306. doi: 10.1053/smrv.2001.0192. [DOI] [PubMed] [Google Scholar]
- 7.Owens JA. The ADHD and sleep conundrum: a review. J Dev Behav Pediatr. 2005;26:312–22. doi: 10.1097/00004703-200508000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Dahl RE. The impact of inadequate sleep on children's daytime cognitive function. Semin Pediatr Neurol. 1996;3:44–50. doi: 10.1016/s1071-9091(96)80028-3. [DOI] [PubMed] [Google Scholar]
- 9.Ring A, Stein D, Barak Y, et al. Sleep disturbances in children with attention-deficit/hyperactivity disorder: a comparative study with healthy siblings. J Learn Disabil. 1998;31:572–8. doi: 10.1177/002221949803100607. [DOI] [PubMed] [Google Scholar]
- 10.Lavigne JV, Arend R, Rosenbaum D, et al. Sleep and behavior problems among preschoolers. J Dev Behav Pediatr. 1999;20:164–9. doi: 10.1097/00004703-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance. A meta-analysis. Sleep. 1996;19:318–26. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- 12.Carskadon MA, Harvey K, Dement WC. Acute restriction of nocturnal sleep in children. Percept Mot Skills. 1981;53:103–12. [Google Scholar]
- 13.Carskadon MA, Harvey K, Dement WC. Sleep loss in young adolescents. Sleep. 1981;4:299–312. doi: 10.1093/sleep/4.3.299. [DOI] [PubMed] [Google Scholar]
- 14.Fallone G, Acebo C, Arnedt JT, Seifer R, Carskadon MA. Effects of acute sleep restriction on behavior, sustained attention, and response inhibition in children. Percept Mot Skills. 2001;93:213–29. doi: 10.2466/pms.2001.93.1.213. [DOI] [PubMed] [Google Scholar]
- 15.Randazzo AC, Muehlbach MJ, Schweitzer PK, Walsh JK. Cognitive function following acute sleep restriction in children ages 10–14. Sleep. 1998;21:861–8. [PubMed] [Google Scholar]
- 16.Touchette E, Petit D, Paquet J, et al. Factors associated with fragmented sleep at night across early childhood. Arch Pediatr Adolesc Med. 2005;159:242–9. doi: 10.1001/archpedi.159.3.242. [DOI] [PubMed] [Google Scholar]
- 17.Dunn LM, Theriault-Whalen CM, Dunn LM. Manuel pour les formes A et B. Toronto: PSYCAN; 1993. Échelle de Vocabulaire en Images Peabody – Adaptation française du Peabody Picture Vocabulary Test – Revised. [Google Scholar]
- 18.Wechsler D. Wechsler Intelligence Scale for Children. Third Edition. San Antonio: The Psychological Corporation; 1991. [Google Scholar]
- 19.Livesey D, Intili D. A gender difference in visual-spatial ability in 4-year-old children: effects on performance of a kinesthetic acuity task. J Exp Child Psychol. 1996;63:436–46. doi: 10.1006/jecp.1996.0057. [DOI] [PubMed] [Google Scholar]
- 20.Ment LR, Vohr B, Allan W, et al. Change in cognitive function over time in very low-birth-weight infants. JAMA. 2003;289:705–11. doi: 10.1001/jama.289.6.705. [DOI] [PubMed] [Google Scholar]
- 21.Oddy WH, Kendall GE, Blair E, et al. Breast feeding and cognitive development in childhood: a prospective birth cohort study. Paediatr Perinat Epidemiol. 2003;17:81–90. doi: 10.1046/j.1365-3016.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- 22.Huijbregts SCJ, Séguin JR, Zelazo PD, Parent S, Japel C, Tremblay RE. Interrelations between maternal smoking during pregnancy, birth weight and sociodemographic factors in the prediction of early cognitive abilities. Infant Child Dev. 2006;15:593–607. doi: 10.1002/icd.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huijbregts SCJ, Séguin JR, Zoccolillo M, Boivin M, Tremblay RE. Associations of maternal prenatal smoking with early childhood physical aggression, hyperactivity-impulsivity, and their co-occurrence. J Abnorm Child Psychol. 2007;35:203–15. doi: 10.1007/s10802-006-9073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farver JA, Kim YK, Lee Y. Cultural differences in Korean- and Anglo-American preschoolers' social interaction and play behaviors. Child Dev. 1995;66:1088–99. [PubMed] [Google Scholar]
- 25.Camp BW. Adolescent mothers and their children: changes in maternal characteristics and child developmental and behavioral outcome at school age. J Dev Behav Pediatr. 1996;17:162–9. [PubMed] [Google Scholar]
- 26.Dollaghan CA, Campbell TF, Paradise JL, et al. Maternal education and measures of early speech and language. J Speech Lang Hear Res. 1999;42:1432–43. doi: 10.1044/jslhr.4206.1432. [DOI] [PubMed] [Google Scholar]
- 27.Cuffe SP, Moore CG, McKeown RE. Prevalence and correlates of ADHD symptoms in the national health interview survey. J Atten Disord. 2005;9:392–401. doi: 10.1177/1087054705280413. [DOI] [PubMed] [Google Scholar]
- 28.Romano E, Tremblay RE, Farhat A, Cote S. Development and prediction of hyperactive symptoms from 2 to 7 years in a population-based sample. Pediatrics. 2006;117:2101–10. doi: 10.1542/peds.2005-0651. [DOI] [PubMed] [Google Scholar]
- 29.Gagnon C, Craig WM, Tremblay RE, Zhou RM, Vitaro F. Kindergarten predictors of boys' stable behavior problems at the end of elementary school. J Abnorm Child Psychol. 1995;23:751–66. doi: 10.1007/BF01447475. [DOI] [PubMed] [Google Scholar]
- 30.Nagin DS. Cambridge, MA: Harvard University Press; 2005. Group based modeling of development. [Google Scholar]
- 31.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29:374–93. [Google Scholar]
- 32.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 33.Sadeh A, Gruber R, Raviv A. The effects of sleep restriction and extension on school-age children: what a difference an hour makes. Child Dev. 2003;74:444–55. doi: 10.1111/1467-8624.7402008. [DOI] [PubMed] [Google Scholar]
- 34.Daniel LM, Lim SB, Clarke L. Eight-year outcome of very-low-birth-weight infants born in KK hospital. Ann Acad Med Singapore. 2003;32:354–61. [PubMed] [Google Scholar]
- 35.Martinez-Cruz CF, Poblano A, Fernadez-Carrocera LA, Jimenez-Quiroz R, Tuyu-Torres N. Association between intelligence quotient scores and extremely low birth weight in school-age children. Arch Med Res. 2006;37:639–45. doi: 10.1016/j.arcmed.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Fallone G, Seifer R, Acebo C, Carskadon MA. Prolonged sleep restriction in 11- and 12-year-old children: Effects on behavior, sleepiness, and mood. Sleep. 2000;23:A28. [Google Scholar]
- 37.Yang P, Jong YJ, Chung LC, Chen CS. Gender differences in a clinic-referred sample of Taiwanese attention-deficit/hyperactivity disorder children. Psychiatry Clin Neurosci. 2004;58:619–23. doi: 10.1111/j.1440-1819.2004.01312.x. [DOI] [PubMed] [Google Scholar]
- 38.Sadeh A. Assessment of intervention for infant night waking: Parental reports and activity-based home monitoring. J Consult Clin Psychol. 1994;62:63–8. doi: 10.1037//0022-006x.62.1.63. [DOI] [PubMed] [Google Scholar]
- 39.Tikotzky L, Sadeh A. Sleep patterns and sleep disruptions in kindergarten. J Clin Child Psychol. 2001;30:581–91. doi: 10.1207/S15374424JCCP3004_13. [DOI] [PubMed] [Google Scholar]
- 40.Sekine M, Chen X, Hamanishi S, Wang H, Yamagami T, Kagamimori S. The validity of sleeping hours of healthy young children as reported by their parents. J Epidemiol. 2002;12:237–42. doi: 10.2188/jea.12.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones K, Harrison Y. Frontal lobe function, sleep loss and fragmented sleep. Sleep Med Rev. 2001;5:463–75. doi: 10.1053/smrv.2001.0203. [DOI] [PubMed] [Google Scholar]
- 42.Mindell JA. Sleep in America. SRS Bulletin. 2004;10:14–5. [Google Scholar]