Abstract
This study was performed to determine whether immunoreactivity of intrarenal hemeoxygenase-1 and angiotensinogen are increased in IgA nephropathy (IgAN) patients. Hemeoxygenase-1 and angiotensinogen immunoreactivity were determined by immunohistochemistry robot system in renal specimens from 39 patients with IgAN. Normal portions of surgically resected kidney served as controls. IgAN patients showed moderate proteinuria (1.1+/−0.2 g/day); however, the control group did not show any proteinuria. Immunoreactivity of intrarenal hemeoxygenase-1 and angiotensinogen in IgAN were significantly increased compared to normal kidneys (2.42+/−0.42 vs 1.00+/−0.26 for hemeoxygenase-1 and 4.05+/−0.40 vs 1.00+/−0.21 for angiotensinogen, arbitrary unit). Even though these IgAN patients did not show massive renal damage, hemeoxygenase-1 and angiotensinogen immunoreactivity were increased in these patients at this time point. These data suggest that activated intrarenal reactive oxygen species-angiotensinogen axis plays some roles in development of IgAN at the early stage and will provide supportive foundation of effectiveness of the renin-angiotensin system blockade in IgAN.
Keywords: IgA nephropathy, angiotensinogen, oxidative stress, angiotensin II, clinical study
Introduction
IgA nephropathy is defined by the predominant deposition of IgA in the glomerular mesangium [1, 2]. IgA nephropathy is the most common primary glomerulopathy among all races from Europe [3-5], Asia [6, 7], and Australia [8] with the exception of the black race [9]. Nair et al demonstrated that IgA nephropathy patients increase as the primary glomerulopathy among young adults (20-39 years old) in the USA [10]. However, it is uncertain about detailed mechanisms of the development of IgA nephropathy or the method of radical cure is not established until now. Patients with isolated hematuria, proteinuria less than 1 g/day and normal renal function have a benign course and are generally just followed up annually. In cases where tonsillitis is the precipitating factor for episodic hematuria, tonsillectomy has been claimed to reduce the frequency of those episodes. However, it does not reduce the incidence of progressive renal failure [11]. As the drug therapeutics, immunosuppressive drugs (steroid, cyclophosphamide, mycophenolic acid, cyclosporine, mizoribine, etc.), anticoagulants (warfarin, heparin, dilazep, etc.), and fish oil (omega-3 fatty acid) had been used in IgA nephropathy patients; however, the results were conflicting [12-16]. Recently, clinical and experimental studies have demonstrated that the blockade of the renin-angiotensin system (RAS) is successful in mitigation and therapy of IgA nephropathy [17], suggesting the activated RAS in the development and progression of IgA nephropathy. Meanwhile, our recent studies demonstrate that reactive oxygen species (ROS)-dependent angiotensinogen enhancement has potent roles in the development and progression of renal injury in salt sensitive hypertension [18, 19] as well as in diabetes [20]. Dahl salt sensitive rats (DS) and resistant rats (DR) were maintained on a high salt diet (HS) or a low salt diet (LS). Systolic blood pressure was significantly increased in DS+HS. Plasma angiotensinogen levels were suppressed by HS in both strains. However, kidney angiotensinogen levels were significantly increased in DS+HS. The evidence suggests that DS fed HS have an inappropriate and paradoxical augmentation of intrarenal angiotensinogen [18]. Recent studies indicate that the inappropriate augmentation of intrarenal angiotensinogen in DS by HS is caused by augmented production of ROS. Systolic blood pressure was significantly increased in DS+HS, and treatment with a superoxide dismutase mimetic, tempol, or treatment with a non-specific vasodilator, hydralazine, attenuated the hypertension to an equivalent extent. Urinary excretion of thiobarbituric acid reactive substances, a marker of oxidative stress, was significantly increased in DS+HS. Tempol treatment prevented this effect but hydralazine treatment only partially prevented the effect. Kidney angiotensinogen levels were significantly increased in DS+HS, and tempol but not hydralazine treatment prevented the intrarenal angiotensinogen augmentation. The evidence suggests that ROS-dependent activation of intrarenal angiotensinogen plays an important role in the development of the hypertension in DS fed HS [19]. The Zucker diabetic fatty (ZDF) obese rat, a model of type 2 diabetes, is well known to show progressive nephropathy; however, the detailed mechanisms have remained unclear. A study was recently performed to examine the possible involvement of angiotensinogen in diabetic nephropathy of ZDF obese rats. Genetic pairs of male ZDF obese rats and ZDF lean rats were maintained on a diet containing high fat. Urinary levels of 8-isoprostane, a marker of oxidative stress, were significantly increased in ZDF obese rats from 15 weeks of age. Kidney angiotensinogen protein levels were significantly increased in ZDF obese rats from 17 weeks of age. The evidence suggests that elevated ROS and ROS-associated augmentation of intrarenal angiotensinogen may initiate the development of diabetic nephropathy in ZDF obese rats [20]. These data imply that the activated intrarenal ROS-angiotensinogen axis may play an important role in the development of IgA nephropathy. Therefore, this study was performed to determine whether intrarenal oxidative stress and angiotensinogen are increased in IgA nephropathy patients.
Materials and Methods
Protocol
The experimental protocol of this clinical study was approved by the Institutional Review Board of Tulane University and Osaka General Medical Center. All samples were obtained from patients with written informed consent.
Sample Collection
Thirty-nine patients (18 males and 21 females) were recruited in Osaka General Medical Center from new outpatients with occasional proteinuria who were later diagnosed as IgA nephropathy by clinical course and renal biopsy. Tissues were obtained by renal biopsy in a general manner. Normal portions of surgically resected kidney served as controls from 5 patients (4 males and 1 female) of renal cell carcinoma in Osaka General Medical Center. All kidney samples were fixed in 10% buffered formalin immediately after removal.
Immunohistochemistry of Hemeoxygenase-1
Using formalin-fixed paraffin-embedded renal sections, immunohistochemistry for hemoxygenase-1 was performed by a robotic system (Dako, Autostainer) to apply the exactly same condition on all slides and counter-stained with hematoxylin-eosin. The primary antibody against human hemoxygenase-1 was purchased from Stressgen Bioreagents (#SPA-896) and the concentration for immunohistochemistry was 1:3,000. The immunoreactivity was quantitatively evaluated by a semi-automatic image analysis system using the Image-Pro plus software (Media Cybernetics). Twenty consecutive microscopic fields were examined for each slide and the averaged intensities were obtained for each slide. The measurements were made in an unbiased blended manner without knowledge of source of the tissue as previously described [20-25].
Immunohistochemistry of 4-Hidroxy-2-nonenal (4-HNE)
Immunohistochemistry for 4-HNE and quantification were performed as described above. The primary antibody against human 4-HNE was purchased from JAICA (#MHN-020P) and the concentration for immunohistochemistry was 1 μg/mL.
Immunohistochemistry of Angiotensinogen
Immunohistochemistry for angiotensinogen and quantification were performed as described above. The primary antibody against human angiotensinogen was raised by Zymed as a custom service and was characterized previously [26] and the concentration for immunohistochemistry was 1:6,000.
Immunohistochemistry of Angiotensin II
Immunohistochemistry for angiotensin II and quantification were performed as described above. The primary antibody against human angiotensin II was purchased from Phoenix Pharmaceuticals (#H-002-12) and the concentration for immunohistochemistry was 1:3,000.
Immunohistochemistry of IgA
Immunohistochemistry for IgA was performed as described above. The primary antibody against human IgA was purchased from Dako (#F0204) and the concentration for immunohistochemistry was 1:2,000.
Statistical Analysis
Statistical analysis was performed using unpaired T test. All data are presented as mean+/−SEM. P<0.05 was considered significant.
Results
Patient Profiles and Clinical Data
Patient profiles were summarized in Table 1. As described in Table 2, IgA nephropathy patients showed higher systolic blood pressure and lower creatinine clearance compared with the control group; however, these changes were not so critical. The control group did not show any proteinuria or hematuria; however, IgA nephropathy patients also showed moderate proteinuria and hematuria. As demonstrated in Figure 1A, immunohistochemical staining was negative for IgA in glomerulus of the control group. In IgA nephropathy patients, the predominant deposition of IgA in the glomerular mesangium were confirmed as shown in Figure 1B.
Table 1.
Patient Profiles
| Control | IgA nephropathy | |
|---|---|---|
| Numbers | 5 | 39 |
| Age | 46±3 | 38±2 |
| Sex, Male/Female | 4/1 | 18/21 |
| Height, cm | 170±6 | 163±2 |
| Body weight, kg | 66±6 | 60±2 |
| Body mass index | 23±1 | 22±1 |
Table 2.
Clinical Data
| Control | IgA nephropathy | |
|---|---|---|
| Systolic blood pressure, mmHg | 115±6 | 123±3 * |
| Diastolic blood pressure, mmHg | 71±4 | 78±2 |
| Serum creatinine, mg/ml | 0.8±0.1 | 0.9±0.1 |
| Creatinine clearance, ml/min | 112±8 | 104±6 * |
| Urinary occult blood, index | Negative | 2.4±0.1 * |
| Urinary protein excretion, g/day | Negative | 1.1±0.2 * |
| Urinary protein-to-creatinine ratio | Negative | 1.1±0.2 * |
P<0.05
Figure 1.
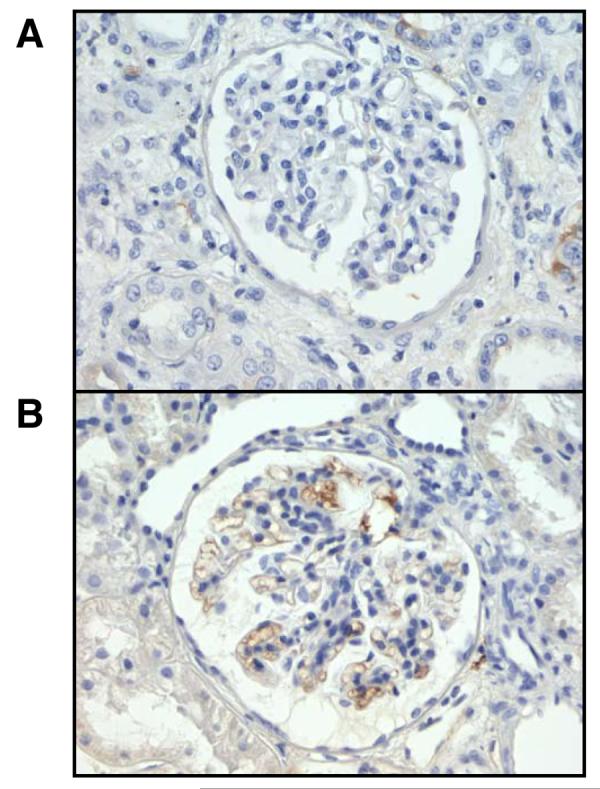
Representative slides for IgA immunostaining from the control group (A) and from the IgA nephropathy group (B).
Immunoreactivity of Hemeoxygenase-1
As illustrated in Figures 2A and 2B, immunoreactivity of hemeoxygenase-1 in tubules was significantly increased in IgA nephropathy patients compared to the control group (Figure 2C, 2.42+/−0.42 vs 1.00+/−0.26, arbitrary unit).
Figure 2.
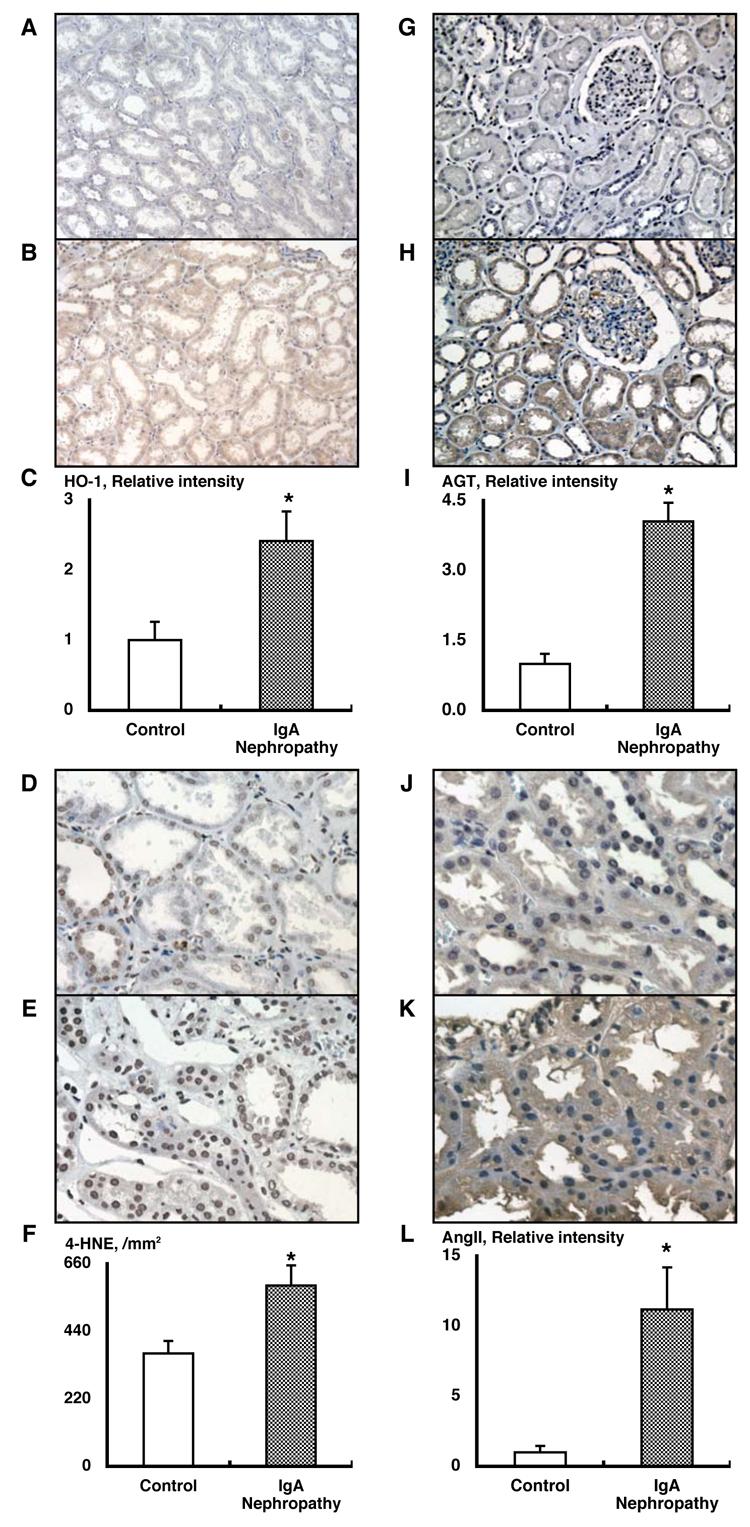
Enhanced intrarenal oxidative stress in IgA nephropathy patients (A-F). Representative slides for hemeoxigenase-1 immunostaining from the control group (A) and from the IgA nephropathy group (B). Densitometric analysis demonstrated that immunoreactivity of hemeoxygenase-1 in tubules was significantly increased in IgA nephropathy patients compared to the control group (C, 2.42+/−0.42 vs 1.00+/−0.26, arbitrary unit). Representative slides for 4-hidroxy-2-nonenal (4-HNE) immunostaining from the control group (D) and from the IgA nephropathy group (E). 4-HNE-positive cell numbers in tubules was significantly increased in IgA nephropathy patients compared to the control group (F, 589+/−65 vs 368+/−41, per mm2). Activated intrarenal renin-angiotensin system in IgA nephropathy patients (G-L). Representative slides for angiotensinogen immunostaining from the control group (G) and from the IgA nephropathy group (H). Densitometric analysis demonstrated that immunoreactivity of angiotensinogen in tubules was significantly increased in IgA nephropathy patients compared to the control group (I, 4.05+/−0.40 vs 1.00+/−0.21, arbitrary unit). Representative slides for angiotensin II immunostaining from the control group (J) and from the IgA nephropathy group (K). Densitometric analysis demonstrated that immunoreactivity of angiotensin II in tubules was significantly increased in IgA nephropathy patients compared to the control group (L, 11.18+/−3.00 vs 1.00+/−0.46, arbitrary unit).
4-HNE-Positive Cell Numbers
As demonstrated in Figures 2D and 2E, 4-HNE-positive cell numbers in tubules was significantly increased in IgA nephropathy patients compared to the control group (Figure 2F, 589+/−65 vs 368+/−41, per mm2).
Immunoreactivity of Angiotensinogen
As illustrated in Figures 2G and 2H, immunoreactivity of angiotensinogen in tubules was significantly increased in IgA nephropathy patients compared to the control group (Figure 2I, 4.05+/−0.40 vs 1.00+/−0.21, arbitrary unit). In normal kidneys, angiotensinogen was predominantly localized in proximal tubular cells; however, angiotensinogen was also expressed in glomerulus in IgA nephropathy patients.
Immunoreactivity of Angiotensin II
As demonstrated in Figures 2J and 2K, immunoreactivity of angiotensin II in tubules was significantly increased in IgA nephropathy patients compared to the control group (Figure 2L, 11.18+/−3.00 vs 1.00+/−0.46, arbitrary unit).
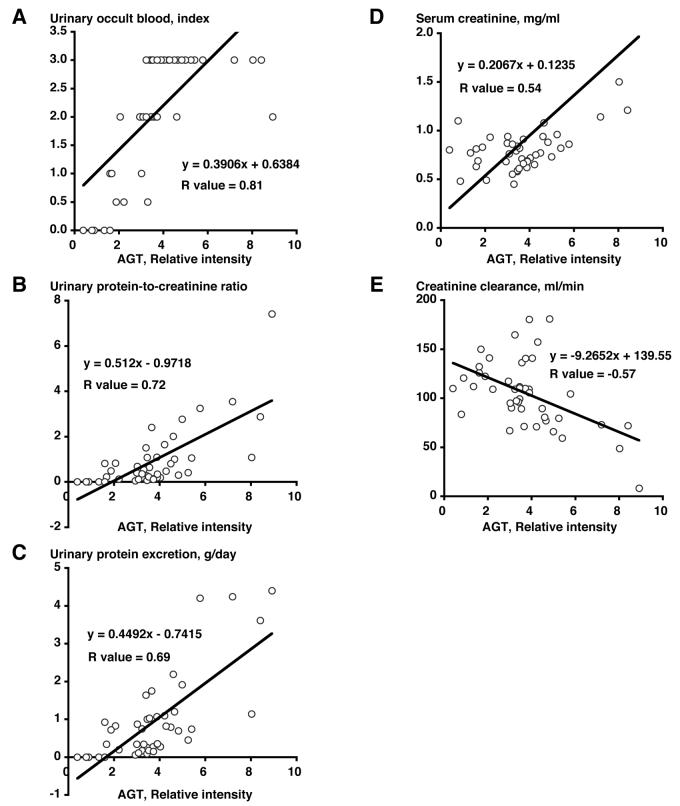
Correlation with Immunoreactivity of Angiotensinogen
Correlation with immunoreactivity of angiotensinogen was calculated with individual clinical data of both groups. Immunoreactivity of angiotensinogen was significantly correlated positively with urinary occult blood (Figure 3A, R value = 0.81), urinary protein-to-creatinine ratio (Figure 3B, R value = 0.72), urinary protein excretion (Figure 3C, R value = 0.69), and serum creatinine (Figure 3D, R value = 0.54). Immunoreactivity of angiotensinogen was also significantly correlated negatively with creatinine clearance (Figure 3E, R value = −0.57).
Figure 3.
Correlation with Immunoreactivity of Angiotensinogen. Correlation with immunoreactivity of angiotensinogen was calculated with individual clinical data of both groups. Immunoreactivity of angiotensinogen was significantly correlated positively with urinary occult blood (A, R value = 0.81), urinary protein-to-creatinine ratio (B, R value = 0.72), urinary protein excretion (C, R value = 0.69), and serum creatinine (D, R value = 0.54). Immunoreactivity of angiotensinogen was also significantly correlated negatively with creatinine clearance (E, R value = −0.57).
Discussion
Recently clinical and experimental studies have demonstrated that the blockade of the RAS is successful in mitigation and therapy of IgA nephropathy [17], suggesting the activated RAS in the development and progression of IgA nephropathy. However, it is uncertain about detailed mechanisms of the development of IgA nephropathy or the method of radical cure is not established until now. In the present study, we provide direct evidence to demonstrate that intrarenal oxidative stress and angiotensinogen are increased in IgA nephropathy patients at the early stage. These data suggest that the activated intrarenal ROS-angiotensinogen axis plays some roles in the development of IgA nephropathy at the early stage and will provide a supportive foundation of the effectiveness of RAS blockade in IgA nephropathy.
A clear linkage between the intrarenal RAS and IgA nephropathy was also recently reported using in vitro models. It was reported that the glomerular angiotensin II type 1 receptor levels were reduced in IgA nephropathy, whereas there was no change in the expression of glomerular angiotensin II type 2 receptors [27]. More recently, it was demonstrated that there is constitutive expression of angiotensin II type 1 receptors and angiotensin II type 2 receptors in renal tubules with increased expression in IgA nephropathy [28]. These data as well as the present data would suggest the activated RAS in the development and progression of IgA nephropathy.
A series of previous studies imply an augmentation of angiotensinogen expression by ROS via several pathways. Rao et al showed that hydrogen peroxide induces extracellular signal-regulated kinase 1/2 in vascular smooth muscle cells [29]. Yoshizumi et al presented that hydrogen peroxide activates c-Jun N-terminal kinase via c-Src-dependent mechanisms in vascular smooth muscle cells [30]. Suzaki et al showed that hydrogen peroxide activates c-Src-mediated extracellular signal-regulated kinase 5 in PC12 cells [31]. She also demonstrated c-Src-dependent extracellular signal-regulated kinase 5 activation in glomeruli of diabetic rats and in glomerular mesangial cells by high glucose conditions [32]. It was also shown by Perona et al that Rho activates nuclear factor kappa beta in 3T3 cells [33]. Schreck et al demonstrated that hydrogen peroxide and oxygen radicals activate nuclear factor kappa beta in a human T cell line [34]. Interestingly, all of these 3 mediators (mitogen-activated protein kinase, ROS, and nuclear factor kappa beta) were reported to activate angiotensinogen expression. Zhang et al showed that angiotensinogen gene expression is stimulated via p38 kinase pathway in immortalized proximal tubular cells of rat kidney [35]. Hsieh et al found that angiotensinogen gene expression is activated via ROS in a proximal tubular cell line [36]. Finally, angiotensinogen gene expression is activated by nuclear factor kappa beta p65 transcription factor in hepatocytes [37]. Moreover, recent papers suggest a possible linkage between mitogen-activated protein kinase activation and nuclear factor kappa beta pathways [38, 39]. These data suggest that the augmentation of angiotensinogen expression by ROS may involve several pathways. Importantly, we previously demonstrated that ROS-dependent activation of intrarenal angiotensinogen plays an important role in vivo of hypertensive rats [18, 19]. Moreover, we recently reported that the elevated ROS and the ROS-associated augmentation of intrarenal angiotensinogen initiate the development of diabetic nephropathy in type 2 diabetic rats [20]. When we take these data into consideration, the present study may suggest that the activated intrarenal ROS-angiotensinogen axis plays some roles in the development of IgA nephropathy at the early stage.
A variety of markers for oxidative stress are available at present. They reflect either level of oxidative DNA degradation products, lipid peroxidation, oxidative protein degradation products, antioxidant enzymes, or antioxidant metabolites. As a marker of oxidative DNA degradation products, 8-oxo-guanine (8-hydroxy-guanine) and 8-oxo-2'-deoxyguanosine (8-hydroxy-2'-deoxyguanosine) are frequently used. The former is generated by reaction of guanine with ROS [40]. The latter is generated by bind 2'-deoxyribose to 8-oxo-guanie [41]. Among the formed aldehydes, 4-HNE is the major product of lipid peroxidation, and it has been suggested to play a major role in tissue toxicity associated with lipid peroxidation [42]. The isoprostanes are a family of eicosanoids of non-enzymatic origin produced by the random oxidation of tissue phospholipids by oxygen radicals. Isoprostanes appear in the plasma and urine under normal conditions and are elevated by oxidative stress. At least one of the isoprostanes, 8-isoprostane (8-epi-prostaglandin F2-alpha), has been proposed as a marker of antioxidant deficiency and oxidative stress and elevated levels have been found in heavy smokers [43]. Advanced oxidation protein products are general chemical names of oxidative protein degradation products found in plasma of uremic patients [44]. Neutrophils and eosinophils play an important role in the defensive system against microbial infection. Myeloperoxidase and eosinophil peroxidase are known to catalyze formation of hypochlorous acid (HOCl) and hypobromous acid (HOBr). These reactive intermediates react with proteins, and are known to form tyrosine halogenation such as dibromotyrosine, which is also recognized as an oxidative stress marker [45]. Under oxidative stress circumstances, antioxidant enzymes such as superoxide dismutase, catalase, glutathione peroxidase, hemeoxygenase-1 (heat shock protein-32) are induced; therefore, these enzymes are also used as markers of oxidative stress. Especially, hemeoxygenase-1 expression is a sensitive oxidative stress marker, which is mediated by the antioxidant response element in the promoter region of hemeoxygenase-1 gene [46, 47]. Fat-soluble antioxidant metabolites such as retinol (vitamin A), alpha-tocopherol (vitamin E), beta-carotene, and ubiquinone (coenzyme Q) and water-soluble antioxidant metabolites such as ascorbic acid (vitamin C), uric acid, lipoic acid, and glutathione are also used as markers of oxidative stress. In this study, we did not examine the level of 8-oxo-guanine or 8-hydroxy-2'-deoxyguanosine. However, 2 markers of oxidative stress, 4-HNE and hemeoxygenase-1, were enhanced in IgA nephropathy patients. Intensive accumulation of in vivo data as well as in vitro data may lead to a standardization of the methodology of the evaluation for oxidative stress.
Correlation analysis in the present study may provide an interesting perspective. Immunoreactivity of intrarenal angiotensinogen was significantly correlated positively with urinary occult blood, urinary protein-to-creatinine ratio, urinary protein excretion and serum creatinine, and correlated negatively with creatinine clearance. The increases in urinary occult blood, urinary protein-to-creatinine ratio, urinary protein excretion and serum creatinine, and the decrease in creatinine clearance may reflect the magnitude of renal diseases in general. Therefore, the present study may suggest that immunoreactivity of intrarenal angiotensinogen can be a marker of the magnitude of IgA nephropathy. This perspective is supported by recent clinical studies [48, 49]. Yamamoto et al provide evidence demonstrating that urinary angiotensinogen levels reflect intrarenal angiotensin II activity associated with increased risk for deterioration of renal function in 80 chronic kidney disease patients [48]. Do et al also provide evidence presenting that angiotensin II type 1 receptor blocker decreases urinary angiotensinogen levels associated with urinary protein excretion in 32 chronic non-diabetic proteinuric patients [49]. We previously reported that urinary excretion of angiotensinogen is of kidney origin in rats [22, 50-53]. We did not collect urine samples in the present study. However, these data may suggest that intrarenal angiotensinogen levels and urinary angiotensinogen levels can be a marker of the magnitude of chronic kidney diseases including IgA nephropathy. Above 2 studies used indirect measurements to assess the urinary angiotensinogen levels because simple and accurate methods to measure human angiotensinogen directly are unavailable at this time. Recently, two independent groups have developed enzyme linked immunosorbent assay system to measure angiotensinogen directly [26, 54]. Outcomes of clinical studies using the direct measurement of human angiotensinogen are expected in the near future.
Acknowledgements
This study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK072408), the National Center for Research Resources (P20RR017659), the National Heart, Lung, and Blood Institute (R01HL026371), the Health Excellence Fund from Louisiana Board of Regents, and Sankyo Co. Ltd. (Tokyo, Japan). The authors acknowledge excellent technical assistances from My-Linh Rauv, Duy V. Tran, Dale M. Seth, and Mark A. Cabrera (Tulane University).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this study were presented in an abstract form at the 38th Annual Meeting of the American Society of Nephrology in Philadelphia, PA (J Am Soc Nephrol 16 (2005) 521A) and the 39th Annual Meeting of the American Society of Nephrology in San Diego, CA (J Am Soc Nephrol 17 (2006) 253A-254A).
References
- 1.Berger J, Hinglais N. Intercapillary deposits of IgA-IgG. J Urol Nephrol (Paris) 1968;74:694–695. [PubMed] [Google Scholar]
- 2.Barratt J, Feehally J. IgA nephropathy. J Am Soc Nephrol. 2005;16:2088–2097. doi: 10.1681/ASN.2005020134. [DOI] [PubMed] [Google Scholar]
- 3.Rivera F, Lopez-Gomez JM, Perez-Garcia R. Frequency of renal pathology in Spain 1994-1999. Nephrol Dial Transplant. 2002;17:1594–1602. doi: 10.1093/ndt/17.9.1594. [DOI] [PubMed] [Google Scholar]
- 4.Schena FP. Survey of the Italian registry of renal biopsies. Frequency of the renal diseases for 7 consecutive years. The Italian group of renal immunopathology. Nephrol Dial Transplant. 1997;12:418–426. doi: 10.1093/ndt/12.3.418. [DOI] [PubMed] [Google Scholar]
- 5.Simon P, Ramee MP, Boulahrouz R, Stanescu C, Charasse C, Ang KS, Leonetti F, Cam G, Laruelle E, Autuly V, Rioux N. Epidemiologic data of primary glomerular diseases in western France. Kidney Int. 2004;66:905–908. doi: 10.1111/j.1523-1755.2004.00834.x. [DOI] [PubMed] [Google Scholar]
- 6.Nationwide and long-term survey of primary glomerulonephritis in Japan as observed in 1,850 biopsied cases. Research group on progressive chronic renal disease. Nephron. 1999;82:205–213. doi: 10.1159/000045404. [DOI] [PubMed] [Google Scholar]
- 7.Li LS, Liu ZH. Epidemiologic data of renal diseases from a single unit in China: Analysis based on 13,519 renal biopsies. Kidney Int. 2004;66:920–923. doi: 10.1111/j.1523-1755.2004.00837.x. [DOI] [PubMed] [Google Scholar]
- 8.Briganti EM, Dowling J, Finlay M, Hill PA, Jones CL, Kincaid-Smith PS, Sinclair R, McNeil JJ, Atkins RC. The incidence of biopsy-proven glomerulonephritis in Australia. Nephrol Dial Transplant. 2001;16:1364–1367. doi: 10.1093/ndt/16.7.1364. [DOI] [PubMed] [Google Scholar]
- 9.Jennette JC, Wall SD, Wilkman AS. Low incidence of IgA nephropathy in blacks. Kidney Int. 1985;28:944–950. doi: 10.1038/ki.1985.222. [DOI] [PubMed] [Google Scholar]
- 10.Nair R, Walker PD. Is IgA nephropathy the commonest primary glomerulopathy among young adults in the USA? Kidney Int. 2006;69:1455–1458. doi: 10.1038/sj.ki.5000292. [DOI] [PubMed] [Google Scholar]
- 11.Xie Y, Chen X, Nishi S, Narita I, Gejyo F. Relationship between tonsils and IgA nephropathy as well as indications of tonsillectomy. Kidney Int. 2004;65:1135–1144. doi: 10.1111/j.1523-1755.2004.00486.x. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi Y, Hiki Y, Kokubo T, Horii A, Tateno S. Steroid therapy during the early stage of progressive IgA nephropathy. A 10-year follow-up study. Nephron. 1996;72:237–242. doi: 10.1159/000188848. [DOI] [PubMed] [Google Scholar]
- 13.Ballardie FW, Roberts IS. Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. J Am Soc Nephrol. 2002;13:142–148. doi: 10.1681/ASN.V131142. [DOI] [PubMed] [Google Scholar]
- 14.Donadio JV, Jr., Bergstralh EJ, Offord KP, Spencer DC, Holley KE. A controlled trial of fish oil in IgA nephropathy. Mayo nephrology collaborative group. N Engl J Med. 1994;331:1194–1199. doi: 10.1056/NEJM199411033311804. [DOI] [PubMed] [Google Scholar]
- 15.Strippoli GF, Manno C, Schena FP. An “Evidence-based” Survey of therapeutic options for IgA nephropathy: Assessment and criticism. Am J Kidney Dis. 2003;41:1129–1139. doi: 10.1016/s0272-6386(03)00344-5. [DOI] [PubMed] [Google Scholar]
- 16.Dillon JJ. Fish oil therapy for IgA nephropathy: Efficacy and interstudy variability. J Am Soc Nephrol. 1997;8:1739–1744. doi: 10.1681/ASN.V8111739. [DOI] [PubMed] [Google Scholar]
- 17.Dillon JJ. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for IgA nephropathy. Semin Nephrol. 2004;24:218–224. doi: 10.1016/j.semnephrol.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension. 2003;41:592–597. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobori H, Nishiyama A. Effects of tempol on renal angiotensinogen production in Dahl salt-sensitive rats. Biochem Biophys Res Commun. 2004;315:746–750. doi: 10.1016/j.bbrc.2004.01.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzaki Y, Ozawa Y, Kobori H. Intrarenal oxidative stress and augmented angiotensinogen are precedent to renal injury in Zucker diabetic fatty rats. Int J Biol Sci. 2007;3:40–46. doi: 10.7150/ijbs.3.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004;43:1126–1132. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol. 2005;16:2073–2080. doi: 10.1681/ASN.2004080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutierrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol. 2005;289:F632–637. doi: 10.1152/ajprenal.00462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozawa Y, Kobori H, Suzaki Y, Navar LG. Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol. 2007;292:F330–339. doi: 10.1152/ajprenal.00059.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzaki Y, Ozawa Y, Kobori H. Quantification of human angiotensinogen by a novel sandwich ELISA. Peptides. 2006;27:3000–3002. doi: 10.1016/j.peptides.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai KN, Chan LY, Tang SC, Tsang AW, Li FF, Lam MF, Lui SL, Leung JC. Mesangial expression of angiotensin II receptor in IgA nephropathy and its regulation by polymeric IgA1. Kidney Int. 2004;66:1403–1416. doi: 10.1111/j.1523-1755.2004.00874.x. [DOI] [PubMed] [Google Scholar]
- 28.Chan LY, Leung JC, Tang SC, Choy CB, Lai KN. Tubular expression of angiotensin II receptors and their regulation in IgA nephropathy. J Am Soc Nephrol. 2005;16:2306–2317. doi: 10.1681/ASN.2004121117. [DOI] [PubMed] [Google Scholar]
- 29.Rao GN. Hydrogen peroxide induces complex formation of shc-grb2-sos with receptor tyrosine kinase and activates ras and extracellular signal-regulated protein kinases group of mitogen-activated protein kinases. Oncogene. 1996;13:713–719. [PubMed] [Google Scholar]
- 30.Yoshizumi M, Abe J, Haendeler J, Huang Q, Berk BC. Src and cas mediate JNK activation but not ERK1/2 and p38 kinases by reactive oxygen species. J Biol Chem. 2000;275:11706–11712. doi: 10.1074/jbc.275.16.11706. [DOI] [PubMed] [Google Scholar]
- 31.Suzaki Y, Yoshizumi M, Kagami S, Koyama AH, Taketani Y, Houchi H, Tsuchiya K, Takeda E, Tamaki T. Hydrogen peroxide stimulates c-src-mediated big mitogen-activated protein kinase 1 (BMK1) and the MEF2C signaling pathway in PC12 cells: Potential role in cell survival following oxidative insults. J Biol Chem. 2002;277:9614–9621. doi: 10.1074/jbc.M111790200. [DOI] [PubMed] [Google Scholar]
- 32.Suzaki Y, Yoshizumi M, Kagami S, Nishiyama A, Ozawa Y, Kyaw M, Izawa Y, Kanematsu Y, Tsuchiya K, Tamaki T. BMK1 is activated in glomeruli of diabetic rats and in mesangial cells by high glucose conditions. Kidney Int. 2004;65:1749–1760. doi: 10.1111/j.1523-1755.2004.00576.x. [DOI] [PubMed] [Google Scholar]
- 33.Perona R, Montaner S, Saniger L, Sanchez-Perez I, Bravo R, Lacal JC. Activation of the nuclear factor-kappaB by Rho, CDC42, and Rac-1 proteins. Genes Dev. 1997;11:463–475. doi: 10.1101/gad.11.4.463. [DOI] [PubMed] [Google Scholar]
- 34.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang SL, Tang SS, Chen X, Filep JG, Ingelfinger JR, Chan JS. High levels of glucose stimulate angiotensinogen gene expression via the p38 mitogen-activated protein kinase pathway in rat kidney proximal tubular cells. Endocrinology. 2000;141:4637–4646. doi: 10.1210/endo.141.12.7844. [DOI] [PubMed] [Google Scholar]
- 36.Hsieh TJ, Zhang SL, Filep JG, Tang SS, Ingelfinger JR, Chan JS. High glucose stimulates angiotensinogen gene expression via reactive oxygen species generation in rat kidney proximal tubular cells. Endocrinology. 2002;143:2975–2985. doi: 10.1210/endo.143.8.8931. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Brasier AR. Angiotensinogen gene activation by angiotensin II is mediated by the rel A (nuclear factor-kappaB p65) transcription factor: One mechanism for the renin angiotensin system positive feedback loop in hepatocytes. Mol Endocrinol. 1996;10:252–264. doi: 10.1210/mend.10.3.8833654. [DOI] [PubMed] [Google Scholar]
- 38.Brinkmann MM, Glenn M, Rainbow L, Kieser A, Henke-Gendo C, Schulz TF. Activation of mitogen-activated protein kinase and NF-kappaB pathways by a kaposi's sarcoma-associated herpesvirus k15 membrane protein. J Virol. 2003;77:9346–9358. doi: 10.1128/JVI.77.17.9346-9358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmann E, Thiefes A, Buhrow D, Dittrich-Breiholz O, Schneider H, Resch K, Kracht M. Mek1-dependent delayed expression of fos-related antigen-1 counteracts c-fos and p65 NF-kappaB-mediated interleukin-8 transcription in response to cytokines or growth factors. J Biol Chem. 2005;280:9706–9718. doi: 10.1074/jbc.M407071200. [DOI] [PubMed] [Google Scholar]
- 40.Aruoma OI, Halliwell B, Gajewski E, Dizdaroglu M. Damage to the bases in DNA induced by hydrogen peroxide and ferric ion chelates. J Biol Chem. 1989;264:20509–20512. [PubMed] [Google Scholar]
- 41.Asagoshi K, Yamada T, Terato H, Ohyama Y, Monden Y, Arai T, Nishimura S, Aburatani H, Lindahl T, Ide H. Distinct repair activities of human 7,8-dihydro-8-oxoguanine DNA glycosylase and formamidopyrimidine DNA glycosylase for formamidopyrimidine and 7,8-dihydro-8-oxoguanine. J Biol Chem. 2000;275:4956–4964. doi: 10.1074/jbc.275.7.4956. [DOI] [PubMed] [Google Scholar]
- 42.Ji C, Kozak KR, Marnett LJ. Ikappab kinase, a molecular target for inhibition by 4-hydroxy-2-nonenal. J Biol Chem. 2001;276:18223–18228. doi: 10.1074/jbc.M101266200. [DOI] [PubMed] [Google Scholar]
- 43.Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ., 2nd Increase in circulating products of lipid peroxidation (f2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332:1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 44.Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–1313. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 45.Kato Y, Kawai Y, Morinaga H, Kondo H, Dozaki N, Kitamoto N, Osawa T. Immunogenicity of a brominated protein and successive establishment of a monoclonal antibody to dihalogenated tyrosine. Free Radic Biol Med. 2005;38:24–31. doi: 10.1016/j.freeradbiomed.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 46.Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kda stress protein induced in human skin fibroblasts by uva radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 1989;86:99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prestera T, Talalay P, Alam J, Ahn YI, Lee PJ, Choi AM. Parallel induction of heme oxygenase-1 and chemoprotective phase 2 enzymes by electrophiles and antioxidants: Regulation by upstream antioxidant-responsive elements (ARE) Mol Med. 1995;1:827–837. [PMC free article] [PubMed] [Google Scholar]
- 48.Yamamoto T, Nakagawa T, Suzuki H, Ohashi N, Fukasawa H, Fujigaki Y, Kato A, Nakamura Y, Suzuki F, Ikegaya N, Hishida A. Urinary angiotensinogen as a marker of intrarenal angiotensin II (AII) activity causing deterioration of renal function in patients with chronic kidney disease (CKD) J Am Soc Nephrol. 2006;17:380A. doi: 10.1681/ASN.2006060554. [DOI] [PubMed] [Google Scholar]
- 49.Do YS, Kim Y-G, Kim YK, Lee JE, Huh WS, Kim DJ, Oh HY. Effects of angiotensin II receptor blocker on the urinary angiotensinogen excretion in chronic non-diabetic proteinuric patients. J Am Soc Nephrol. 2006;17:575A. [Google Scholar]
- 50.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–1335. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal angiotensin status in hypertension. Hypertension. 2003;41:42–49. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lantelme P, Rohrwasser A, Vincent M, Cheng T, Gardier S, Legedz L, Bricca G, Lalouel JM, Milon H. Significance of urinary angiotensinogen in essential hypertension as a function of plasma renin and aldosterone status. J Hypertens. 2005;23:785–792. doi: 10.1097/01.hjh.0000163147.20330.f5. [DOI] [PubMed] [Google Scholar]