Abstract
Ependymal overexpression of brain-derived neurotrophic factor (BDNF) stimulates neuronal addition to the adult striatum, from subependymal progenitor cells. Noggin, by suppressing subependymal gliogenesis and increasing progenitor availability, potentiates this process. We asked whether BDNF/Noggin overexpression might be used to recruit new striatal neurons in R6/2 huntingtin transgenic mice. R6/2 mice injected with adenoviral BDNF and adenoviral Noggin (AdBDNF/AdNoggin) recruited BrdU+βIII-tubulin+ neurons, which developed as DARPP-32+ and GABAergic medium spiny neurons that expressed either enkephalin or substance P and extended fibers to the globus pallidus. Only AdBDNF/AdNoggin-treated R6/2 mice harbored migrating doublecortin-defined neuroblasts in their striata, and the new neurons expressed p27 as a marker of mitotic quiescence after parenchymal integration. AdBDNF/AdNoggin-treated R6/2 mice sustained their rotarod performance and open-field activity and survived longer than did AdNull-treated and untreated controls. Neither motor performance nor survival improved in R6/2 mice treated only with AdBDNF, and intraventricular infusion of the mitotic inhibitor Ara-C completely blocked the performance and survival effects of AdBDNF/AdNoggin, suggesting that the benefits of AdBDNF/AdNoggin derived from neuronal addition. Thus, BDNF and Noggin induced striatal neuronal regeneration, delayed motor impairment, and extended survival in R6/2 mice, suggesting a new therapeutic strategy in Huntington disease.
Introduction
Neural stem cells persist throughout the ventricular subependyma of the adult vertebrate brain (1–5). These adult neural stem cells can be induced to differentiate and survive as neurons under the influence of brain-derived neurotrophic factor (BDNF) (6–9). When administered intraventricularly, BDNF increases neuronal addition to the olfactory bulb, a typical neurogenic site (10). In addition, BDNF administration and overexpression are both associated with heterotopic neuronal addition to the neostriatum, a region of the brain that is typically non-neurogenic in adults (11–13). These results indicated the feasibility of recruiting new neurons from resident progenitor cells in otherwise non-neurogenic regions of the adult forebrain. However, the vast majority of cells generated from adult subependymal cells either die (14) or differentiate as glia. This raised the possibility that neuronal production by resident progenitor cells might be potentiated by concurrently suppressing glial differentiation. Previous studies had shown that the bone morphogenetic proteins (BMPs) can direct neural progenitors to glial fate in both the adult and the late fetal brain (15, 16). On this basis, we overexpressed Noggin, a soluble inhibitor of the BMPs (17), so as to suppress astroglial differentiation by subependymal zone (SZ) progenitor cells, thereby promoting their neuronal differentiation. We found that Noggin indeed suppressed subependymal gliogenesis, and in so doing expanded the pool of SZ cells responsive to neuronal instruction by BDNF (18, 19). In normal adult rats, concurrent Noggin and BDNF overexpression yielded a substantial increase in the number of new neurons recruited to the neostriatum. Most were added as medium spiny neurons (MSNs), which expressed both the medium spiny protein dopamine- and cAMP-regulated phosphoprotein–32 kDa (DARPP-32) and the principal synthetic enzyme for GABA, glutamic acid dehydrogenase–67 kDa (GAD67). Fluorogold (FG) backfills then revealed that most new MSNs extended fibers to their usual target, the globus pallidus. Together, these data suggested that BDNF-induced neurons could mature as medium spiny pallidal projection neurons (18).
MSNs comprise the predominant striatal phenotype lost in Huntington disease (HD). HD is an autosomal-dominant disorder associated with multifocal degeneration; striatal atrophy is its hallmark. HD is associated with CAG repeat expansions in the first exon of the huntingtin gene; these lead to the production of a mutant huntingtin with marked polyglutamine expansions (20, 21). Mutant huntingtin exerts a neurotoxic effect manifested in the selective degeneration of several discrete neuronal populations, the most dramatically affected of which is the MSN of the caudate-putamen. Because viral overexpression of BDNF and Noggin induced the selective addition of new neurons in normal rats (18), we asked whether this strategy might be used to restore MSNs to the afflicted HD neostriatum. To this end, we overexpressed both BDNF and Noggin in R6/2 transgenic mice. These mice express a mutant exon 1 of the human huntingtin gene, engineered to include an approximately 145 CAG repeat expansion (22, 23). R6/2 mice phenocopy much of the neuropathology and behavioral manifestations of clinical HD, its juvenile-onset form in particular (24, 25). They exhibit motor deficits as early as 5–6 wk of age, display overt behavioral abnormalities at 8–9 wk, and typically die between 11 and 13 wk (25).
We report here that adenoviral (Ad) overexpression of BDNF and Noggin in the striatal ventricular wall induced substantial neuronal addition to the striata of R6/2 huntingtin mutant mice. The newly recruited striatal neurons developed as DARPP-32+ and GAD67+ GABAergic MSNs and matured to extend fibers to the ipsilateral globus pallidus. The new striopallidal projection neurons survived and integrated as GABAergic MSNs and included both enkephalin- and substance P–expressing (SP-expressing) neurons, respective constituents of the indirect and direct pallidofugal pathways. The AdBDNF/AdNoggin-treated animals exhibited delayed motor deterioration, with substantially improved and sustained rotarod performance and open-field activity relative to untreated R6/2 mice. Moreover, they survived significantly longer than did untreated control R6/2 mice, maintaining volitional activity and self care significantly beyond the point at which all untreated R6/2 mice had died. Importantly, BDNF treatment alone, which provides neurotrophic support but only a limited stimulus to neuronal addition, failed to confer any performance or survival benefit to R6/2 mice. Furthermore, the mitotic inhibitor cytosine-β-D-arabinofuranoside (Ara-C) completely blocked both the performance and the survival benefits of AdBDNF/AdNoggin in R6/2 mice, pari passu with its suppression of mitotic neurogenesis. These findings strongly suggest that the benefits of AdBDNF/AdNoggin treatment were due to neuronal addition per se, rather than to any neuroprotective effect of the expressed neurotrophins, thereby implicating induced neurogenesis as an important contributor to the functional improvement of treated R6/2 mice.
Results
AdBDNF induced striatal neuronal addition in both WT and R6/2 mice.
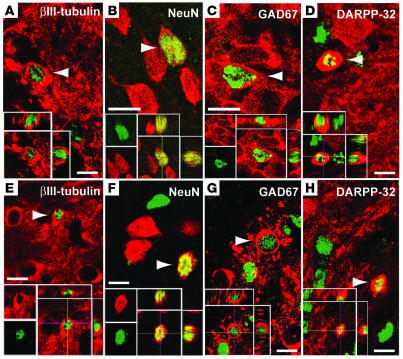
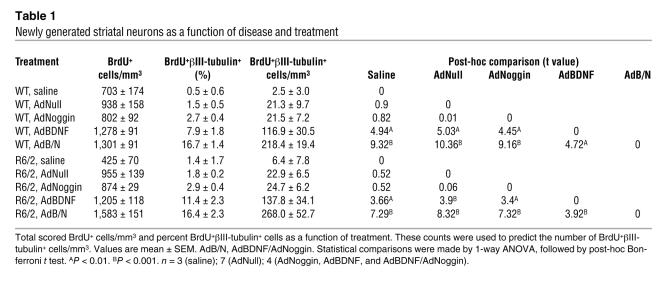
A total of 44 mice, 22 R6/2 and 22 WT controls, were used to assess histologically the ability of BDNF combined with Noggin treatment to elicit striatal neurogenesis in R6/2 mice relative to WT controls. At 6 wk of age, matched groups of R6/2 and WT mice received bilateral 1.5 μl intraventricular injections of AdBDNF/AdNoggin (n = 4 per group); AdBDNF (n = 4 per group); AdNoggin (n = 4 per group); AdNull (n = 7 per group); or saline (n = 3 per group). All mice were treated with BrdU once daily for 4 wk thereafter, and then sacrificed at 10 wk of age. Their brains were sectioned and their striata immunolabeled for BrdU together with either βIII-tubulin or NeuN, so as to identify newly generated neurons, using confocal validation as described previously (11, 26). Among the R6/2 mice, AdBDNF-treated animals exhibited 137.8 ± 34.1 BrdU+βIII-tubulin+ cells/mm3, significantly more than their counterparts given AdNoggin (24.7 ± 6.2 cells/mm3), AdNull (22.9 ± 6.5 cells/mm3), or saline (6.4 ± 7.8 cells/mm3; P < 0.05 for each comparison, ANOVA followed by post-hoc Bonferroni t tests; Figure 1A and Figure 2A). Similarly, WT mice injected solely with AdBDNF exhibited 116.9 ± 30.5 BrdU+βIII-tubulin+ cells/mm3, significantly more than mice given AdNoggin (21.5 ± 7.2 cells/mm3), AdNull (21.3 ± 9.7 cells/mm3), or saline (2.5 ± 3.0 cells/mm3; P < 0.05 for each comparison; Figure 1E and Figure 2A). Thus, AdBDNF elicited substantial neuronal addition to both R6/2 and WT neostriata. In each, the neuronal identity of AdBDNF-induced BrdU+βIII-tubulin+ cells was confirmed by labeling for NeuN (Figure 1, B and F), GAD67 (Figure 1, C and G), and DARPP-32 (Figure 1, D and H).
Figure 1. AdBDNF induced striatal neuronal recruitment in both R6/2 and WT mice.
At 1 mo after AdBDNF injection (6 wk), R6/2 striata were double-stained for BrdU (green) and βIII-tubulin (red, A), NeuN (red, B), GAD67 (red, C), or DARPP-32 (red, D). Identically treated WT mice stained for the same markers are shown in E–H. Colabeling of BrdU with each neuronal marker was confirmed by confocal optical sectioning, with orthogonal views in the xz and yz planes (insets). Arrowheads denote double-labeled cells. Scale bars: 10 μm.
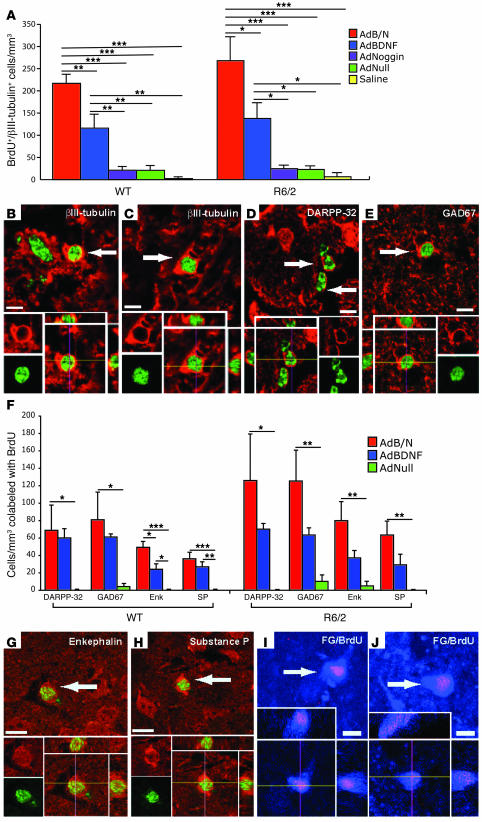
Figure 2. AdNoggin potentiated AdBDNF-induced neuronal addition and integration.
(A) Density of BrdU+βIII-tubulin+ cells in the striatum of R6/2 and WT mice injected once at 4 wk of age with AdBDNF/AdNoggin (AdB/N), AdBDNF, AdNoggin, AdNull, or saline. (B–E) Newly generated neurons were recognized in both WT (B) and R6/2 (C–E) mice by confocal imaging of BrdU (green) colabeling with βIII-tubulin (red, B and C), DARPP-32 (red, D), or GAD67 (red, E). (F) Density of BrdU+ cells (green) coexpressing either DARPP-32, GAD67, enkephalin (Enk), or SP in the striatum of AdBDNF/AdNoggin-, AdBDNF-, or AdNull-injected WT and R6/2 mice. (G and H) BrdU-tagged (green) enkephalinergic (red, G) and SP (red, H) neurons in R6/2 mice. (I and J) FG injection of the globus pallidus revealed BrdU-tagged striatal projection neurons in AdBDNF/AdNoggin-injected 11-wk-old WT (I) and R6/2 (J) mice. *P < 0.05, **P < 0.01, ***P < 0.001, 1-way ANOVA followed by post-hoc Bonferroni t tests. Arrows denote double-labeled cells. Scale bars: 10 μm.
AdNoggin potentiated AdBDNF-induced striatal neuronal recruitment.
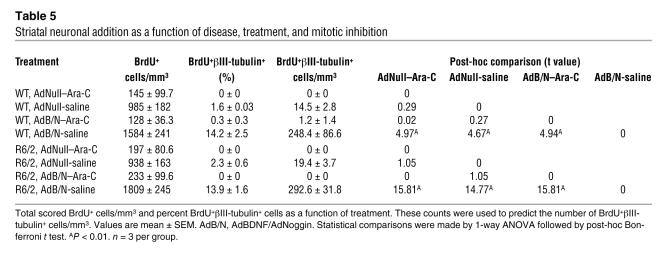
We next postulated that by suppressing gliogenesis, Noggin might increase the pool of R6/2 ventricular zone progenitors responsive to neuronal instruction by BDNF. WT mice treated with AdBDNF/AdNoggin exhibited 218.4 ± 19.4 BrdU+βIII-tubulin+ cells/mm3, significantly more than WT mice injected solely with AdBDNF, AdNoggin, AdNull, or saline (P < 0.01 for each comparison; Figure 2, A and B, and Table 1). Similarly, the neostriata of AdBDNF/AdNoggin-treated R6/2 mice harbored 268.0 ± 52.7 BrdU+βIII-tubulin+ cells/mm3, significantly more than that observed in R6/2 mice given only AdBDNF (P < 0.05) or AdNoggin, AdNull, or saline (P < 0.001 for each comparison; Figure 2, A and C). These data indicated that in both WT and R6/2 mice, BDNF and Noggin acted cooperatively to induce striatal neuronal recruitment. AdBDNF/AdNoggin treatment elicited as strong a neurogenic response in R6/2 mice as in WT mice, suggesting that neither the mutant huntingtin phenotype nor any antecedent compensatory progenitor response had depleted or exhausted the progenitor pool in R6/2 mice. Thus, the concurrent use of AdBDNF and AdNoggin, by effectively providing a permissive and instructive environment for striatal neurogenesis, greatly enhanced neuronal recruitment to the striatum.
Table 1 .
Newly generated striatal neurons as a function of disease and treatment
New striatal neurons were recruited as MSNs.
We previously noted in rats that AdBDNF-induced striatal neurons expressed markers characteristic of GABAergic MSNs, such as calbindin, GAD67, and DARPP-32 (11). To determine whether newly recruited neurons in the R6/2 striatum likewise differentiated as MSNs, we immunolabeled sections of BrdU-tagged R6/2 striata for BrdU and either GAD67 or DARPP-32, the latter being the most definitive marker for the GABAergic MSN phenotype within the striatum (27, 28). We compared the resultant counts to those obtained from R6/2 mice injected with either AdBDNF alone or AdNull. In addition, counts were obtained from matched WT controls similarly injected with either AdNull, AdBDNF, or AdBDNF/AdNoggin.
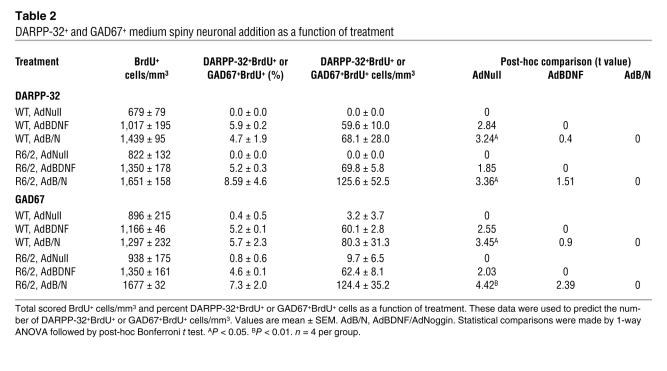
In R6/2 mice injected with AdBDNF/AdNoggin at 6 wk and killed at 10 wk, 8.6% ± 4.6% of the imaged BrdU+ cells, or 125.6 ± 52.5 cells/mm3, were DARPP-32+, while 124.4 ± 35.2 cells/mm3 expressed GAD67 (Figure 2, D–F, and Table 2). In contrast, only 69.8 ± 5.8 DARPP-32+BrdU+ and 62.4 ± 8.1 GAD67+ striatal neurons/mm3 were noted in R6/2 mice treated with AdBDNF alone. Moreover, AdNull-injected mice exhibited either no (DARPP-32+BrdU+) or insignificantly few (GAD67+BrdU+) new MSNs, consistent with their lack of spontaneous striatal neurogenesis at this age. The difference between AdBDNF/AdNoggin-treated and AdNull-treated R6/2 mice in their number of newly recruited striatal DARPP-32+ and GAD67+ neurons, like that demonstrated previously in their total number of new neurons, proved significant (F = 10.58, P < 0.01, and F =15.78, P < 0.001, respectively, 1-way ANOVA with post-hoc Bonferroni t tests). WT mice exhibited the same effect, with AdBDNF/AdNoggin yielding a significant recruitment of both DARPP-32+BrdU+ and GAD67+BrdU+ neurons (Figure 2F).
Table 2 .
DARPP-32+ and GAD67+ medium spiny neuronal addition as a function of treatment
Both enkephalinergic and SP+ MSNs were induced by AdBDNF/AdNoggin.
MSNs include nonoverlapping pools of pallidal projection neurons that express either enkephalin or SP. Enkephalinergic neurons largely project to the external globus pallidus, and thence to the subthalamus, while SP+ cells project to the internal segment of the pallidum and subsequently to the substantia nigra and thalamus (29). As a result, SP+ and enkephalinergic MSNs contribute to the functionally distinct direct and indirect pallidofugal pathways, respectively (30). Enkephalinergic MSNs are especially vulnerable in HD, such that striatal input to the external segment of the globus pallidus is preferentially lost (30). To predict the relative reconstitution of the indirect and direct pallidofugal pathways by AdBDNF/AdNoggin-mediated neuronal addition, we asked whether and to what extent newly generated MSNs assumed enkephalinergic or SP+ phenotypes. Sections of R6/2 striata (n = 4) were sampled from mice sacrificed at 10 wk of age, after AdBDNF/AdNoggin injection at 6 wk and daily BrdU tagging for a month thereafter. The sections were then stained for BrdU together with either enkephalin or SP. In addition, to assess the possibility that the AdBDNF/AdNoggin-induced production of enkephalinergic and SP+ neurons might be influenced by disease state, we compared matched samples of R6/2 mice and WT controls (n = 4 per group).
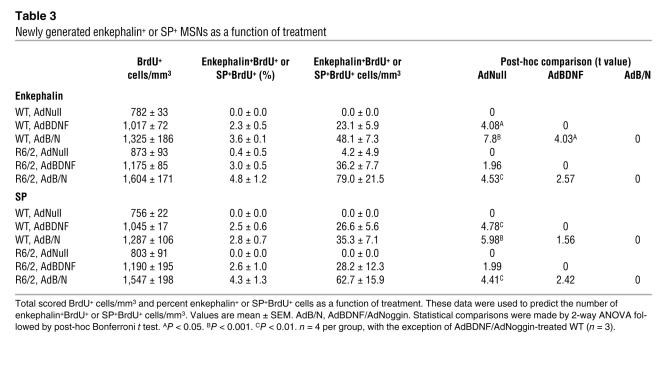
We found that both enkephalin+ and SP+ MSNs were generated in response to AdBDNF/AdNoggin in roughly equal numbers (Figure 2, F–H, and Table 3). We counted 79.0 ± 21.5 BrdU+enkephalin+ striatal cells/mm3 (Figure 2G), and 62.7 ± 15.9 SP+ cells/mm3 in the same animals (Figure 2H), 4 wk after AdBDNF/AdNoggin injection. In contrast, no BrdU-tagged SP+ neurons, and 4.2 ± 4.9 BrdU+enkephalin+ neurons/mm3 (the latter not significantly different from 0), were noted in the AdNull-treated R6/2 mice. Thus, both enkephalinergic and SP+ MSNs were generated in response to AdBDNF/AdNoggin treatment, mirroring the typical loss of both of these phenotypes in the R6/2 model and suggesting that both direct and indirect pallidofugal pathways may be supplemented by progenitor-derived MSNs. The relative numbers and proportions of BrdU-incorporating enkephalinergic and SP+ neurons did not differ significantly between AdBDNF/AdNoggin-treated R6/2 and WT mice, indicating that the phenotypes assumed by new neurons were not influenced by the R6/2 disease environment.
Table 3 .
Newly generated enkephalin+ or SP+ MSNs as a function of treatment
New neurons were in fact new neurons.
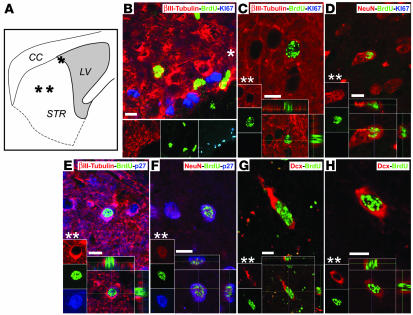
Recent reports have noted instances of BrdU incorporation in the setting of neuronal disease or death, which have been associated with the aborted reentry of threatened neurons into cell cycle (31–33). To assess whether R6/2 HD mice exhibit BrdU incorporation by neurons dying as a result of aborted cell cycle reentry, we performed a number of additional experiments. First, we used both caspase-3 and TUNEL immunostaining at 10 wk to assess the incidence of apoptotic neuronal death in the striata of R6/2 mice. Despite abundant caspase labeling in the brains of 1-d-old mice, which served as positive controls because of their abundant perinatal cell death, we observed few caspase+ neurons in 10-wk-old R6/2 striata (Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI31778DS1). Similarly, we observed only rare TUNEL+ cells. These observations were consistent with previous reports that R6/2 mice exhibit little apoptotic neuronal death (23, 34, 35), despite past reports of both caspase transcription (36) and striatal involution (35). Thus, we found no evidence of appreciable apoptotic cell death in the R6/2 striatum, arguing against artifactual uptake of BrdU occurring on this basis. Next, we focused on previous observations that neurons dying of hypoxic ischemia can express markers consistent with G1/S phase transition, such as Ki67, while incorporating BrdU, and that these dying cells may lose expression of the G1-cyclin-dependent kinase (CDK) inhibitor protein p27 (32). To assess the incidence of such nonproliferative BrdU incorporation by striatal neurons in AdBDNF/AdNoggin-treated R6/2 mice, we immunostained R6/2 striata for the mitotic marker Ki67. Of a confocal-analyzed sample of 37 BrdU+βIII-tubulin+ neurons randomly imaged in Ki67 costained sections, none coexpressed Ki67 (Figure 3, A–D). To the contrary, these BrdU+βIII-tubulin+ neurons invariably expressed the CDK inhibitor p27, as would be expected of postmitotic neurons that had earlier incorporated BrdU (Figure 3, E and F). Indeed, of 33 BrdU+βIII-tubulin+ neurons randomly identified and confocal imaged in p27 costained sections, all expressed p27; none failed to do so. Thus, the BrdU+βIII-tubulin+ neurons generated in AdBDNF/AdNoggin-treated R6/2 striata expressed the postmitotic CDK inhibitor p27, but did not express the active cell cycle marker Ki67, consistent with the mitotic generation and then cell cycle exit of those BrdU+βIII-tubulin+ and BrdU+NeuN+ neurons observed in the striata of AdBDNF/AdNoggin-treated R6/2 mice.
Figure 3. New neurons were the product of antecedent neuronal mitogenesis.
(A) Schematic of a mouse brain section through the striatum (STR) and lateral ventricle (LV) showing the locations of images in B–H (asterisks). CC, corpus callosum. (B) Striatal ventricular wall of an AdBDNF/AdNoggin-treated R6/2 mouse, given BrdU for 3 wk after viral injection at 6 wk of age and sacrificed at 10 wk, immunostained for BrdU (green), βIII-tubulin (red), and Ki67 (blue), which is expressed by mitotically active cells. In the subventricular zone, actively dividing subependymal cells expressed Ki67, whereas BrdU+ daughter cells, the products of earlier divisions, did not. In the neostriatum within the same section, newly generated BrdU+ neurons identified by βIII-tubulin (C) or NeuN (D) did not express Ki67 and thus showed no evidence of either persistent mitotic competence or aberrant cell cycle reentry. To the contrary, newly generated BrdU+βIII-tubulin+ (E) and BrdU+NeuN+ (F) neurons expressed the tumor suppressor p27kip1 (blue, E and F), a marker of mitotic quiescence. A cohort of BrdU+ cells in AdBDNF/AdNoggin-treated mice coexpressed the developmental migratory neuroblastic marker DCX (red, G and H); importantly, these BrdU+DCX+ cells were not found in AdNull-treated R6/2 striata at this age, indicating that the immigration of migrating neuroblasts into the R6/2 striatum was a function of AdBDNF/AdNoggin treatment. Scale bars: 10 μm (B–F); 5 μm (G and H).
Only AdBDNF/AdNoggin-treated R6/2 striata harbored migrating neuroblasts.
As an independent means of establishing BrdU+ neurons as newly generated, we immunostained both AdBDNF/AdNoggin-treated mice and their AdNull-injected controls for doublecortin (DCX) protein. DCX is transiently expressed during neuroblast migration (37); although expressed by parenchymal progenitors (38), it is not known to be misexpressed by damaged neurons. We reasoned that if BrdU incorporation was limited to new neurons, and if only AdBDNF/AdNoggin-treated R6/2 mice exhibited significant striatal recruitment of new neurons, then only AdBDNF/AdNoggin-treated mice, and not untreated controls, would exhibit double-labeled BrdU+DCX+ striatal neuroblasts. To test this postulate, an observer blinded as to treatment compared the incidence of BrdU+DCX+ cells in striatal sections (12 per mouse) sampled from 10-wk-old AdBDNF/AdNoggin- and AdNull-treated R6/2 mice (n = 6–8 per group). In the AdBDNF/AdNoggin-injected mice, we identified, and verified by confocal imaging, a total of 23 BrdU+DCX+ cells among 1,231 BrdU+ cells scored, most of a typical bipolar neuroblastic morphology (Figure 3, G and H). In contrast, of 630 BrdU+ cells identified in the AdNull-injected R6/2 striata, we failed to identify a single BrdU+DCX+ cell. Analysis of the difference between these observed frequencies yielded a c2 value of 11.9, indicating a highly significant difference (P = 0.0006) in the frequency of BrdU+DCX+ cells in the striata of AdBDNF/AdNoggin- and AdNull-treated R6/2 mice. The apparent restriction of BrdU+DCX+ cells to the striata of AdBDNF/AdNoggin-treated R6/2 mice, and the absence of these cells from their untreated controls, suggests that these cells represent migrating neuroblasts, newly generated in response to BDNF and Noggin overexpression.
Newly generated striatal neurons projected to the globus pallidus.
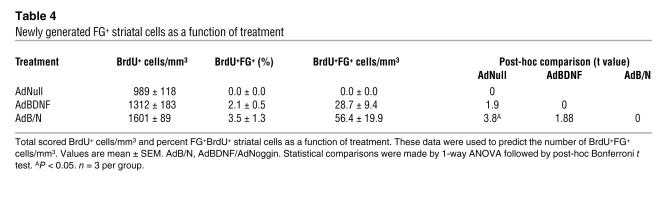
We next asked whether new MSNs extended processes to their normal developmental target, the globus pallidus. Mice treated with AdBDNF/AdNoggin, AdBDNF, or AdNull at 4 wk of age (n = 3 per group) and tagged with BrdU for 3 wk thereafter (100 mg/kg daily i.p.) were injected at 10 wk of age with the retrograde tracer FG delivered into the globus pallidus. The mice were sacrificed 10 d later, at 7.5 wk after initial virus administration. Their striata were assessed for the incidence of BrdU+FG+ cells, which we defined as newly generated pallidal projection neurons. To avoid spread of the FG tracer beyond the pallidum and thus ensure the identity of retrograde-labeled neurons, we injected only 1 μl of a 1% solution; this would have underestimated the incidence of BrdU+FG+ neurons in these striata. Nonetheless, 56.4 ± 19.9 neurons/mm3 in AdBDNF/AdNoggin-treated animals and 28.7 ± 9.4 neurons/mm3 in mice treated with AdBDNF alone were BrdU+FG+, and thus identifiable as new pallidal projection neurons (Figure 2, I and J, and Table 4). In contrast, no BrdU+FG+ neurons were noted in AdNull-treated striata; only those mice treated with AdBDNF or AdBDNF/AdNoggin generated new striatal neurons whose projections achieved pallidal innervation (P < 0.05, 1-way ANOVA; Table 4).
Table 4 .
Newly generated FG+ striatal cells as a function of treatment
To exclude the possibility that physical diffusion of FG from the pallidal injection sites to the striatum might have yielded any false-positive BrdU+FG+ striatal neurons, we immunostained the striata of AdBDNF/AdNoggin-treated R6/2 mice for choline acetyltransferase (ChAT), a marker of cholinergic neurons. Cholinergic neurons comprise a minor, but readily apparent, fraction of striatal neurons. However, unlike the MSNs, striatal cholinergic neurons do not project to the globus pallidus. As a result, they do not incorporate FG delivered to the globus pallidus, but would be expected to label with FG were any of the tracer to enter the striatal parenchyma. We therefore reasoned that if diffusion of FG to the striatum were to account for any of the BrdU+FG+ striatal neurons we observed, then we should expect FG+ChAT+ neurons to be present as well, whereas the absence of the latter would be inconsistent with significant striatal penetration of the injected FG. To test this possibility, we immunostained the brains of 3 FG-injected R6/2 mice, each given an intrapallidal injection of FG 10 d before sacrifice, for ChAT. We then assessed the incidence of ChAT+ striatal neurons and the fraction of these that were FG+. We found that only 2 of 96 ChAT+ neurons sampled within the dorsal two-thirds of the mouse striata, the major region of neuronal addition, had incorporated FG, despite virtually uniform FG incorporation by neighboring, noncholinergic striatal neurons (Supplemental Figure 2). These results provide additional evidence that the BrdU+FG+ neurons noted in AdBDNF/AdNoggin-treated mice represent newly generated neurons that have extended axons though the striatal parenchyma to their normal targets in the adult globus pallidus.
AdBDNF/AdNoggin treatment slowed motor deterioration.
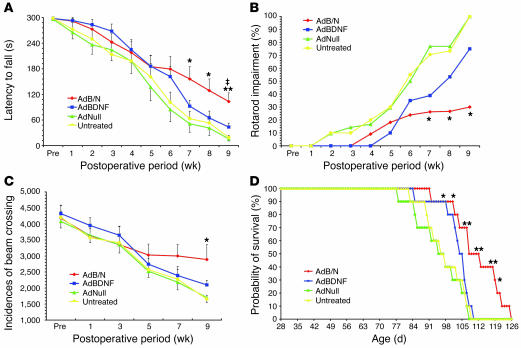
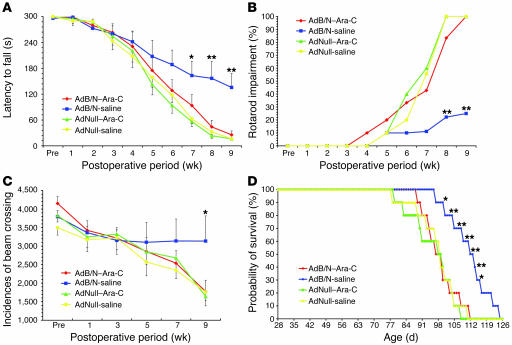
To assess the effect of AdBDNF/AdNoggin injection upon the functional deterioration of R6/2 mice, we used both rotarod testing and open-field analysis of volitional locomotion. We also asked whether AdBDNF/AdNoggin cotreatment, which yields substantially more neuronal recruitment than that afforded by AdBDNF alone, would result in better motor performance than that achieved solely by BDNF (30). To these ends, we established new cohorts of R6/2 mice treated with AdBDNF/AdNoggin (n = 22), AdBDNF alone (n = 20), and AdNull (n = 15) as well as saline-treated controls (n = 14). All mice were trained on the rod by 4 wk of age, and then tested weekly at a constant 12 rpm (39). We found that the AdBDNF/AdNoggin-treated mice exhibited a significant slowing in their rate of motor deterioration, relative to the AdNull-treated R6/2 controls (Figure 4A and Supplemental Videos 1–5). When the latency to fall off the rotarod (y) was plotted as a function of postoperative survival (x), curves were generated for AdBDNF/AdNoggin- and AdNull-injected mice that appeared to diverge at approximately 5 wk after treatment. Simple regression analysis revealed that whereas the motor performance of AdBDNF/AdNoggin-treated animals could be described by the line y = –28.6x + 322.7, that of the AdNull-treated controls was described by y = –44.0x + 321.4. ANOVA of these regressions revealed that the rate of deterioration of motor performance, as reflected in the regression slopes, was significantly influenced by treatment (F = 4.95 [3, 79 df]; P = 0.003). Post-hoc analysis showed that the rate of motor deterioration was significantly greater in AdNull-injected R6/2 mice than in either their AdBDNF/AdNoggin- or AdBDNF-treated counterparts (P = 0.003 and P = 0.023, respectively). When rotarod performance was compared between groups at each time point, again using ANOVA with post-hoc tests, we found that by 7 wk after treatment, the AdBDNF/AdNoggin-injected R6/2 mice performed significantly better than did AdNull-treated and untreated controls (P = 0.003 and P = 0.004, respectively). In addition, the AdBDNF/AdNoggin-treated R6/2 mice exhibited a performance advantage over mice treated only with AdBDNF, which achieved statistical significance by 9 wk after treatment, at 13 wk of age (P < 0.001; Figure 4A). When assessed with regards to their ability to sustain 60 s of rotarod performance, the AdBDNF/AdNoggin-treated R6/2 mice performed significantly better than did controls by 11 wk of age, or 7 wk after viral injection (F = 4.49 [3, 63 df], P = 0.006). This difference was sustained through 13 wk of age (F = 9.51 [3, 38 df], P < 0.001; Figure 4B). Post-hoc comparisons thereafter became difficult because the control animals died, yielding a disproportionate representation of AdBDNF/AdNoggin-treated animals; rotarod testing was halted at that point. Similarly, open-field testing revealed that locomotion was relatively preserved in AdBDNF/AdNoggin-treated R6/2 mice, which exhibited significantly more volitional horizontal locomotion than did AdNull-treated or untreated controls at 13 wk of age (F = 4.39 [3, 29 df], P = 0.012; Figure 4C and Supplemental Videos 1–5).
Figure 4. Induced neurogenesis was associated with delayed disease progression and prolonged survival.
(A and B) AdBDNF/AdNoggin-cotreated R6/2 mice exhibited slower deterioration of motor performance than did AdBDNF-treated or untreated control R6/2 mice. (A) Time of sustained rotarod performance as a function of time point and treatment, using a 300-s rotarod challenge. (B) Results of the same challenge as in A presented as the percent rotarod impairment (see Methods). (C) Open-field testing revealed that AdBDNF/AdNoggin-treated R6/2 mice sustained volitional explorative behavior, as manifested in spontaneous horizontal locomotion, longer than did mice treated with AdBDNF only or AdNull or left untreated. (D) Kaplan-Meier analysis revealed that survival was significantly extended in R6/2 mice injected with AdBDNF/AdNoggin compared with R6/2 mice injected with AdBDNF only or AdNull or left untreated. Pre, preoperative. *P < 0.05, **P < 0.01 versus AdNull and untreated; ‡P < 0.01 versus AdBDNF.
Importantly, the increments in both rotarod and open-field performance measures noted in AdBDNF/AdNoggin-treated mice were not replicated by AdBDNF alone (Figure 4, A–C). To the contrary, AdBDNF treatment alone never yielded a significant increment in either measure, relative to AdNull-treated or untreated R6/2 controls. These data suggest that the robust neuronal recruitment associated with AdBDNF/AdNoggin correlated with functional improvement, while the more limited neurogenic and neurotrophic effects of BDNF alone failed to do so.
Neuronal addition was associated with longer survival.
In light of the motor performance increments noted in AdBDNF/AdNoggin-treated R6/2 mice, we asked whether treatment influenced survival. In addition, we again asked whether the effects of AdBDNF/AdNoggin cotreatment were significantly better than those achieved with BDNF alone. To this end, we compared the mean survival of additional matched groups of R6/2 mice (n = 10 per group), treated at 4 wk of age with a single AdBDNF/AdNoggin injection; AdBDNF alone; AdNull:GFP control; or no treatment at all. The mice were then returned to their cages and followed with supportive husbandry until death. We found that AdBDNF/AdNoggin coinjected R6/2 mice survived significantly longer than both AdNull-treated and untreated controls. Moreover, the net survival of R6/2 mice treated with AdBDNF alone was no different than that of AdNull-treated and untreated controls (Figure 4D). Survival analysis revealed that AdBDNF/AdNoggin-treated R6/2 mice survived a mean of 110.0 ± 3.3 d, whereas AdNull-treated and untreated controls survived 94.5 ± 3.2 and 96.6 ± 2.7 d, respectively. ANOVA revealed that the overall effect of treatment upon survival was significant (F = 4.45 [3, 38 df], P = 0.011). Post-hoc analysis confirmed the difference in mean survival between AdBDNF/AdNoggin-treated R6/2 mice and AdNull-treated and untreated controls (P < 0.01 and P < 0.05, respectively), such that AdBDNF/AdNoggin-treated R6/2 mice survived an average of 16.8% longer than did AdNull controls. Although the effect of treatment achieved statistical significance at day 98 (P = 0.013), it was largely because of the relative survival of AdBDNF/AdNoggin-treated mice; R6/2 mice treated only with AdBDNF survived 102.0 ± 2.2 d, not significantly longer than AdNull-treated or untreated R6/2 controls. Thus, the survival benefit associated with AdBDNF/AdNoggin treatment was not provided by AdBDNF alone (Figure 4D). Importantly, while the weight loss expected in response to central BDNF administration (11, 40) was noted to equal degree in both AdBDNF- and AdBDNF/AdNoggin-treated mice (Supplemental Figure 3), this BDNF-associated weight loss was transient, and evidently did not impair survival of the treated mice.
Mitotic inhibition abrogated BDNF/Noggin-associated treatment benefits.
The improved motor performance and survival of AdBDNF/AdNoggin-treated R6/2 mice, relative not only to untreated controls but to AdBDNF-treated animals, suggested that the benefits of BDNF and Noggin co-overexpression derived specifically from induced neurogenesis, rather than from any neuroprotective effects of either agent. To better establish the causal relationship of induced neurogenesis to the performance and survival benefits of treatment, we assessed the effects of AdBDNF/AdNoggin while concurrently inhibiting induced neurogenesis. To this end, we gave AdBDNF/AdNoggin-treated R6/2 mice chronic intraventricular infusions of the mitotic inhibitor Ara-C (2% via Alzet minipump, 0.25 μl/h), so as to block mitotic neurogenesis (41). We then compared the performance and survival of these mice to matched AdBDNF/AdNoggin-treated R6/2 mice infused with saline, as well as to additional R6/2 mice given an AdNull control vector and then infused with either Ara-C or saline. One set of 40 R6/2 mice were thereby distributed to 4 groups of 10 each, designated AdBDNF/AdNoggin–Ara-C, AdBDNF/AdNoggin-saline, AdNull–Ara-C, and AdNull-saline; these were compared in regard to their motor performance as assessed by rotarod and locomotor activity. A second set of 40 R6/2 mice were then distributed into 4 analogous groups of 10 each and entered into a prospective survival study, as assessed by Kaplan-Meier statistics. These 2 studies were designed so as to define whether the motor performance and survival increments associated with AdBDNF/AdNoggin could be reversed by Ara-C blockade of induced neurogenesis. Accordingly, a third set of mice was evaluated in regard to the actual incidence of striatal, neuronal addition in AdBDNF/AdNoggin- or AdNull-treated R6/2 and WT mice, as a function of the presence or absence of concurrent Ara-C infusion. To this end, matched cohorts of 4-wk-old R6/2 and WT mice were grouped as AdBDNF/AdNoggin–Ara-C, AdBDNF/AdNoggin-saline, AdNull–Ara-C, and AdNull-saline (n = 3 per group). Infusion of Ara-C or saline was begun 1 wk after viral injection, at 5 wk of age; 1 d later, daily injections of BrdU (100 mg/kg i.p.) were initiated and maintained for 4 wk. All mice (24 total, 12 R6/2 and 12 WT) were then sacrificed, and their brains were assessed for the incidence of BrdU+βIII-tubulin+ neurons among all striatal neurons, so as to evaluate the suppression of neurogenesis afforded by Ara-C.
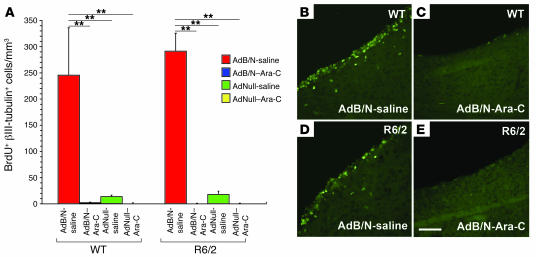
We found that Ara-C infusion yielded an almost complete suppression of mitotic neurogenesis and neuronal addition to the striata of both R6/2 and WT animals (Figure 5). Histologically, whereas AdBDNF/AdNoggin-treated R6/2 mice infused with saline exhibited 292.6 ± 31.8 BrdU+βIII-tubulin+ neurons/mm3, those infused with Ara-C exhibited no BrdU-tagged neurons whatsoever (P < 0.001, ANOVA followed by post-hoc Bonferroni t tests). Similarly, while AdBDNF/AdNoggin-treated WT mice manifested 248.4 ± 86.6 neurons/mm3, those infused with Ara-C had no new quantifiable striatal neurons (P < 0.001; Table 5).
Figure 5. Ara-C suppressed neuronal mitogenesis.
(A) Number of newly generated striatal neurons in both R6/2 and WT mice after treatment with AdBDNF/AdNoggin or AdNull, both with and without infusion of the mitotic inhibitor Ara-C. Ara-C essentially abrogated AdBDNF/AdNoggin-associated neuronal addition. (B–E) AdBDNF/AdNoggin-treated WT (B) and R6/2 (D) mice exhibited robust incorporation of BrdU (green) by subependymal progenitor cells. In contrast, Ara-C infusion potently suppressed mitotic activity within the subependyma, essentially abrogating neuronal addition to both WT (C) and R6/2 (E) neostriata. **P < 0.001. Scale bar: 100 μm.
Table 5 .
Striatal neuronal addition as a function of disease, treatment, and mitotic inhibition
Along with this Ara-C–associated inhibition of striatal neurogenesis, we observed an essentially complete abrogation of the performance and survival benefits of AdBDNF/AdNoggin treatment in Ara-C–infused R6/2 mice. Both the rotarod performance (Figure 6, A and B) and open-field locomotor activity (Figure 6C) of AdBDNF/AdNoggin-treated R6/2 mice were substantially better than those of AdNull-treated controls, replicating our initial results of Figure 4. However, Ara-C treatment completely inhibited the salutatory effects of AdBDNF/AdNoggin treatment on both rotarod performance and open-field volitional activity (Figure 6, A–C, and Supplemental Videos 1–5). Similarly, whereas AdBDNF/AdNoggin-treated R6/2 mice lived significantly longer than did the untreated controls, matched Ara-C–infused animals lived no longer than did AdNull-injected, untreated R6/2 mice (Figure 6D). In this case, Kaplan-Meier survival analysis revealed that AdBDNF/AdNoggin-treated R6/2 mice survived a mean of 110.7 ± 2.8 d, whereas matched AdBDNF/AdNoggin-treated mice infused with Ara-C survived only 97.9 ± 2.3 d, suggesting that the survival increment blocked by Ara-C, and hence attributable to neurogenesis, was 13.1%. The difference in survival between AdBDNF/AdNoggin-treated mice and AdNull-treated and Ara-C–inhibited controls achieved significance by day 100 (P = 0.035) and persisted through day 116 (P = 0.024). Indeed, R6/2 mice treated with Ara-C as well as AdBDNF/AdNoggin survived no longer, at 97.9 ± 2.3 d, than did AdNull-treated control mice given either Ara-C (94.9 ± 3.1 d) or saline (96.6 ± 2.9 d). ANOVA confirmed that AdBDNF/AdNoggin significantly improved the survival of R6/2 mice relative both to those given AdNull alone (t = 12.7, P < 0.01), and to those treated with AdNull together with Ara-C (t = 16.0, P < 0.01). Remarkably, Ara-C addition to AdBDNF/AdNoggin abolished this survival benefit (P > 0.05 compared with AdNull), as revealed by a significant difference in the survival of Ara-C–infused AdBDNF/AdNoggin-treated R6/2 mice compared with those treated with AdBDNF/AdNoggin but infused only with saline (t = 10.5, P < 0.05). These results indicated that both the performance and the survival benefits associated with AdBDNF/AdNoggin treatment were dependent upon mitotic neurogenesis, and thus could be abrogated by the suppression of neuronal addition to the diseased R6/2 neostriatum.
Figure 6. Ara-C inhibition of neurogenesis blocked the treatment-associated delay in disease progression.
(A) Ara-C infusion blocked the relative preservation of R6/2 rotarod performance otherwise afforded by AdBDNF/AdNoggin treatment. (B) Rotarod impairment is shown as a function of time point and treatment. (C) Ara-C infusion similarly inhibited the relative maintenance of volitional explorative behavior exhibited by AdBDNF/AdNoggin-treated R6/2 mice. (D) Kaplan-Meier analysis confirmed that survival was significantly extended in R6/2 mice injected with AdBDNF and AdNoggin, compared with AdNull-injected R6/2 mice, and revealed that the Ara-C–mediated inhibition of mitotic neurogenesis completely abrogated that survival benefit. *P < 0.05, **P < 0.01 versus Ara-C–infused AdBDNF/AdNoggin and saline-infused controls.
Discussion
These results indicate that the concurrent overexpression of BDNF and Noggin may be used to induce quantitatively substantial and functionally significant neuronal recruitment from endogenous progenitor cells into the R6/2 huntingtin mutant neostriatum. When R6/2 mice were assessed at 10 wk of age, BDNF overexpression beginning at 6 wk was found to have induced the addition of 138 ± 34 new neurons/mm3. Although significant, this represents just 0.6% of the striatal neuronal population. On this basis, we then coexpressed BDNF together with Noggin so as to suppress non-neurogenic pathways of subependymal cell differentiation and thereby increase the pool of progenitor cells potentially responsive to BDNF. By this means, 268 ± 53 neurons/mm3 were added to the AdBDNF/AdNoggin-treated R6/2 striatum within a month of viral injection, representing an increment of 1.2% of the mean striatal neuronal pool during that month. Whether such numbers can be uniformly recruited over longer periods of time using more sustained expression vectors will depend upon the capacity for sustained neurogenesis by the R6/2 neural stem cell pool, as well as the sustained receptivity of the tissue. Nonetheless, recent reports of expanded endogenous neural stem cell populations in both R6/2 mice (42) and HD patients (43) suggest that subependymal progenitor cell populations potentially receptive to BDNF/Noggin-induced mobilization indeed remain abundant and active in the HD brain.
Adenoviral gene expression is transient, so that meaningful BDNF and Noggin expression persists for less than 2 mo after intraventricular viral injection (11). Nonetheless, a pronounced functional benefit was noted in these AdBDNF/AdNoggin-treated mice that persisted long after their effective period of BDNF and Noggin overexpression: AdBDNF/AdNoggin treatment delayed the onset and slowed the course of motor deterioration of the R6/2 mice, just as it extended their survival. Interestingly, the enhanced performance and prolonged survival of the treated mice was thus associated with neuronal addition that had occurred during the discrete period of BDNF and Noggin overexpression, which likely ceased months before the treated animals actually died. This argues for the sustained functional benefits potentially associated with antecedent neuronal addition and suggests the even greater likely value of more sustained BDNF and Noggin overexpression strategies.
In this regard, it is important to note that the AdBDNF/AdNoggin-associated survival benefit was entirely abolished by concurrent intraventricular infusion of the mitotic inhibitor Ara-C, delivered at low doses that impede central neurogenesis (41) but otherwise lack known central or systemic toxicity (44). These results argue that induced restorative neurogenesis can delay symptom progression in a prototypic mouse model of HD. In addition, the inhibition of this effect by the concurrent Ara-C–mediated suppression of neurogenesis indicated that the improved motor performance and survival of AdBDNF/AdNoggin-treated R6/2 mice required neuronal addition; both BDNF and Noggin might still have exercised neuroprotective effects on threatened striatal neurons, but if such effects were operative, they were alone insufficient to significantly improve either motor performance or survival in these mice.
Our evidence strongly points to mitotic neurogenesis as the basis for BrdU incorporation by the BrdU+ neurons that we noted. This point bears scrutiny, given several recent reports of BrdU incorporation in the setting of neuronal injury and degeneration. Kuan and colleagues (32) noted that neurons dying of hypoxic ischemia may reenter the cell cycle, and as a result express markers consistent with G1/S phase transition, such as Ki67, while incorporating BrdU and losing expression of the CDK proteins p16 and p27. However, no such evidence of aberrant cell cycle reentry was noted in a variety of other insults, which included adrenalectomy, kainite toxicity, and major trauma. Kuan et al. assessed the temporal relationship of ectopic cell cycle reentry to neuronal death in detail and noted that BrdU-incorporating neurons appeared by 5 d after ischemic injury, but were no longer evident by 28 d. When apparent at 5 d, they expressed the mitotic marker Ki67 and had lost expression of the mitotic inhibitor p27, suggesting cell cycle reentry. In addition, Kuan et al. noted that BrdU-incorporating neurons arising after ischemia quickly lost expression of the neuronal skeletal constituent βIII-tubulin, so that the cells had to be identified as such by colabeling with the more stable marker NeuN. As a result, these authors found no evidence of BrdU+βIII-tubulin+ cells after the first week after ischemic injury (32).
In contrast to the failure of Kuan et al. to observe BrdU+βIII-tubulin+ neurons at 4 wk after ischemia (32), we noted abundant BrdU+βIII-tubulin+ neurons in the AdBDNF/AdNoggin-treated neostriata of both R6/2 and WT mice as long as 6 wk after viral injection. These cells expressed DARPP-32 and either enkephalin or SP and maintained projections to the globus pallidus that could be FG backfilled as long as 7 wk after viral treatment. Furthermore, all of our quantitative scoring was done using an BrdU+βIII-tubulin+ end point at 4 wk after viral injection; Kuan et al. noted the complete disappearance of this antigenic phenotype by 4 wk (32). To emphasize this point, we reliably noted over 300 BrdU+βIII-tubulin+ neurons/mm3 added to both R6/2 and WT mice after AdBDNF/AdNoggin treatment, a number that vastly surpassed the few BrdU+NeuN+ striatal neurons (and lack of BrdU+βIII-tubulin+ neurons) noted by Kuan et al. 4 wk after ischemic injury (32). In this regard, it is also worth noting that we have previously reported the persistence of these cells as long as 56 d after production in rats, with little fall-off from the numbers noted at 21 d in that study (18). Thus, unlike the transient appearance and rapid clearance of a BrdU-incorporating βIII-tubulin+ neuronal phenotype immediately following hypoxic ischemia, the striatal neurons generated in response to BDNF and Noggin overexpression in the R6/2 brain were long lasting and stably integrating.
In studies that paralleled those of Kuan et al. (32), Herrup and colleagues reported that neuronal cell death in both Alzheimer disease and ataxia telangiectasia can be preceded by cell cycle reentry (33, 45). These observations again raise the possibility that neuronal death in some pathologies might be heralded by aborted cell cycle reentry, with attendant neuronal incorporation of BrdU. To assess whether R6/2 HD mice exhibit BrdU incorporation by neurons dying as a result of aborted cell cycle reentry, we performed a number of additional experiments. First, TUNEL staining at 10 wk revealed very few dying neurons, consistent with previous reports that R6/2 mice exhibit little if any apoptotic neuronal death (23, 34, 35). Second, immunostaining for the mitotic marker Ki67 revealed no evidence of Ki67-expressing βIII-tubulin+ or NeuN+ striatal neurons; to the contrary, BrdU+βIII-tubulin+ neurons were noted to invariably express the CDK inhibitor p27, as would be expected of postmitotic neurons that had incorporated BrdU previously. In addition, it is important to note that we observed BrdU+βIII-tubulin+ neurons as a function of BDNF/Noggin treatment, but not as a function of disease; at 10 wk, these cells were present in both AdBDNF/AdNoggin-treated WT and HD striata. Indeed, the similar incidence of BrdU-tagged neurons in the WT and HD striata is hard to reconcile with any disease-associated aberration in neuronal mitotic quiescence. Together, these observations strongly suggest that the BrdU+βIII-tubulin+ and BrdU+NeuN+ neurons we observed in the AdBDNF/AdNoggin-treated R6/2 striata were the products of mitotic neurogenesis.
Although the mechanisms of neurotoxicity in HD remain unclear, BDNF transcription has been reported to be impeded by CAG-expanded huntingtin protein (46). Because BDNF supports MSNs via its anterograde transport and release from corticostriatal afferents (47), mutant huntingtin’s suppression of corticostriatal BDNF or loss of WT huntingtin function may deprive MSNs of their trophic support. As a result, BDNF has been investigated as a neuroprotective agent for MSNs in the setting of HD (48–50). The improved motor performance of our AdBDNF/AdNoggin-treated mice may derive in part from BDNF’s neurotrophic effects on resident MSNs, over and above its induction of new neurons from resident progenitors (51, 52). Yet neither rotarod performance nor survival was significantly improved in R6/2 mice treated with AdBDNF alone, save for a minor delay in their rotarod deterioration rate. This contrasted with the robust increments in each outcome measure noted in R6/2 mice treated with AdBDNF and AdNoggin together. Because Noggin itself has no known neurotrophic actions and exhibited no significant induction of neuronal addition relative to AdNull-treated mice, the beneficial effects of Noggin’s addition to BDNF in this study would appear to derive from its potentiation of BDNF-induced striatal neuronal recruitment. In this regard, in pilot studies of the effects of AdNoggin on the motor performance and survival of R6/2 mice, we noted no significant differences from AdNull- or saline-treated mice (our unpublished observations), suggesting that AdNoggin’s suppression of subependymal gliogenesis did not provide observable benefit to R6/2 mice. These data argue that the performance and survival increments of AdBDNF/AdNoggin-treated R6/2 mice were specifically caused by the AdBDNF/AdNoggin-associated addition of new MSNs to the R6/2 striatum and did not derive from BDNF’s neuroprotective effects. That being said, the neurotrophic actions of BDNF delivered to the striatal wall may have potentiated the relative contribution of the new neurons by preserving the functional competence of the network into which they integrated.
Together, these observations suggest that BDNF/Noggin-stimulated neurogenesis may represent a means of both replacing neurons lost to striatal neurodegeneration and conferring therapeutic benefit in HD. By using this fundamentally restorative strategy in parallel with independent neuroprotective approaches, such as minocycline inhibition of caspase activity (53), histone deacetylase inhibitor support of neuronal transcription (24), Coenzyme Q and creatine support of neuronal energy reserves (39, 54–56), and FGF2 infusion (57), we might reasonably hope to establish a protocol for combination therapy of HD in affected individuals. In this regard, the 17% net increase in R6/2 survival that we observed following a single intraventricular injection of AdBDNF/AdNoggin is as robust an effect as any previously noted using any of these alternative neuroprotective strategies, alone or in combination. Yet because BDNF and Noggin were likely overexpressed for no longer than 1–2 mo after viral injection (11), we can reasonably postulate that more sustained BDNF and Noggin delivery, whether afforded by protein delivery or more persistent transgene expression, may yield proportionately greater benefits to the performance and survival of affected subjects. Used in tandem with these other, mechanistically independent, approaches toward the protection of threatened striatal neurons, induced neurogenesis may prove an effective strategy for delaying both extrapyramidal dysfunction and overall disease progression in HD. More broadly, these findings suggest that induced neurogenesis from resident progenitor cells may comprise a feasible strategy reconstituting lost multinuclear circuits in the diseased adult brain.
Methods
Ad construction.
Replication-incompetent AdBDNF and AdNull were constructed and raised as described previously (11). In brief, we constructed an Ad vector bearing BDNF under the control of the CMV promoter, placed upstream to the gene encoding humanized GFP, with an intervening internal ribosomal entry site. Using the same techniques, a ΔE1, ΔE3 type 5 adenovirus was made to encode, under CMV control, human NogginΔB2, from which the B2 heparin binding domain had been deleted, yielding AdNogginΔB2 (58). Viral concentrations and effective titers were as follows: AdBDNF, 9.3 × 1011 particles/ml, 5 × 109 pfu/ml; AdNoggin, 1.1 × 1012 particles/ml, 5.3 × 109 pfu/ml; AdNull, 1 × 1012 particles/ml, 5 × 109 pfu/ml. All animals received total viral injections of 3 μl, delivered as 1.5 μl into each lateral ventricle. When AdBDNF and AdNoggin were delivered together, they were injected at a 1:1 ratio (1.5 μl of each virus, mixed together before intraventricular injection) and hence brought to a final titer of 2.5 × 109 pfu/ml each within the same injectate.
Experimental design and stereotaxic injection.
This study was approved by both the Institutional Animal Care and Use Committee of the Weill Medical College of Cornell University and the University Committee on Animal Resources of the University of Rochester. In one series of experiments, 6-wk-old R6/2 and WT mice received bilateral 1.5 μl intraventricular injections of AdBDNF/AdNoggin, AdNoggin, AdBDNF, AdNull, or saline. In a second series, 4-wk-old mice were injected with either AdBDNF/AdNoggin or AdNull and then infused beginning 1 wk later with either Ara-C or saline (see below). In each case, virus was stereotaxically delivered to the following coordinates: from Bregma, AP –0.5 mm, ML ±0.7 mm; from dura, DV –2.0 mm. In both series of experiments, mice dedicated to histological analysis were injected daily for 30 d with the mitotic marker BrdU (100 mg/kg, i.p.). BrdU injections were given for 30 d beginning at 6 wk for the first series and at 5 wk for the second series. All mice were sacrificed the day after the last BrdU injection.
FG labeling.
A cohort of 10 mice received intraventricular injection of AdBDNF/AdNoggin (1 R6/2 and 3 WT), AdBDNF (3 WT), or AdNull (3 WT), followed by daily injections of BrdU for 3 wk. Three wk after the last BrdU injection, they were injected with 1 μl of 1% FG (Biotium) bilaterally into the globus pallidus (from Bregma, AP –0.8 mm, ML ± 2.25 mm; from dura, DV –3.25 mm), according to the coordinates of Paxinos (59). The injected animals were sacrificed 10 d later and perfused with 2% paraformaldehyde; their brains were cryosectioned and stained for BrdU, followed by confocal identification of BrdU+FG+ striatal cells.
Immunohistochemistry.
The animals were sacrificed, perfusion fixed, and their brains removed a day after their last BrdU injection unless otherwise noted. Fixation was accomplished with 4% paraformaldehyde in 0.1M phosphate buffer, pH 7.4, followed by serial immersion in 6% and 30% sucrose in phosphate buffer. The brains were cryosectioned as 15-μm sagittal sections, then stained for BrdU and neuronal markers as previously described (11, 60, 61). Individual sections were first stained for one of the following neuronal markers: βIII-tubulin, using mAb TuJ1 (mouse IgG, 1:400; Promega); DARPP-32 (rabbit antisera, 1:5,000; provided by H. Hemmings, Cornell University); GAD67 (mouse IgG, 1:100; Chemicon); NeuN (mouse IgG, 1:400; Chemicon); met-enkephalin (Chemicon); SP (Chemicon); (g) DCX (mouse IgG, 1:200; BD Biosciences — Pharmingen); and ChAT (goat antisera, 1:200; Chemicon). For costaining with BrdU, the sections were further washed and denatured in 2 N HCl at 37°C for 30 min, then exposed to monoclonal rat anti-BrdU (1:200; Serotec), followed by a goat anti-rat Alexa 488 secondary antibody (1:400; Molecular Probes Inc.). For triple staining, sections were treated with Ki67 (1:200; Lab Vision) or p27Kip1 (rabbit antisera, 1:800; Chemicon) followed by anti-rabbit Alexa 647 secondary antibody (1:400; Molecular Probes Inc.). For caspase-3 immunostaining, sections were first washed with cold PBS with 0.1% Triton X-100 and 0.01% sodium citrate, followed by anti–caspase-3 (1:100; BD Biosciences — Pharmingen) and peroxidase-conjugated secondary antibodies, which were subsequently detected using diaminobenzidine and H2O2. DNA fragmentation was detected by TUNEL, which was performed according to the manufacturer’s protocol (TUNEL Apoptosis Detection Kit; Upstate Biologicals).
Confocal imaging.
In sections double-stained for BrdU and either βIII-tubulin, DARPP-32, GAD67, NeuN, or DCX, single striatal BrdU+ cells were randomly selected for confocal imaging. Using an Olympus Fluoview confocal microscope, images were acquired using an argon-krypton laser and analyzed as previously described (11). Briefly, the images of every cell double-immunostained with BrdU and a neuronal marker were observed orthogonally in both the vertical and the horizontal plane. Each potentially double-labeled cell underwent independent review by 2 observers. Only when both observers deemed a cell as double-labeled, with central BrdU immunoreactivity surrounded by neuronal staining from all observation angles in every serial optical section and in each merged and rotated composite, were the cells scored as newly generated neurons.
Scoring and quantification.
Striatal BrdU+ cells counts were done on 6 15-μm sagittal sections per animal; every sixteenth section was analyzed at 240-μm intervals, as previously described (11). The striatal region sampled began with the first appearance of striatal fascicles. In each striatum, total BrdU+ nuclei and βIII-tubulin+ neurons were counted at ×20 magnification; these results were converted into BrdU+ cells/mm3 or neurons/mm3 after determining the striatal surface area using BioQuant image analysis software, with which the net volume of each striatum was estimated. The number of striatal BrdU+βIII-tubulin+ cells/mm3 in a given section was then determined by multiplying the percentage of BrdU+ cells that coexpressed βIII-tubulin in confocal-verified sample fields by the total number of BrdU+ cells/mm3. Comparisons of the number of BrdU+βIII-tubulin+ cells/mm3 in AdBDNF/AdNoggin-, AdBDNF-, AdNull-, and saline-injected animals were performed using ANOVA and post-hoc Bonferroni t tests. Statistical analyses were performed using GB-Stat and SPSS.
Rotarod performance.
Motor coordination and balance were measured using rotarod analysis (39). In our initial series, AdBDNF/AdNoggin- (n = 22), AdBDNF- (n = 20), and AdNull-treated (n = 21) R6/2 mice were assessed by rotarod, as were untreated controls (n = 20), beginning at 4 wk of age. In a second series, we used R6/2 mice treated with AdBDNF/AdNoggin or AdNull (n = 20 per group), 10 of which in each group were subjected to mitotic inhibition by Ara-C. The 4 distinct treatment groups were as follows: AdBDNF/AdNoggin with Ara-C infusion; AdBDNF/AdNoggin with saline infusion; AdNull and Ara-C; and AdNull and saline (n = 10 per group). In each series, the mice were trained 3 times daily for 2 consecutive days on a rotarod (catalog no. 7650; UGO Basile), at a constant speed of 12 rpm; they were subsequently tested weekly at same speed. At each weekly test, each mouse was given 3 trials on the rod, and their latencies to fall measured. We defined a maximum latency of 300 s, at which the individual test was terminated and scored as 300 s; for every 3-trial test, the best result, i.e., the longest time spent on the rod without falling, was recorded. All mice were tested from the day before stereotaxic surgery, at 4 wk of age, until either 13 wk of age, or until they were unable to maintain their body posture, whichever was later. Rotarod scores of less than 60 s were considered neurologically abnormal; we used this as an additional criterion to define rotarod impairment (62), the incidence of which was evaluated weekly. Rotarod impairment, expressed as a percentage, was calculated as (60 s — latency to fall [in s])/60 s. Comparisons of the mean duration of rotarod performance as a function of age were performed by ANOVA, followed by post-hoc Bonferroni t tests.
Open-field activity test.
Volitional locomotion was evaluated in an activity cage (catalog no. 7420; UGO Basile) at 2-wk intervals after surgery. In our first series, AdBDNF/AdNoggin-, AdBDNF-, and AdNull-treated R6/2 mice as well as saline-treated controls (n = 15 per group) were assessed by placing the individuals into the infrared beam-arrayed cage (41 × 41 × 33 cm) for 1 h. Spontaneous horizontal locomotion was analyzed as counts per unit testing time, as measured by the number of infrared beams a mouse crossed. The latter were read by 2 facing blocks containing an infrared array of 16 emitters and receivers, and the data were collected automatically by a recording machine. A second series of experiments used mice treated with either AdBDNF/AdNoggin or AdNull, half of which were subjected to mitotic inhibition by Ara-C, as described above (n = 10 per group).
Survival.
In our initial survival series, AdBDNF/AdNoggin-, AdBDNF-, and AdNull-treated as well as untreated R6/2 mice (n = 10 per group) were assessed for viability twice daily beginning at 4 wk of age. In a second series of experiments, AdBDNF/AdNoggin- and AdNull-treated mice were compared with their Ara-C–infused counterparts, as described above (n = 10 per group). To exclude the possibility that net survival might be affected by rotarod or open-field testing, the mice in each survival study were not subjected to behavioral assessment. Survival data were analyzed by Kaplan-Meier survival curves.
Infusion of a mitotic inhibitor.
We infused 2% Ara-C (Sigma-Aldrich) in 0.9% saline, or saline alone, into the brains of R6/2 or WT mice with an Alzet micro-osmotic pump (model 1002, flow rate 0.25 μl/hr, 14 d; Durect) as previously described (41). The mice were first treated with Ara-C or saline 1 wk after AdBDNF/AdNoggin or AdNull injections, which were given at 4 wk of age. A cannula (Brain Infusion Kit 3; Alzet) was implanted into the right ventricle, stereotaxically at AP –0.5 mm, ML –0.7 mm from Bregma and DV –2.0 mm from dura. The osmotic pump was preincubated overnight in sterile saline at 37°C, so as to allow flow to begin immediately upon implantation, and then implanted subcutaneously along the animal’s back. After 2 wk of infusion, the osmotic pump was replaced with a fresh pump to continue antimitotic treatment for another 2 wk, so as to provide a total of 4 wk of mitotic suppression, spanning 5–9 wk of age. Matched cohorts of Ara-C–treated R6/2 mice and their AdNull-treated R6/2 controls were assigned to either behavioral testing (n = 10 per group) or survival analysis (n = 10 per group), while additional sets of Ara-C–treated and untreated R6/2 and WT mice (n = 3 per group) were assessed for immunohistochemistry after BrdU injection for 4 wk, as described above.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke of the NIH, by the Cure HD Initiative of the Hereditary Disease Foundation, and by the HiQ Foundation Inc. We are grateful to Martha Windrem and Fraser Sim for advice and to Ethan Craig, Stephanie Tyler, Bonhee Koo, and Eric Robbins for technical assistance. We thank Regeneron Pharmaceuticals and Frederick Kaplan and Eileen Shore (University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA) for AdNoggin and Hugh Hemmings (Cornell University) for anti–DARPP-32. We also thank Sue Brown and Flint Beal (Cornell University) for advice in the behavioral evaluation of R6/2 mice.
Footnotes
Nonstandard abbreviations used: Ad, adenoviral; Ara-C, cytosine-β-D-arabinofuranoside; BDNF, brain-derived neurotrophic factor; CDK, G1-cyclin-dependent kinase; ChAT, choline acetyltransferase; DARPP-32, dopamine- and cAMP-regulated phosphoprotein–32 kDa; DCX, doublecortin; FG, Fluorogold; GAD67, glutamic acid dehydrogenase–67 kDa; HD, Huntington disease; MSN, medium spiny neuron; SP, substance P; SZ, subependymal zone.
Conflict of interest: A. Economides is an employee of Regeneron Pharmaceuticals. The remaining authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 117:2889–2902 (2007). doi:10.1172/JCI31778
Sung-Rae Cho and Abdellatif Benraiss contributed equally to this work.
Sung-Rae Cho’s present address is: Department of Rehabilitation Medicine, Yonsei University College of Medicine, Seoul, Repulic of Korea.
Amer Samdani’s present address is: Shriners Children’s Hospital, Philadelphia, Pennsylvania, USA.
References
- 1.Goldman S.A., Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc. Natl. Acad. Sci. U. S. A. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morshead C.M., et al. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Buylla A., Garcia-Verdugo J.M. Neurogenesis in adult subventricular zone. J. Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gage F.H. Neurogenesis in the adult brain. J. Neurosci. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldman S. Glia as neural progenitor cells. Trends Neurosci. 2003;26:590–596. doi: 10.1016/j.tins.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Thoenen H., Sendtner M. Neurotrophins: from enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nat. Neurosci. 2002;5(Suppl.):1046–1050. doi: 10.1038/nn938. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed S., Reynolds B.A., Weiss S. BDNF enhances the differentiation but not the survival of CNS stem cell- derived neuronal precursors. J. Neurosci. 1995;15:5765–5778. doi: 10.1523/JNEUROSCI.15-08-05765.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirschenbaum B., Goldman S.A. Brain-derived neurotrophic factor promotes the survival of neurons arising from the adult rat forebrain subependymal zone. Proc. Natl. Acad. Sci. U. S. A. 1995;92:210–214. doi: 10.1073/pnas.92.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindholm D., Carroll P., Tzimagiogis G., Thoenen H. Autocrine-paracrine regulation of hippocampal neuron survival by IGF-1 and the neurotrophins BDNF, NT-3 and NT-4. Eur. J. Neurosci. 1996;8:1452–1460. doi: 10.1111/j.1460-9568.1996.tb01607.x. [DOI] [PubMed] [Google Scholar]
- 10.Zigova T., Pencea V., Wiegand S.J., Luskin M.B. Intraventricular administration of BDNF increases the number of newly generated neurons in the adult olfactory bulb. Mol. Cell. Neurosci. 1998;11:234–245. doi: 10.1006/mcne.1998.0684. [DOI] [PubMed] [Google Scholar]
- 11.Benraiss A., Chmielnicki E., Lerner K., Roh D., Goldman S.A. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J. Neurosci. 2001;21:6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pencea V., Bingaman K.D., Wiegand S.J., Luskin M.B. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J. Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bedard A., Gravel C., Parent A. Chemical characterization of newly generated neurons in the striatum of adult primates. Exp. Brain Res. 2005;170:501–512. doi: 10.1007/s00221-005-0233-5. [DOI] [PubMed] [Google Scholar]
- 14.Morshead C., van der Kooy D. Postmitotic death is the fate of constitutively proliferating cells in the subependymal layer of the adult mouse brain. J. Neurosci. 1992;12:249–256. doi: 10.1523/JNEUROSCI.12-01-00249.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross R.E., et al. Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron. 1996;17:595–606. doi: 10.1016/s0896-6273(00)80193-2. [DOI] [PubMed] [Google Scholar]
- 16.Lim D.A., et al. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 17.Zimmerman L.B., De Jesus-Escobar J.M., Harland R.M. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 18.Chmielnicki E., Benraiss A., Economides A.N., Goldman S.A. Adenovirally expressed noggin and brain-derived neurotrophic factor cooperate to induce new medium spiny neurons from resident progenitor cells in the adult striatal ventricular zone. J. Neurosci. 2004;24:2133–2142. doi: 10.1523/JNEUROSCI.1554-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chmielnicki E., Goldman S.A. Induced neurogenesis by endogenous progenitor cells in the adult mammalian brain. Prog. Brain Res. 2002;138:451–464. doi: 10.1016/s0079-6123(02)38093-2. [DOI] [PubMed] [Google Scholar]
- 20.Margolis R.L., Ross C.A. Diagnosis of Huntington disease. Clin. Chem. 2003;49:1726–1732. doi: 10.1373/49.10.1726. [DOI] [PubMed] [Google Scholar]
- 21.DiFiglia M., et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- 22.Mangiarini L., et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- 23.Davies S.W., et al. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90:537–548. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- 24.Rubinsztein D.C. Lessons from animal models of Huntington’s disease. Trends Genet. 2002;18:202–209. doi: 10.1016/s0168-9525(01)02625-7. [DOI] [PubMed] [Google Scholar]
- 25.Menalled L.B., Chesselet M.F. Mouse models of Huntington’s disease. Trends Pharmacol. Sci. 2002;23:32–39. doi: 10.1016/s0165-6147(00)01884-8. [DOI] [PubMed] [Google Scholar]
- 26.Kuhn H.G., Winkler J., Kempermann G., Thal L.J., Gage F.H. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J. Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivkovic S., Ehrlich M. Expression of the striatal DARPP-32/ARPP-21 phenotype in GABAergic neurons requires neurotrophins in vivo and in vitro. J. Neurosci. 1999;19:5409–5419. doi: 10.1523/JNEUROSCI.19-13-05409.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivkovic S., Polonskaia O., Farinas I., Ehrlich M.E. Brain-derived neurotrophic factor regulates maturation of the DARPP-32 phenotype in striatal medium spiny neurons: studies in vivo and in vitro. Neuroscience. 1997;79:509–516. doi: 10.1016/s0306-4522(96)00684-7. [DOI] [PubMed] [Google Scholar]
- 29.Gerfen C. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992;15:133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- 30.Canals J., et al. BDNF regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington’s disease. J. Neurosci. 2004;24:7727–7739. doi: 10.1523/JNEUROSCI.1197-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herrup K., Neve R., Ackerman S., Copani A. Divide and die: cell cycle events as triggers of nerve cell death. J. Neurosci. 2004;24:9232–9239. doi: 10.1523/JNEUROSCI.3347-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuan C.-Y., et al. Hypoxia-ischemia induces DNA synthesis without cell proliferation in dying neurons in adult rodent brain. J. Neurosci. 2004;24:10763–10772. doi: 10.1523/JNEUROSCI.3883-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y., Mufson E., Herrup K. Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer disease. J. Neurosci. 2003;23:2557–2563. doi: 10.1523/JNEUROSCI.23-07-02557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turmaine M., et al. Nonapoptotic neurodegeneration in a transgenic mouse model of Huntington’s disease. Proc. Natl. Acad. Sci. U. S. A. 2000;97:8093–8097. doi: 10.1073/pnas.110078997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu Z., et al. Mutant huntingtin causes context-dependent neurodegeneration in mice with Huntington’s disease. J. Neurosci. 2003;23:2193–2202. doi: 10.1523/JNEUROSCI.23-06-02193.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez Mejia R., Friedlander R. Caspases in Huntington’s Disease. Neuroscientist. 2001;7:480–489. doi: 10.1177/107385840100700604. [DOI] [PubMed] [Google Scholar]
- 37.Gleeson J., Lin P., Flanagan L., Walsh C. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- 38.Sim F., et al. Complementary patterns of gene expression by adult human oligodendrocyte progenitor cells and their white matter environment. Ann. Neurol. 2006;59:763–779. doi: 10.1002/ana.20812. [DOI] [PubMed] [Google Scholar]
- 39.Andreassen O.A., et al. Creatine increase survival and delays motor symptoms in a transgenic animal model of Huntington’s disease. Neurobiol. Dis. 2001;8:479–491. doi: 10.1006/nbdi.2001.0406. [DOI] [PubMed] [Google Scholar]
- 40.Pelleymounter M., Cullen M., Wellman C. Characteristics of BDNF-induced weight loss. Exp. Neurol. 1995;131:229–238. doi: 10.1016/0014-4886(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 41.Doetsch F., Garcia-Verdugo J.M., Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc. Natl. Acad. Sci. U. S. A. 1999;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Batista C., et al. A progressive and cell non-autonomous increase in striatal neural stem cells in the Huntington’s disease R6/2 mouse. J. Neurosci. 2006;26:10452–10460. doi: 10.1523/JNEUROSCI.2850-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Curtis M.A., et al. Increased cell proliferation and neurogenesis in the adult human Huntington’s disease brain. Proc. Natl. Acad. Sci. U. S. A. 2003;100:9023–9027. doi: 10.1073/pnas.1532244100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Groothuis D., et al. Comparisons of cytosine arabinoside delivery to rat brain by intravenous, intrathecal, intraventricular, and intraparenchymal routes of administration. Brain Res. 2000;856:281–290. doi: 10.1016/s0006-8993(99)02089-2. [DOI] [PubMed] [Google Scholar]
- 45.Yang Y., Herrup K. Loss of neuronal cell cycle control in ataxia-telangiectasia: A unified disease mechanism. J. Neurosci. 2005;25:2522–2529. doi: 10.1523/JNEUROSCI.4946-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fusco F.R., et al. Co-localization of brain-derived neurotrophic factor (BDNF) and wild-type huntingtin in normal and quinolinic acid-lesioned rat brain. Eur. J. Neurosci. 2003;18:1093–1102. doi: 10.1046/j.1460-9568.2003.02844.x. [DOI] [PubMed] [Google Scholar]
- 47.Altar C.A., et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- 48.Nakao N., Brundin P., Funa K., Lindvall O., Odin P. Trophic and protective actions of brain-derived neurotrophic factor on striatal DARPP-32-containing neurons in vitro. Brain Res. Dev. Brain Res. 1995;90:92–101. doi: 10.1016/0165-3806(96)83489-4. [DOI] [PubMed] [Google Scholar]
- 49.Bemelmans A.P., et al. Brain-derived neurotrophic factor-mediated protection of striatal neurons in an excitotoxic rat model of Huntington’s disease, as demonstrated by adenoviral gene transfer. Hum. Gene Ther. 1999;10:2987–2997. doi: 10.1089/10430349950016393. [DOI] [PubMed] [Google Scholar]
- 50.Ferrer I., Goutan E., Marin C., Rey M.J., Ribalta T. Brain-derived neurotrophic factor in Huntington disease. Brain Res. 2000;866:257–261. doi: 10.1016/s0006-8993(00)02237-x. [DOI] [PubMed] [Google Scholar]
- 51.Zuccato C., et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science. 2001;293:493–498. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]
- 52.Zuccato C., et al. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat. Genet. 2003;35:76–83. doi: 10.1038/ng1219. [DOI] [PubMed] [Google Scholar]
- 53.Chen M., et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat. Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- 54.Dedeoglu A., et al. Creatine therapy provides neuroprotection after onset of clinical symptoms in Huntington’s disease transgenic mice. J. Neurochem. 2003;85:1359–1367. doi: 10.1046/j.1471-4159.2003.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrante R.J., et al. Neuroprotective effects of creatine in a transgenic mouse model of Huntington’s disease. J. Neurosci. 2000;20:4389–4397. doi: 10.1523/JNEUROSCI.20-12-04389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stack E., et al. Combination therapy using minocycline and coenzyme Q10 in R6/2 transgenic Huntington’s disease mice. Biochem. Biophys. Acta. 2006;1762:373–380. doi: 10.1016/j.bbadis.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Jin K., et al. FGF2 promotes neurogenesis and neuroprotection in a transgenic mouse model of Huntingtons disease. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18189–18194. doi: 10.1073/pnas.0506375102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paine-Saunders S., Viviano B.L., Economides A.N., Saunders S. Heparan sulfate proteoglycans retain Noggin at the cell surface: a potential mechanism for shaping BMP gradients. J. Biol. Chem. 2001;277:2089–2096. doi: 10.1074/jbc.M109151200. [DOI] [PubMed] [Google Scholar]
- 59. Paxinos, G., and Watson, C. 1986.The rat brain in stereotaxic coordinates. Academic Press. Orlando, Florida, USA. 264 pp. [Google Scholar]
- 60.Roy N.S., et al. Identification, isolation, and promoter-defined separation of mitotic oligodendrocyte progenitor cells from the adult human subcortical white matter. J. Neurosci. 1999;19:9986–9995. doi: 10.1523/JNEUROSCI.19-22-09986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roy N.S., et al. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat. Med. 2000;6:271–277. doi: 10.1038/73119. [DOI] [PubMed] [Google Scholar]
- 62.Laforet G.A., et al. Changes in cortical and striatal neurons predict behavioral and electrophysiological abnormalities in a transgenic murine model of Huntington’s disease. J. Neurosci. 2001;21:9112–9123. doi: 10.1523/JNEUROSCI.21-23-09112.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.