Abstract
Background and purpose:
Airway wall remodelling in asthma is characterised by a number of structural changes, including an increase in the volume of airway smooth muscle (ASM), and the abundance of the extracellular matrix (ECM) protein, collagen, is increased. We have investigated the mechanism of collagen-induced glucocorticoid resistance of proliferation, and migration of ASM.
Experimental approach:
ASM cultured from human airways has been seeded on to either type I monomeric collagen or a laminin pentapeptide, YIGSR. The role of α2β1 integrin in the collagen-induced glucocorticoid resistance was investigated using a function blocking monoclonal antibody.
Key results:
Culture of ASM on collagen I, but not laminin, led to a greater proliferative response that was insensitive to regulation by dexamethasone (100 nM). The anti-migratory effects of the glucocorticoid, fluticasone propionate (1 nM) were also impaired by contact of ASM with collagen. The impaired anti-mitogenic action of dexamethasone was associated with a failure to reduce the levels of the rate-limiting cell cycle regulatory protein, cyclin D1. When signalling through the α2β1 integrin was reduced, dexamethasone-mediated reductions in proliferation and cyclin D1 levels were restored.
Conclusions and implications:
In the collagen-rich microenvironment of the inflamed and fibrotic asthmatic airway, integrin/ECM interactions may contribute to glucocorticoid resistance.
Keywords: asthma, steroids, smooth muscle, extracellular matrix, glucocorticoid resistance, COPD, laminin
Introduction
Asthma is a chronic, episodic inflammatory disorder of the airways, involving leucocytes, lymphocytes and structural cell types. Airway hyper-responsiveness (AHR), an exaggerated airway narrowing response to chemically and biologically diverse stimuli, plays a key role in the frequency and severity of asthma symptoms (Boulet, 2003). Airway wall remodelling (AWR) thickens the airway wall, and amplifies airway narrowing in response to airway smooth muscle (ASM) shortening and therefore contributes to AHR (James and Carroll, 2000). The most important cellular changes occurring in the remodelled airway are ASM hypertrophy and hyperplasia (Ebina et al., 1993), which together with increased extracellular matrix (ECM) secretion increase the volume of ASM in the asthmatic airway. Postmortem studies show that the airways of fatal asthmatics have a greater abundance of ASM than those of controls or of asthmatics dying of non-respiratory causes (Carroll et al., 1993). Analysis of biopsy specimens reveals that patients with severe, steroid-requiring asthma have a greater area occupied by ASM than non-asthmatics or patients with milder asthma (Mast et al., 2003). These findings suggest that the extent of ASM hyperplasia appears to increase with asthma severity. Although the mechanism(s) for the increase in volume of ASM in the airways is not clearly established, the ASM growth-promoting actions of several factors, including the ECM and mitogens have been implicated (Hirst et al., 2004; Stewart et al., 2004). Furthermore, the observation that circulating mesenchymal precursor cells migrate to the airways following allergen challenge in both humans and mice (Schmidt et al., 2003) has intensified the interest in cellular migration as a mechanism for hyperplasia (Stewart, 2004).
In the remodelled airway, the ECM is expanded by the deposition of collagen, not only in the subepithelial spaces, but also throughout the airways (Redington, 2000). Within the muscle bundles, the amount of ECM is increased around individual cells. The contraction of ASM strips is increased significantly when the ECM surrounding ASM is degraded by collagenase, indicating that collagen is likely to influence ASM mechanical function (Bramley et al., 1995). Collagen deposition not only reduces airways distensibility by increasing the structural rigidity of airways, but is also likely to play an important role in ASM proliferation via integrin/ECM interactions (Hirst et al., 2000; Bonacci et al., 2003). In addition, ECM proteins, including collagen type I, protect cultured ASM cells against apoptosis induced by the protein synthesis inhibitor, cycloheximide (Freyer et al., 2001).
Culture on monomeric collagen type I promotes growth of several cell types, including vascular smooth muscle, bovine ASM and human ASM (Hirst et al., 2000; Bonacci et al., 2003) by a mechanism involving integrins (Nguyen et al., 2005). Cells communicate with the ECM via a family of cell surface heterodimeric receptors comprised of membrane-spanning α and β subunits known as integrins. Different cell types express distinct collagen-binding integrins, including the α1β1 and α2β1 integrins, that interact with the arginine, aspartate and glutamate residue motifs (RGD) in the collagen chains. However, the α2β1 integrin has a greater selectivity for type I collagen than the α1β1 integrin (Yamamoto and Yamamoto, 1994). In human ASM, the α2 and α5 integrin subunits are expressed in a greater proportion than are the α1 subunits, whereas the β1 is the most abundant β integrin subunit (Freyer et al., 2001; Nguyen et al., 2005), suggesting that the α2β1 integrin may be the dominant integrin influencing ASM function. Nguyen et al. (2005) showed that members of the β1-integrin family, including α2β1, α4β1 and α5β1, contributed to the collagen type I-induced enhancement of ASM proliferation, whereas the α2β1 and αvβ3 integrins were predominantly responsible for mediating cell attachment to collagen type I (Nguyen et al., 2005).
Glucocorticoids reduce inflammatory cell infiltration, increase lung function and improve quality of life in the majority of asthmatic patients. However, a small population of severe steroid-requiring asthmatics remain symptomatic and are more likely to be admitted to hospital and die from asthma, despite therapy with both inhaled and oral glucocorticoids (Barnes, 2004). These patients constitute the most significant therapeutic challenge in asthma. We have recently reported that monomeric collagen I enhanced the proliferation of ASM and impaired anti-mitogenic actions of glucocorticoids (Bonacci et al., 2003).
In this study, we have elucidated a mechanism by which collagen interferes with the anti-mitogenic actions of glucocorticoids. The loss of glucocorticoid efficacy was associated with a failure to inhibit retinoblastoma protein phosphorylation and the failure to reduce cyclin D1 protein and transcript levels, when cells were cultured on collagen I. The growth-promoting actions of collagen and impairment of glucocorticoid actions were dependent on α2β1 integrin/ECM signalling in ASM cells.
Methods
Cell culture
Human ASM was cultured from macroscopically normal bronchi (0.5–2 cm diameter), obtained from resection specimens from lung transplant recipients. ASM was dissected from the bronchial wall, enzymatically digested and maintained in culture as described previously (Fernandes et al., 1999).
Cell enumeration
Cells were seeded onto six-well culture plates containing a flexible-silastic membrane, pre-coated by the manufacturer (Flexcell Corporation, McKeesport, PA, USA) with type I collagen (200 μg ml−1) or a laminin penta-peptide sequence (1 μg ml−1) representing the integrin receptor attachment site. In separate experiments, cells were cultured on plastic culture plates and treated as described below. Cells were grown to a subconfluent density, washed with PBS and serum-deprived for 24 h before drug treatment. In experiments with α2β1 integrin-blocking antibodies (Dako, Copenhagen, Denmark, clone P1E6), cells were incubated with a concentration shown to interfere with integrin signalling (1:1000) (Scaffidi et al., 2001) or negative isotype immunoglobulin G (IgG)1 control, once seeded, and the antibody remained in the medium for the duration of the experiment. Cells were then incubated with a maximally effective concentration of dexamethasone, 100 nM (Sigma, St Louis, MO, USA) or fluticasone propionate, 1 nM (GSK, UK) for 30 min before the addition of a maximally effective concentration of the mitogen, basic fibroblast growth factor (bFGF, 300 pM, Promega, Madison, WI, USA) and the growth supplement, monomed A (CSL, Australia), comprising insulin, transferrin and selenium. After the stimulation period (described in each experiment), cells were detached from plates and manually counted as described previously (Fernandes et al., 1999).
Immunoblot analysis
Cells were cultured as described previously, serum-deprived for 24 h and incubated for various times described in each experiment with bFGF in the presence or absence of dexamethasone (added 30 min before mitogen). Lysates were prepared and assayed as described previously (Fernandes et al., 1999) and Western analysis was performed to identify cyclin D1 levels (Upstate Biotechnology, Charlottsville, VA, USA, rabbit anti-human cyclin D1), retinoblastoma protein phosphorylation (Cell Signalling, Danvers, MA, USA, rabbit anti-human phospho-Ser780 retinoblastoma protein) and p21cip1 (Transduction Labs, San Jose, CA, USA, mouse anti-human p21cip1) by a protocol described previously (Fernandes et al., 1999). Cyclin D1 levels and retinoblastoma protein phosphorylation were measured at 20 h, whereas p21cip1 levels were measured at 16 h. These time points during the G1 phase of the cell cycle correspond to the times at which there are maximum changes and when cyclin activity is essential for S-phase entry.
RNA extraction and real-time reverse transcription-polymerase chain reaction
To quantify gene expression, RNA was extracted from stimulated human ASM cells, and reverse transcribed for real-time reverse transcription–polymerase chain reaction (RT-PCR) analysis using TaqMan chemistry. Total RNA isolation was performed using Qiagen RNeasy Mini-kits (Qiagen, Australia) according to the manufacturer's instructions. RNA was reverse transcribed into cDNA using random primers with TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA, USA). Levels of cyclin D1 target transcript and 18S rRNA were assayed by real-time PCR according to the manufacturer's instructions and reagents (Applied Biosystems), using the ABI Prism 7900 HT sequence analyzer (Applied Biosystems). Assays for cyclin D1 and 18S rRNA were performed in triplicate using Platinum qPCR Supermix-UDG reagents (Invitrogen, Carlsbad, CA, USA) in 384-well plates. Cycle threshold (CT) values for each reaction were determined using SDS software (Applied Biosystems). Cyclin D1 data were normalized against 18S rRNA transcript levels. The 18S rRNA levels were unaffected by drug treatment (data not shown).
ASM migration assay
Human ASM cells were grown to confluence in 175 cm2 culture flasks, then deprived of serum for 24 h. Cells were trypsinized, counted and 100 μl of the cell suspension was plated in the upper compartment of Costar transwell inserts (Edward Keller, Australia) at a density of approximately 7 × 105 cells ml−1. Both the upper and lower surfaces of the transwell inserts were coated overnight at 4°C with 0.1% v v−1 bovine bone gelatin (Sigma, USA) before the commencement of the assay. Six hundred microlitre of Dulbecco's modified Eagle's medium (DMEM) or chemoattractant (platelet-derived growth factor (PDGF)-BB, Sapphire Biosciences, Australia) was then added to the lower compartments, and the cells were allowed to incubate at 37°C in an atmosphere of 5% CO2 in room air for 5 h. Cells were incubated with fluticasone propionate (1 nM) or vehicle for 30 min before plating the cells in the upper compartment of the Transwell inserts.
In a separate experiment, cells were incubated in the upper compartment (100 μl) at a density of 3 × 105 cells ml−1 for a total of 3 days before the migration assay, initially in DMEM supplemented with 1% v v−1 foetal calf serum (FCS), and L-glutamine, non-essential amino acids and sodium pyruvate to facilitate adhesion (24 h), then for a further 2 days in serum-free DMEM. Cells were pretreated with fluticasone propionate for 30 min before being stimulated to migrate towards PDGF-BB (1 ng ml−1, 5 h incubation). These cells were referred to as substrate-adherent.
Following the 5 h chemotaxis assays, the inserts were gently washed with PBS, and cells were fixed with DiffQuick stain (Lab Aids, Australia) according to the manufacturer's instructions. The membranes were cut out of the Transwell inserts and mounted onto slides using xylene, and after drying were visualized by light microscopy. The number of migrated cells in five fields (at magnification × 400) was counted in triplicate.
Statistical analysis
All results are expressed as the mean±s.e.m. of n individual cultures obtained from different donors. Differences were determined by one-way analysis of variance (ANOVA) with repeated measures, followed by a post hoc Bonferroni test. All statistical analyses were performed using GraphPad Prism (for Windows, Version 3), and differences were considered to be statistically significant when P<0.05.
Results
Glucocorticoids inhibit proliferation of cells grown on laminin, but not collagen type I
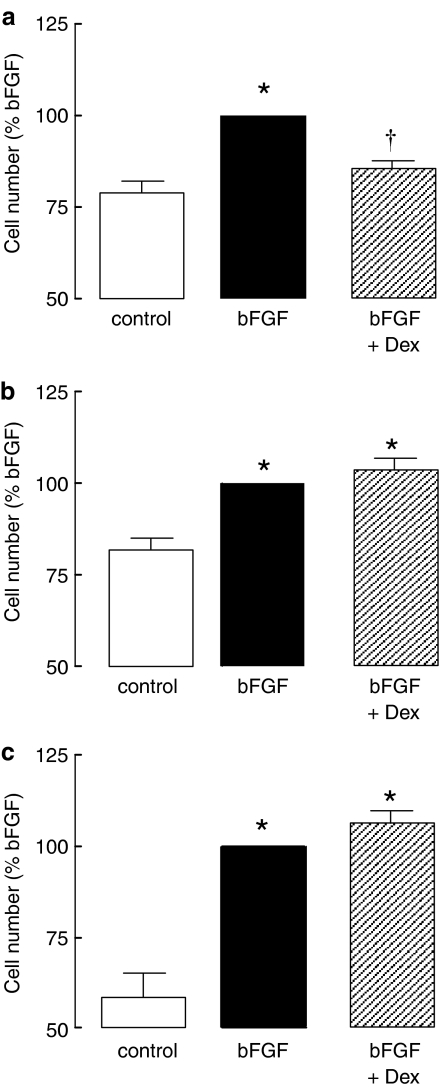
Dexamethasone (100 nM) inhibited bFGF-induced proliferation of cells grown on a laminin, but not collagen ECM (Figure 1). To establish whether prolonged glucocorticoid treatment was required to achieve anti-proliferative efficacy on collagen, cells were enumerated after 7 days of bFGF and glucocorticoid exposure. Under these conditions, neither dexamethasone (Figure 1c) nor fluticasone propionate (100 nM, data not shown), reduced bFGF-stimulated increases in ASM cell number. Furthermore, an extended pretreatment duration with dexamethasone of 24 h also failed to prevent increases in cell number in response to bFGF (4±4% inhibition, P>0.05, n=4).
Figure 1.
Effect of glucocorticoids on proliferation of ASM grown on (a) laminin and (b and c) collagen. Cells were incubated with dexamethasone (Dex, 100 nM) for 30 min before the addition of bFGF (300 pM) and were counted (a and b) 48 h (a and b) or 7 days (c) after the addition of the mitogen. Data are normalized to the number of cells in the presence of bFGF for each culture condition (a, 15.8±4.1 × 104 cells; b, 16.7±3.8 × 104 cells; c, 28.7±10.4 × 104 cells). *P<0.05 cf. control; †P<0.05 cf. bFGF response.
Resistance to glucocorticoid anti-mitogenic actions is associated with a failure to regulate cell cycle regulatory protein levels
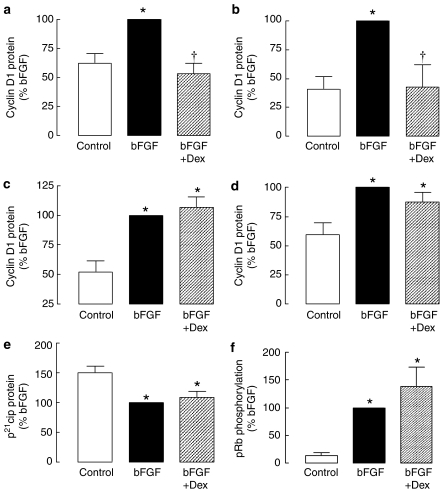
The effect of dexamethasone (100 nM) on bFGF-induced increases in cyclin D1 protein expression was examined, as our previous studies of ASM cultured on plastic culture plates established that glucocorticoids reduce cyclin D1 mRNA and protein expression. Dexamethasone completely inhibited bFGF-induced increases in cyclin D1 protein expression in cells grown on laminin (Figure 2a, P<0.05), but was without effect in cells grown on collagen (Figure 2c, P>0.05). We studied the expression of cyclin D1 mRNA to establish whether the inhibition of cyclin D1 protein by dexamethasone was evident at the transcript level. Dexamethasone significantly inhibited bFGF-induced increases in cyclin D1 mRNA in cells grown on laminin (Figure 2b, P<0.05), but not on collagen (Figure 2d, P>0.05). Cyclin D1 protein levels in cells seeded on collagen were greater than the levels in cells seeded on laminin (P<0.01, two-way ANOVA with repeated measures). Basal levels of cyclin D1 were not different (1.7±0.7-fold collagen/laminin) in cells cultured on collagen or laminin. In the presence of bFGF, the increase in cyclin D1 in cells cultured on collagen compared with those on laminin (2.1±0.5-fold) became significant (P<0.05, Bonferroni post hoc test) and the difference was greater (3.7±0.6-fold) in cells incubated with bFGF and dexamethasone (P<0.05, Bonferroni post hoc test).
Figure 2.
The effect of dexamethasone on bFGF-induced changes in cyclin D1 protein(a and c) and mRNA expression (b and d), p21cip1 protein levels (e) and retinoblastoma protein phosphorylation (f). Cells were incubated with dexamethasone (Dex, 100 nM) for 30 min before the addition of bFGF (300 pM). mRNA levels were measured by real-time RT-PCR after 6 h and protein was measured by Western analysis after 16 (p21cip1) or 20 h (Cyclin D1, retinoblastoma protein). Cells were maintained on (a and b) laminin- or (c–f) collagen-coated plates. *P<0.05 cf. control. †P<0.05 cf. bFGF response.
We investigated whether dexamethasone affected the levels of the cyclin-dependent kinase inhibitor, p21cip1. The magnitude of the bFGF-induced reduction in p21cip1 levels was unaffected by incubation with dexamethasone (Figure 2e, P<0.05). The propagation of cell cycle signalling through cyclins results in the phosphorylation of the retinoblastoma protein. In cells maintained on collagen, bFGF-induced phosphorylation of the retinoblastoma protein was unaffected by treatment with dexamethasone (Figure 2f, P>0.05, n=4), whereas retinoblastoma protein phosphorylation was reduced by glucocorticoids when cells cultured on laminin were treated with dexamethasone (58±16% inhibition, P<0.05, n=4).
Reducing integrin signalling restores glucocorticoid inhibition of cyclin D1 and anti-mitogenic actions
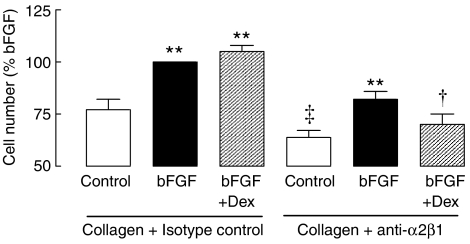
The role of integrin signalling in human ASM proliferation and glucocorticoid responsiveness was investigated with a function-blocking antibody to the α2β1 integrin, an integrin responsible for proliferation induced by collagen I (Nguyen et al., 2005). The negative isotype control antibody (IgG1) did not influence the number of ASM cells maintained on collagen. The function-blocking antibody had no effect on the number of cells when ASM was grown on plastic culture plates under basal conditions (plastic control 100 %, anti-α2β1 93±6%, n=3, P>0.05). However, in ASM grown on a collagen ECM, incubation with the α2β1 function-blocking antibody from the time of seeding (and then throughout the experiment) reduced cell number by at least 20% (P<0.05, n=6, Figure 3), irrespective of the presence of mitogen. Dexamethasone inhibited bFGF-induced proliferation when cells were grown on collagen in the presence of the α2β1 function-blocking antibody (P<0.05, n=6, Figure 3), but not in cells grown in the same concentration of the negative isotype control antibody. We investigated whether the reversal of glucocorticoid resistance was simply due to the blocking antibody-induced reduction in cell number response to collagen/bFGF. For cells grown on collagen, the proliferation of ASM induced by 30 pM bFGF, matched the response to bFGF (300 pM) in the presence of anti-α2β1. This matched proliferation response to 30 pM bFGF was unaffected by pretreatment with dexamethasone (P>0.05, Table 1). In separate experiments, the effects of the function-blocking antibody on proliferative responses of cells seeded on laminin were investigated. The small response to bFGF under these conditions was completely prevented by the addition of the function-blocking antibody, so it was not possible to ascertain what impact there was on the anti-proliferative effects of glucocorticoids (data not shown).
Figure 3.
The effects of the function-blocking monoclonal antibody to the α2β1 integrin (anti-α2β1, 15 μg ml−1) on the proliferation of ASM cultured on collagen type I. Inhibition of bFGF (300 pM)-induced proliferation by the glucocorticoid, dexamethasone (Dex, 100 nM) was examined in cells grown on collagen type I in the presence of negative isotype control or anti-α2β1 (n=6). Cells were counted after 72 h incubation, and data are normalized to the number of cells in the presence of bFGF and isotype control (15.3±6.7 × 104 cells). ‡P<0.05 cf. collagen control in the absence of anti-α2β1; **P<0.01 cf. respective control; †P<0.05 cf. bFGF response in the presence of the anti-α2β1.
Table 1.
Effect of dexamethasone on the proliferation of ASM cells grown on collagen-coated plates and stimulated by submaximal concentrations of bFGF
| Incubation conditions | Cell number (% unstimulated) |
|---|---|
| Control (unstimulated) | 100 |
| bFGF 3 pM | 106±4 |
| bFGF 3 pM+Dex (100 nM) | 103±5 |
| bFGF 30 pM | 128±6* |
| bFGF 30 pM+Dex (100 nM) | 128±6* |
Abbreviations: ASM, airway smooth muscle; bFGF, basic fibroblast growth-factor; Dex, dexamethasone.
Cell number data are expressed as mean ±s.e.m. percentages of the number of cells maintained on a collagen matrix in the absence of additional mitogen. Cells were pretreated with dexamethasone (100 nM) for 30 min before stimulation with either 3 or 30 pM.
bFGF *P<0.05 cf. control. n=3–5.
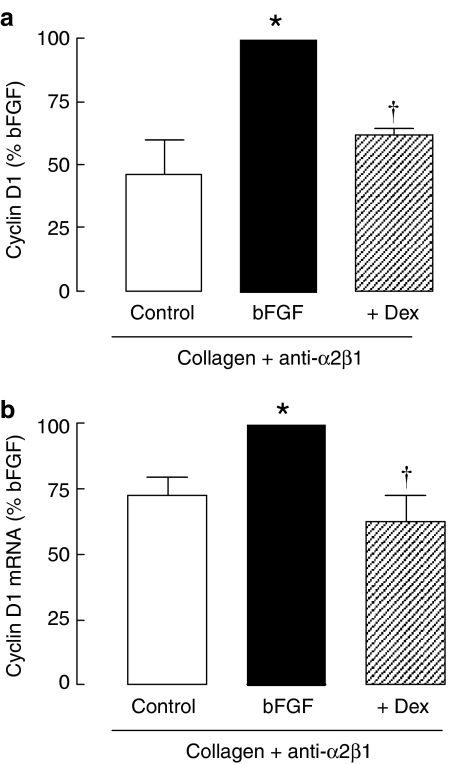
As the function-blocking antibody reversed resistance to the anti-mitogenic effects of the glucocorticoid, we expected that the inhibition of cyclin D1 levels would also be restored if this action of the glucocorticoid was important for reducing proliferation. In cells grown on collagen in the presence of the α2β1 function-blocking antibody, dexamethasone (100 nM) significantly inhibited bFGF-induced expression of cyclin D1 protein (Figure 4a, P<0.05). The inhibition of bFGF-induced cyclin D1 mRNA expression by dexamethasone (100 nM) was also restored (Figure 4b, P<0.05).
Figure 4.
The effects of dexamethasone on cyclin D1 expression in the presence of α2β1 integrin function-blocking antibody (anti-α2β1). Cells were incubated with dexamethasone for 30 min before the addition of bFGF (300 pM). Cyclin D1 protein (a) was measured by Western analysis after 20 h (n=3) and mRNA (b) expression was measured by real-time PCR after 6 h incubation (n=5). *P<0.05 cf. control; †P<0.05 cf. bFGF response.
Regulation of ASM migration by fluticasone propionate
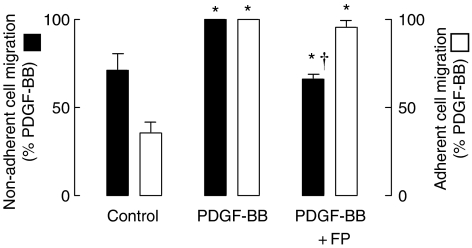
We investigated whether glucocorticoids were effective in regulating the migration of ASM cells in a well-characterized chemotaxis migration assay. PDGF-BB, when used at a concentration previously shown to induce migration of human ASM cells (1 ng ml−1), significantly increased migration as compared to unstimulated cells (P<0.05, n=4). Pretreatment with fluticasone propionate (1 nM) for 30 min resulted in an attenuation of the PDGF-BB-induced migration (P<0.05, Figure 5). In situ, ASM cells make direct contacts with the ECM and other ASM cells. Therefore, the assay was repeated with cells allowed to attach to each other and to the gelatin-coated transwell inserts for 72 h before exposure to fluticasone propionate or PDGF-BB. Incubation with PDGF-BB increased ASM migration by approximately 30% over that of unstimulated cells (P<0.05, n=3). However, pretreatment with fluticasone propionate (1 nM) for 30 min did not inhibit the migration of substrate-adherent ASM induced by PDGF-BB (P>0.05, Figure 5).
Figure 5.
The effect of the glucocorticoid, fluticasone propionate (FP, 1 nM), on the chemotactic migration of non-adherent and substrate-adherent ASM cells induced by platelet-derived growth factor (PDGF-BB, 1 ng ml−1). Cells were incubated with fluticasone propionate for 30 min before the addition of PDGF-BB, and were allowed to migrate for 5 h (n=3–4). In the adherent cell experiment, cells were allowed to attach to the gelatin-coated transwell inserts for 72 h before pretreatment with FP and exposure to PDGF-BB. *P<0.05 cf. control; †P<0.05 cf. PDGF-BB response.
Discussion
Modulation of ASM proliferation may have therapeutic benefit by regulating AWR and AHR in asthma (Stewart et al., 1993). There is extensive evidence for a beneficial effect of glucocorticoids on inflammatory cytokine levels (Barnes and Adcock, 2003), but less is known about the glucocorticoid-responsiveness of long-term remodelling events in asthma. Moreover, while more extensively investigated, the impact of glucocorticoids on fibrosis remains controversial (Redington, 2000). The development of marked ASM hyperplasia in patients with severe asthma (Mast et al., 2003), a patient population that is likely to include those who have used high doses of inhaled or oral glucocorticoid, is difficult to reconcile with the well-confirmed and substantial inhibitory effects of glucocorticoids on ASM maintained on plastic culture plates (Stewart et al., 1995; Fernandes et al., 1999). Our current data showing that glucocorticoids do not regulate the proliferation of ASM in a collagen-rich ECM may partly explain the development of ASM hyperplasia in glucocorticoid-treated, moderate to severe asthma.
Although type I collagen is deposited in the airways as fibrils, the characteristic influx of inflammatory cells into asthmatic airways results in the release of proteases such as MMPs (Ohbayashi, 2002), which degrade fibrillar collagen into a monomeric form. In the subepithelial space and within muscle bundles, it is conceivable that ASM are in direct contact with a form of degraded collagen that is recognized in the same way as the monomeric type I collagen used in the present study. Fibrillar and monomeric collagen have been shown to differentially influence cell function in vitro: monomeric collagen enhances mitogen-stimulated proliferation of human ASM as compared to rates of proliferation on fibrillar collagen or laminin (Hirst et al., 2000; Bonacci et al., 2003; Nguyen et al., 2005).
Our previous finding that dexamethasone prevented bFGF-induced proliferation of cells cultured on laminin, but not monomeric collagen (Bonacci et al., 2003) has been extended by showing that neither prolonged pretreatment, nor a longer growth period, restored the anti-mitogenic actions of glucocorticoids on ASM grown on collagen. Inflammatory and structural cells in the airway release different mitogens that may contribute to ASM hyperplasia. Our evidence suggests that the impairment by monomeric collagen of the anti-mitogenic actions of glucocorticoids is not mitogen specific, because it was observed when bFGF (present study), thrombin or FCS (Bonacci et al., 2003) was used as a mitogen.
The induction by culture on collagen of a primary biochemical defect in glucocorticoid action is not consistent with our earlier observation that dexamethasone inhibited granulocyte–macrophage colony-stimulating factor (GM-CSF) levels of human ASM cultured on collagen and laminin to a similar extent when the cells were exposed to either thrombin or FCS (Bonacci et al., 2003). This conclusion is reinforced by the observation that, in ASM cultured on collagen, the ability of glucocorticoids to induce translocation of the glucocorticoid receptor to the nucleus is maintained (Bonacci and Stewart, 2006). The impairment of glucocorticoid actions is not restricted to ASM proliferation, as the migration of collagen-adherent ASM was also resistant to inhibition by glucocorticoids. The resistance to some, but not other actions of glucocorticoids suggests that the collagen ECM may influence signalling to render it resistant to modulation by glucocorticoids, either by virtue of an increase in signal strength or by the recruitment of new signalling pathways that are inherently glucocorticoid-insensitive.
In bronchial fibroblasts derived from mild asthmatics, glucocorticoids are reported to increase DNA synthesis (measured as 3H-thymidine incorporation) (Kraft et al., 2001). Moreover, cultured ASM derived from asthmatics proliferates faster than smooth muscle derived from non-asthmatic subjects, and is considered to be unresponsive to glucocorticoids (Johnson et al., 2001). Glucocorticoids also fail to prevent the production of ECM proteins by ASM cells exposed to serum from asthmatics (Johnson et al., 2000). Thus, glucocorticoid-insensitive ECM remodelling may promote proliferation to amplify and accelerate the development of AWR and AHR in asthma.
When the communication between collagen and ASM was intercepted with a function-blocking monoclonal antibody to the collagen receptor, the α2β1 integrin, proliferation was reduced and glucocorticoid actions were restored. These restored glucocorticoid actions do not appear to result from a reduced bFGF response, as collagen impairment of glucocorticoid anti-mitogenic effects did not appear to be related to the magnitude of the proliferation. Nguyen et al. (2005) showed that collagen-mediated ASM proliferation was prevented by neutralizing the activity of the α2β1 integrin, α4β1 or α5β1 integrins (Nguyen et al., 2005). Synergy between these integrin isoforms in enhancing proliferation may explain why blockade of each reduces the effects of collagen. Thus, we cannot exclude a role for α4β1 or α5β1 integrins in collagen actions on ASM. A neutralizing antibody to β1 integrin subunit had no effect on the collagen response. This finding has a number of interpretations, including the possibility that there are confounding effects of the block of multiple β1-type integrins; the α2 subunit of α2β1 integrin retains activity in the presence of this β1-blocking antibody; the anti-α2β1 recognizes α2 subunits in uncharacterized or unreported dimers with other β subunits; the concentration used (1 μg ml−1) was inadequate. Nguyen and colleagues used concentrations of anti-β1 ranging from 0.1 to 10 μg ml−1 with 10 μg ml−1of anti-β1 causing a 50% reduction in cell attachment to collagen. The use of interventions that influence cellular attachment is likely to confound interpretation of subsequent outcomes such as proliferation or migration.
Glucocorticoid-mediated inhibition of ASM proliferation is not attributable to apoptosis (Fernandes et al., 1999). Glucocorticoids inhibited the proliferation of human ASM cells grown on plastic in association with a reduction in cyclin D1 mRNA and protein levels, and the phosphorylation of the retinoblastoma protein (Fernandes et al., 1999). Cyclin D1 accumulation enables cyclin-dependent kinase 4-mediated hyperphosphorylation of the retinoblastoma protein, leading to de-repression of E2F transcription factors and synthesis of genes required for S-phase entry. Cyclin-dependent kinase inhibitors, such as p21cip1, can arrest cell cycle progression by preventing retinoblastoma protein phosphorylation. However, glucocorticoid-mediated inhibition of thrombin- or epidermal growth factor-induced proliferation of ASM cultured on plastic occurs independently of an elevation in p21cip1 protein or mRNA levels. Furthermore, the bFGF-induced reduction in p21cip1 protein levels in cells maintained on collagen type I was unaffected by glucocorticoids. Therefore, changes in p21cip1 levels do not appear to offer an explanation of the anti-mitogenic actions of glucocorticoids when cultured on plastic (Vlahos et al., 2003), or of the impairment of action of glucocorticoids on collagen (current study). Dexamethasone reduced cyclin D1 protein and mRNA levels when cells were cultured on laminin, but not on collagen type I. Although an elevation in the levels of cyclin-dependent kinase inhibitors other than p21cip1 may be involved in the anti-mitogenic actions of glucocorticoids, the observed changes in the regulation of cyclin D1 levels offers the most likely explanation. The resistance of the retinoblastoma protein phosphorylation to inhibition by glucocorticoids is readily explained by the lack of effect of glucocorticoids on growth factor-induced cyclin D1 levels.
The interaction between the collagen ECM and integrin receptors results in greater accumulation of cyclin D1, providing an explanation for the enhanced proliferation on collagen as compared to laminin. When cells maintained on collagen were incubated with the anti-α2β1 monoclonal antibody, the glucocorticoid-mediated reduction in cyclin D1 levels was restored, in parallel with the anti-mitogenic actions. Integrin and ECM signalling can promote the survival and proliferation of a variety of cell types (Freyer et al., 2001). The intracellular proteins that associate with integrins include integrin-linked kinase (ILK) and focal adhesion kinase. Although focal adhesion kinase activates cyclin D1 promoter activity, likely through the activation of extracellular signal-regulated kinase (ERK) (Zhao et al., 2001), our previous studies have excluded ERK as a regulatory target for glucocorticoids (Fernandes et al., 1999). Signalling through ILK increases the levels of cyclin D1 mRNA and protein, ultimately accelerating proliferation. Furthermore, ILK signalling inhibits glycogen synthase kinase 3β activity, which marks cyclin D1 for proteasomal degradation (D'Amico et al., 2000). Regardless of the precise mechanism, the increased strength of signalling that elevates cyclin D1 levels when cells are grown on collagen appears to reduce the capacity of glucocorticoids to regulate this step of cell-cycle progression.
Cell migration may contribute to the accumulation of ASM in the remodelled airway (Gizycki et al., 1997). In asthmatics, allergen challenge increases the number of fibroblasts showing a contractile phenotype. These cells appear within 24 h, which is too soon for them to have been the result of cell division and the numbers exceed the number of fibroblasts within the region of the airway wall. These observations are consistent with the myofibroblasts having migrated either from a pool of mesenchymal precursor cells in the circulation/bone marrow (Schmidt et al., 2003) or from the muscle bundle into the collagen-rich subepithelial space (Stewart, 2001). In non-adherent cells, fluticasone propionate halved the number of cells that migrated in response to PDGF-BB, a finding consistent with those of Goncharova et al. (2003). However, the study of substrate-adherent cells may provide more relevant insights into the pharmacological regulation of the migration process, as ASM in situ is in contact with other cells and ECM. In migration studies in which ASM were adherent to type I monomeric collagen (gelatin)-coated membranes, the migration induced by PDGF-BB was unresponsive to glucocorticoids. Collagen type I has not been shown to directly influence the chemotactic response of ASM (Parameswaran et al., 2004). The PI3-K and p38MAPK pathways have been implicated in the signalling of ASM migration (Goncharova et al., 2002), the latter having recently been shown to be sensitive to inhibition by glucocorticoids (Tran et al., 2005). However, it is unclear as to whether these inhibitory effects are preserved when cells are maintained on collagen, or in the presence of the chemoattractant, PDGF-BB.
In conclusion, a collagen-rich microenvironment renders ASM remodelling-associated responses, such as proliferation and migration, insensitive to regulation by glucocorticoids in vitro. Whether the increased collagen expression in asthmatic airways influences ASM proliferation and/or interferes with glucocorticoid therapy is difficult to ascertain. Especially since it is not known whether the fibrillar collagen state dominates over the monomeric collagen generated by MMP-mediated degradation.
Acknowledgments
This work was supported by the NHMRC (#299823) and GSK (UK). We thank Dr Darryl Knight (University of British Columbia, Canada) for provision of function-blocking antibody to the α2β1 integrin. We also thank the cardiothoracic surgeons and anatomical pathologists of the Alfred Hospital (Melbourne, Victoria, Australia) for the provision of human airway specimens.
Abbreviations
- ASM
airway smooth muscle
- AWR
airway wall remodelling
- bFGF
basic fibroblast growth factor
- DMEM
Dulbecco's modified Eagle's medium
- ECM
extracellular matrix
- ERK
extracellular signal-regulated kinase
- FCS
foetal calf serum
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- ILK
integrin-linked kinase
- PDGF
platelet-derived growth factor
Conflict of interest
Alastair Stewart has received grant support and consultancies for GSK(UK).
References
- Barnes PJ. New drugs for asthma. Nat Rev Drug Discov. 2004;3:831–844. doi: 10.1038/nrd1524. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Adcock IM. How do corticosteroids work in asthma. Ann Intern Med. 2003;139:359–370. doi: 10.7326/0003-4819-139-5_part_1-200309020-00012. [DOI] [PubMed] [Google Scholar]
- Bonacci JV, Harris T, Wilson JW, Stewart AG. Collagen-induced resistance to glucocorticoid anti-mitogenic actions: a potential explanation of smooth muscle hyperplasia in the asthmatic remodelled airway. Br J Pharmacol. 2003;138:1203–1206. doi: 10.1038/sj.bjp.0705135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonacci JV, Stewart AG. Regulation of human airway mesenchymal cell proliferation by glucocorticoids and beta(2)-adrenoceptor agonists. Pulm Pharmacol Ther. 2006;19:32–38. doi: 10.1016/j.pupt.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Boulet LP. Physiopathology of airway hyperresponsiveness. Curr Allergy Asthma Rep. 2003;3:166–171. doi: 10.1007/s11882-003-0030-9. [DOI] [PubMed] [Google Scholar]
- Bramley AM, Roberts CR, Schellenberg RR. Collagenase increases shortening of human bronchial smooth muscle in vitro. Am J Respir Crit Care Med. 1995;152:1513–1517. doi: 10.1164/ajrccm.152.5.7582286. [DOI] [PubMed] [Google Scholar]
- Carroll N, Elliot J, Morton A, James A. The structure of large and small airways in nonfatal and fatal asthma. Am Rev Respir Dis. 1993;147:405–410. doi: 10.1164/ajrccm/147.2.405. [DOI] [PubMed] [Google Scholar]
- D'Amico M, Hulit J, Amanatullah DF, Zafonte BT, Albanese C, Bouzahzah B, et al. The integrin-linked kinase regulates the cyclin D1 gene through glycogen synthase kinase 3beta and cAMP-responsive element-binding protein-dependent pathways. J Biol Chem. 2000;275:32649–32657. doi: 10.1074/jbc.M000643200. [DOI] [PubMed] [Google Scholar]
- Ebina M, Takahashi T, Chiba T, Motomiya M. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial asthma: a 3-D morphometric study. Am Rev Respir Dis. 1993;148:720–726. doi: 10.1164/ajrccm/148.3.720. [DOI] [PubMed] [Google Scholar]
- Fernandes D, Guida E, Koutsoubos V, Harris T, Vadiveloo P, Wilson JW, et al. Glucocorticoids inhibit proliferation, cyclin D1 expression, and retinoblastoma protein phosphorylation, but not activity of the extracellular-regulated kinases in human cultured airway smooth muscle. Am J Respir Cell Mol Biol. 1999;21:77–88. doi: 10.1165/ajrcmb.21.1.3396. [DOI] [PubMed] [Google Scholar]
- Freyer AM, Johnson SR, Hall IP. Effects of growth factors and extracellular matrix on survival of human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2001;25:569–576. doi: 10.1165/ajrcmb.25.5.4605. [DOI] [PubMed] [Google Scholar]
- Gizycki MJ, Adelroth E, Rogers AV, O'Byrne PM, Jeffery PK. Myofibroblast involvement in the allergen-induced late response in mild atopic asthma. Am J Respir Cell Mol Biol. 1997;16:664–673. doi: 10.1165/ajrcmb.16.6.9191468. [DOI] [PubMed] [Google Scholar]
- Goncharova EA, Ammit AJ, Irani C, Carroll RG, Eszterhas AJ, Panettieri RA, et al. PI3K is required for proliferation and migration of human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002;283:L354–L363. doi: 10.1152/ajplung.00010.2002. [DOI] [PubMed] [Google Scholar]
- Goncharova EA, Billington CK, Irani C, Vorotnikov AV, Tkachuk VA, Penn RB, et al. Cyclic AMP-mobilizing agents and glucocorticoids modulate human smooth muscle cell migration. Am J Respir Cell Mol Biol. 2003;29:19–27. doi: 10.1165/rcmb.2002-0254OC. [DOI] [PubMed] [Google Scholar]
- Hirst SJ, Martin JG, Bonacci JV, Chan V, Fixman ED, Hamid QA, et al. Proliferative aspects of airway smooth muscle. J Allergy Clin Immunol. 2004;114:S2–S17. doi: 10.1016/j.jaci.2004.04.039. [DOI] [PubMed] [Google Scholar]
- Hirst SJ, Twort CH, Lee TH. Differential effects of extracellular matrix proteins on human airway smooth muscle cell proliferation and phenotype. Am J Respir Cell Mol Biol. 2000;23:335–344. doi: 10.1165/ajrcmb.23.3.3990. [DOI] [PubMed] [Google Scholar]
- James A, Carroll N. Airway smooth muscle in health and disease; methods of measurement and relation to function. Eur Respir J. 2000;15:782–789. doi: 10.1034/j.1399-3003.2000.15d25.x. [DOI] [PubMed] [Google Scholar]
- Johnson PR, Black JL, Carlin S, Ge Q, Underwood PA. The production of extracellular matrix proteins by human passively sensitized airway smooth-muscle cells in culture: the effect of beclomethasone. Am J Respir Crit Care Med. 2000;162:2145–2151. doi: 10.1164/ajrccm.162.6.9909111. [DOI] [PubMed] [Google Scholar]
- Johnson PR, Roth M, Tamm M, Hughes M, Ge Q, King G, et al. Airway smooth muscle cell proliferation is increased in asthma. Am J Respir Crit Care Med. 2001;164:474–477. doi: 10.1164/ajrccm.164.3.2010109. [DOI] [PubMed] [Google Scholar]
- Kraft M, Lewis C, Pham D, Chu HW. IL-4, IL-13, and dexamethasone augment fibroblast proliferation in asthma. J Allergy Clin Immunol. 2001;107:602–606. doi: 10.1067/mai.2001.113760. [DOI] [PubMed] [Google Scholar]
- Mast A, Bamford TL, Wilson JW, Pain MC, Robertson CF, Smallwood D, et al. Smooth muscle area is increased in bronchial biopsies from steroid-resistant asthma. Am J Respir Crit Care Med. 2003;167:A33. [Google Scholar]
- Nguyen TT, Ward JP, Hirst SJ. beta1-Integrins mediate enhancement of airway smooth muscle proliferation by collagen and fibronectin. Am J Respir Crit Care Med. 2005;171:217–223. doi: 10.1164/rccm.200408-1046OC. [DOI] [PubMed] [Google Scholar]
- Ohbayashi H. Matrix metalloproteinases in lung diseases. Curr Protein Pept Sci. 2002;3:409–421. doi: 10.2174/1389203023380549. [DOI] [PubMed] [Google Scholar]
- Parameswaran K, Radford K, Zuo J, Janssen LJ, O'Byrne PM, Cox PG. Extracellular matrix regulates human airway smooth muscle cell migration. Eur Respir J. 2004;24:545–551. doi: 10.1183/09031936.04.00113103. [DOI] [PubMed] [Google Scholar]
- Redington AE. Airway fibrosis in asthma: mechanisms, consequences, and potential for therapeutic intervention. Monaldi Arch Chest Dis. 2000;55:317–323. [PubMed] [Google Scholar]
- Scaffidi AK, Moodley YP, Weichselbaum M, Thompson PJ, Knight DA. Regulation of human lung fibroblast phenotype and function by vitronectin and vitronectin integrins. J Cell Sci. 2001;114:3507–3516. doi: 10.1242/jcs.114.19.3507. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- Stewart AG.Airway smooth muscle as a target for novel anti-asthma drugs acting on airway wall remodelling Airway Wall Remodeling 2001Marcel Dekker Inc.: New York; 217–243.In: Howarth P, Wilson JW, Bousquet J, Rak S, Pauwels RA (eds) [Google Scholar]
- Stewart AG. Emigration and immigration of mesenchymal cells: a multicultural airway wall. Eur Respir J. 2004;24:515–517. doi: 10.1183/09031936.04.00067404. [DOI] [PubMed] [Google Scholar]
- Stewart AG, Bonacci JV, Quan L. Factors controlling airway smooth muscle proliferation in asthma. Curr Allergy Asthma Rep. 2004;4:109–115. doi: 10.1007/s11882-004-0055-8. [DOI] [PubMed] [Google Scholar]
- Stewart AG, Fernandes D, Tomlinson PR. The effect of glucocorticoids on proliferation of human cultured airway smooth muscle. Br J Pharmacol. 1995;116:3219–3226. doi: 10.1111/j.1476-5381.1995.tb15127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AG, Tomlinson PR, Wilson J. Airway wall remodelling in asthma: a novel target for the development of anti-asthma drugs. Trends Pharmacol Sci. 1993;14:275–279. doi: 10.1016/0165-6147(93)90130-c. [DOI] [PubMed] [Google Scholar]
- Tran T, Fernandes DJ, Schuliga M, Harris T, Landells L, Stewart AG. Stimulus-dependent glucocorticoid-resistance of GM-CSF production in human cultured airway smooth muscle. Br J Pharmacol. 2005;145:123–131. doi: 10.1038/sj.bjp.0706174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahos R, Lee KS, Guida E, Fernandes DJ, Wilson JW, Stewart AG. Differential inhibition of thrombin- and EGF-stimulated human cultured airway smooth muscle proliferation by glucocorticoids. Pulm Pharmacol Ther. 2003;16:171–180. doi: 10.1016/S1094-5539(02)00183-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Yamamoto M. Cell adhesion receptors for native and denatured type I collagens and fibronectin in rabbit arterial smooth muscle cells in culture. Exp Cell Res. 1994;214:258–263. doi: 10.1006/excr.1994.1256. [DOI] [PubMed] [Google Scholar]
- Zhao J, Pestell R, Guan JL. Transcriptional activation of cyclin D1 promoter by FAK contributes to cell cycle progression. Mol Biol Cell. 2001;12:4066–4077. doi: 10.1091/mbc.12.12.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]