Abstract
Background and purpose:
The types of hepatic microsomal cytochrome P450 (CYP) isozymes responsible for the metabolism of metformin in humans and rats have not been published to date. Therefore, a series of experiments using various inducers and inhibitors of CYP isozymes was conducted to find out what types of CYP isozymes are involved in the metabolism of metformin in rats.
Experimental approach:
Metformin at a dose of 100 mg kg−1 was administered intravenously to rats. The rats were pretreated with CYP inducers such as 3-methylcholanthrene, orphenadrine, isoniazid, and dexamethasone (major inducers of CYP1A1/2, 2B1/2, 2E1, and 3A1/2, respectively, in rats), or CYP inhibitors such as SKF-525 (a non-specific inhibitor of CYP isozymes), and sulfaphenazole, quinine, and troleandomycin (major inhibitors of CYP2C11, 2D1, and 3A1/2, respectively, in rats). The time-averaged non-renal clearance (CLNR) of metformin was compared with that of controls.
Key results:
In rats pretreated with dexamethasone, the CLNR was significantly faster (57% increase) than for the controls. In rats pretreated with SKF-525-A, sulfaphenazole, quinine, and troleandomycin, the CLNR was significantly slower (24.3, 62.9, 77.6, and 78.7% decrease, respectively) than for the controls. However, the CLNR values did not significantly different in the rats pretreated with 3-methylencholanthrene, orphenadrine, and isoniazid compared with the controls.
Conclusions and implications:
Our data suggest that metformin was metabolized mainly via CYP2C11, 2D1, and 3A1/2 in rats. This result could contribute to understanding of the possible changes in metformin pharmacokinetics in disease models where CYP2C11 and/or 3A1/2 are altered.
Keywords: metformin; pharmacokinetics; CYP2C11, 2D1 and 3A1/2; CYP inducers and inhibitors; rats
Introduction
Metformin, a biguanide antihyperglycemic agent, is widely used in the management of type II diabetes mellitus. It lowers the blood glucose concentration without causing hypoglycemia (Scheen, 1996). After intravenous (i.v.) administration of metformin at doses of 0.25–1.0 g to healthy volunteers, the terminal half-life of the drug was 1.5–4.5 h, and 79–100% of the dose was excreted in urine via active renal tubular secretion (Scheen, 1996 and references therein). After oral administration of metformin at doses of 0.5–1.5 g to healthy volunteers, absorption was not complete (20–30% of the oral dose was recovered from feces) possibly due to an active, saturable absorption process, and the absolute oral bioavailability was 33–55% (Scheen, 1996 and references therein). Metformin does not bind with human plasma proteins (Sirtori et al., 1978; Pentikäinen et al., 1979; Tucker et al., 1981). Metabolism of metformin was suggested in humans (Scheen, 1996), based on its incomplete recovery in the urine after its i.v. administration (Sirtori et al., 1978) and on a further study in which 20% of the dose was not accounted for (Tucker et al., 1981). Studies in vitro incubating metformin with the 9000 g supernatant fractions of livers from male Sprague–Dawley rats showed that approximately 20% of the added metformin (10 μg) disappeared after 30-min incubation (Choi et al., 2006). This suggested that rat liver could metabolize metformin.
However, the types of hepatic microsomal cytochrome P450 (CYP) isozymes responsible for the metabolism of metformin in humans and rats have not yet been identified. Therefore, this study was performed to find out what types of CYP isozymes are responsible for the metabolism of metformin in rats. Our results show that CYP2C11, 2D1 and 3A1/2 were involved in the metabolism of metformin in male Sprague–Dawley rats.
Methods
Animals
Male Sprague–Dawley rats (weighing 195–300 g) purchased from Taconic Farms Inc. (Samtako Bio Korea, O-San, South Korea) were housed in a light-controlled room (light: 0700–1900 hours, dark: 1900–0700 hours) kept at a temperature of 22±2°C and a relative humidity of 55±5% (Animal Center for Pharmaceutical Research, College of Pharmacy, Seoul National University, Seoul, South Korea). The rats were housed in metabolic cages (Tecniplast, Varese, Italy) with a supply of filtered pathogen-free air, and food (Samyang Company, Seoul, South Korea) and water ad libitum. The protocol of this study was approved by the Animal Care and Use Committee of the College of Pharmacy of Seoul National University.
Pretreatment with enzyme inducers and inhibitors
The rats received a single i.v. injection of 80 mg (2 ml) kg−1 of sulfaphenazole (dissolved in distilled water with a minimum amount of NaOH to yield a pH of approximately 8.0 in the SPT group (Ogiso et al., 1999)), a single intraperitoneal (i.p.) injection of 50 mg (3.3 ml) kg−1 of SKF 525-A (dissolved in 0.9% NaCl-injectable solution in the SKT group (Conney, 1971)), 500 mg (5 ml) kg−1 of troleandomycin (dissolved in 0.9% NaCl-injectable solution, acidified to pH 4.0 with HCl in the TMT group (Sinclair et al., 2000)), 20 mg (5 ml) kg−1 of quinine hydrochloride (dissolved in 0.9% NaCl-injectable solution in the QNT group (Tomkins et al., 1997)), three daily i.p. injections of 50 mg (5 ml) kg−1 of dexamethasone phosphate (dissolved in 0.9% NaCl-injectable solution in the DXT group (Arlotto et al., 1987; Ross et al., 1993; Correia, 1995)), 150 mg (3 ml) kg−1 of isoniazid (dissolved in 0.9% NaCl-injectable solution in the INT group (Ryan et al., 1985)), or 60 mg (5 ml) kg−1 of orphenadrine (OP) citrate (dissolved in 0.9% NaCl-injectable solution in the OPT group (Murray et al., 2003)), or four daily i.p. injections of 20 mg (3.3 ml) kg−1 of 3-methylcholanthrene (3-MC) (dissolved in corn oil in the MCT group (Williams et al., 1979; Choi et al., 1991)). Control groups received an i.p. (or i.v.) injection of 5 ml kg−1 of a 0.9% NaCl-injectable solution for the TMC, SKC, SPC, QNC, OPC, PBC or INC groups, or 3.3 ml kg−1 of corn oil for the MCC group. ‘T' and ‘C' refer to pretreatment and control, respectively. During the pretreatment, the rats had free access to food and water.
Intravenous study
In the early morning, the jugular vein (for drug administration) and the carotid artery (for blood sampling) of each rat were cannulated with a polyethylene tube (Clay Adams, Parsippany, NJ, USA), while each rat was under light ether anesthesia (Kim et al., 1993). Both cannulae were exteriorized to the dorsal side of the neck, where each cannula was terminated with a long silastic tube (Dow Corning, Midland, MI, USA). Both silastic tubes were inserted into a wire sheath to allow free movement of the rat. Each rat was housed individually in a rat metabolic cage (Daejong Scientific Company, Seoul, South Korea) and allowed to recover from the anesthesia over 4–5 h before the study was begun. They were not restrained during the study. An experiment was performed just after the injection for the SPC and SPT groups (Ogiso et al., 1999; Bae et al., 2004), during the first hour for the SKT and SKC groups (Conney, 1971) and the QNC and QNT groups (Tomkins et al., 1997), after 2 h for the TMC and TMT groups (Wrighton et al., 1985; Arlotto et al., 1987; Sinclair et al., 2000), on the fourth day for the DXC, DXT, INC, INT, OPC and OPT groups (Ryan et al., 1985; Arlotto et al., 1987; Ross et al., 1993; Sinclair et al., 2000; Murray et al., 2003), and on the fifth day for the MCT and MCC groups (Williams et al., 1979; Choi et al., 1991).
Metformin (metformin hydrochloride was dissolved in a 0.9% NaCl-injectable solution) at a dose of 100 mg kg−1 was administered i.v. over 1 min via the jugular vein of the control groups (n=6 for the MCC group, n=8 for the DXC, INC, OPC, SKC and QNC groups, n=9 for the TMC and SPC groups), and the pretreatment groups (n=8 for the SPT, DXT and INT groups, n=9 for the OPT, TMT, QNT and MCT groups, n=12 for the SKT group). The total injection volume was approximately 0.6 ml. Blood samples (approximately 0.12 ml) were collected via the carotid artery at 0 (to serve as a control), 1 (at the end of the infusion), 5, 15, 30, 60, 90, 120, 180, 240, 360, 480, 600 and 720 min. About 0.25 ml of a heparinized 0.9% NaCl-injectable solution (20 U ml−1) was used to flush the cannula after each blood sample to prevent blood clotting. The blood samples were immediately centrifuged at 16 000 g for 10 min, and a 50-μl aliquot of each plasma sample was stored in a –70°C freezer (Model DF8517, Ilshin Laboratory Company, Seoul, South Korea) until the high-pressure liquid chromatography (HPLC) analysis of metformin (Hale et al., 2002). At the end of 24 h, each metabolic cage was rinsed with 20 ml of distilled water, and the rinsed material was combined with the 24-h urine sample. After the exact volume of the combined urine sample was measured, a 50-μl aliquot of the sample was stored in a –70°C freezer until the HPLC analysis of metformin (Hale et al., 2002). At the same time (24 h), each rat was killed by cervical dislocation and exsanguinated.
Plasma protein binding of metformin using the equilibrium dialysis technique
Plasma protein binding of metformin in the control rats with or without the presence of quinine (QNT) or sulfaphenazole (SPT) was measured using the equilibrium dialysis technique (Shim et al., 2000). One milliliter of plasma was dialyzed against 1 ml of isotonic Sørensen phosphate buffer (pH 7.4) containing 3% (w/v) dextran in a 1-ml dialysis cell (Spectrum Medical Industries, Los Angeles, CA, USA) using a Spectra/Por 4 membrane (molecular weight cutoff of 12 000–14 000, Spectrum Medical Industries). After 24 h incubation, two 50 μl aliquots were removed from each compartment and stored in a –70°C freezer until the HPLC analysis of metformin (Hale et al., 2002).
HPLC analysis of metformin
The concentrations of metformin in the above samples were determined from a slight modification of a reported HPLC method (Hale et al., 2002), and ipriflavone instead of hydrocodeine was used as the internal standard. A 50 μl aliquot of the biological sample was deproteinized with a 100 μl aliquot of acetonitrile, and a 50 μl aliquot of methanol that contained 10 μg ml−1 of ipriflavone (an internal standard) was added. After vortex-mixing and centrifugation at 16 000 g for 10 min, a 50-μl aliquot of supernatant was injected directly onto a reversed-phase (C18) HPLC column. The mobile phases (pH=6), 10 mM KH2PO4: acetonitrile, at the ratios of 47.8:52.2 (v/v) for the rat plasma samples and 28:72 (v/v) for the urine samples were run at a flow rate of 1.5 ml min−1. The column effluent was monitored with an ultraviolet detector set at 235 nM.
Pharmacokinetic analysis
The total area under the plasma concentration–time curve from time zero to time infinity (AUC) was calculated using the trapezoidal-rule-extrapolation method. This method uses the logarithmic trapezoidal rule (Chiou, 1978) to calculate the area during the phase of declining plasma level, and the linear trapezoidal rule for the phase of rising plasma level. The area from the last datum point to time infinity was estimated by dividing the last measured plasma concentration by the terminal phase rate constant.
Standard methods (Gibaldi and Perrier, 1982) were used to calculate the time-averaged total body (CL), renal (CLR) and non-renal (CLNR) clearances, the terminal half-life (t1/2), the total area under the first moment of the plasma-concentration–time curve from time zero to time infinity (AUMC), the mean residence time (MRT), and the apparent volume of distribution at a steady state (Vss) (Kim et al., 1993). The mean values of each clearance (Chiou, 1980), Vss (Chiou, 1979) and t1/2 (Eatman et al., 1977) were calculated using the harmonic mean method.
Statistical analysis
A P-value of less than 0.05 was considered to be statistically significant using the unpaired t-test. All the results are expressed as mean±s.d.
Materials
Metformin hydrochloride and ipriflavone (an internal standard for HPLC analysis of metformin) were supplied by Dalim Medical (Seoul, South Korea) and Research Laboratory of Dong-A Pharmaceutical Company (Yongin, South Korea), respectively. 3-MC, OP citrate, isoniazid and dexamethasone phosphate (major inducers of CYP1A1/2, 2B1/2, 2E1 and 3A1/2, respectively, in rats (Williams et al., 1979; Ryan et al., 1985; Arlotto et al., 1987; Choi et al., 1991; Ross et al., 1993; Correia, 1995; Murray et al., 2003)), and SKF 525-A, sulfaphenazole, quinine hydrochloride and troleandomycin (a nonspecific inhibitor of CYP isozymes and inhibitors of CYP2C11, 2D1 and 3A1/2, respectively, in rats (Conney, 1971; Wrighton et al., 1985; Correia, 1995; Tomkins et al., 1997; Ogiso et al., 1999; Tyndale et al., 1999; Sinclair et al., 2000)) were purchased from Sigma-Aldrich Corporation (St Louis, MO, USA). Other chemicals were of reagent or HPLC grade.
Results
Pharmacokinetics of metformin in rats pretreated with various enzyme inducers
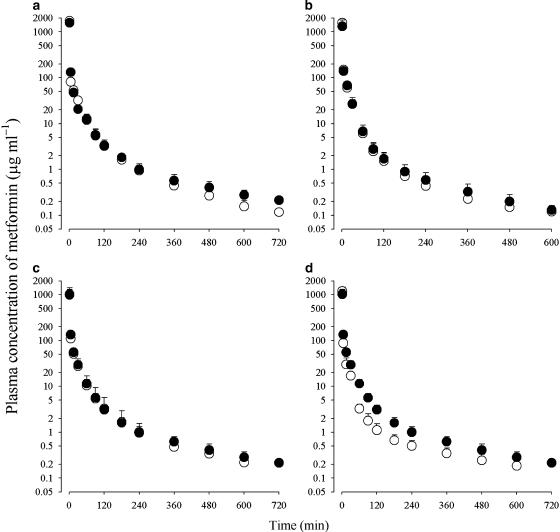
The mean arterial plasma concentration–time profiles of metformin after 1 min i.v. administration at a dose of 100 mg kg−1 to rats pretreated with 3-MC, orphenadrine, isoniazid or dexamethasone and to their respective control rats are shown in Figure 1, and some relevant pharmacokinetic parameters are listed in Table 1. After i.v. administration, the plasma concentrations of metformin declined in a polyexponential manner for all groups of rats.
Figure 1.
Arterial plasma concentration–time profiles of metformin after 1-min i.v. administration at a dose of 100 mg kg−1 to rats pretreated with various enzyme inducers (open circles), 3-methylcholanthrene (a), orphenadrine (b), isoniazid (c) or dexamethasone (d) and their respective control rats (filled circles). Data are presented as mean±s.d.
Table 1.
Pharmacokinetic parameters of metformin after i.v. administration at a dose of 100 mg kg−1 to rats pretreated with various enzyme inducers, 3-methylcholanthrene (MCT), orphenadrine (OPT), isoniazid (INT) and dexamethasone (DXT), and their respective control rats (MCC, OPC, INC and DXC)
| Parameter | MCC | MCT | OPC | OPT | DXC, INC | INT | DXT |
|---|---|---|---|---|---|---|---|
| Initial body weight (g) | 246±12.8 | 246±10.1 | 251±6.09 | 243±4.53 | 239±11.3 | 235±8.64 | 242±9.49 |
| Final body weight (g) | 270±16.1 | 261±4.43 | 283±10.7 | 261±5.63* | 263±11.6 | 243±14.1* | 222±7.99* |
| AUC (μg min ml−1) | 5840±1040 | 5700±1230 | 5220±945 | 5610±1050 | 5220±680 | 4730±1350 | 3780±460* |
| t1/2 (min) | 183±43.2 | 158±45.0 | 176±67.8 | 180±38.7 | 218±31.8 | 185±44.8 | 212±73.0 |
| MRT (min) | 44.7±16.2 | 36.2±7.28 | 28.9±8.56 | 22.4±4.18 | 54.0±13.1 | 44.8±12.3 | 38.1±14.6*** |
| Vss (ml kg−1) | 657±448 | 608±237 | 642±256 | 387±114 | 814±252 | 858±185 | 766±222 |
| CL (ml min−1 kg−1) | 17.2±3.21 | 17.6±4.25 | 18.8±2.77 | 17.8±3.64 | 19.2±2.50 | 21.2±9.12 | 26.8±3.52* |
| CLR (ml min−1 kg−1) | 10.5±3.01 | 12.3±5.45 | 11.8±2.09 | 12.0±4.00 | 11.3±2.58 | 11.7±5.46 | 14.0±2.99 |
| CLNR (ml min−1 kg−1) | 5.94±1.93 | 4.48±2.03 | 6.53±2.03 | 5.10±1.38 | 7.58±0.723 | 8.93±4.50 | 11.9±2.46* |
| Ae0–24 h (% of i.v. dose) | 63.0±11.2 | 72.9±6.33 | 63.6±7.74 | 69.7±9.60 | 60.2±6.52 | 56.8±8.29 | 54.2±7.67 |
Abbreviations: AUC, total area under the plasma-concentration–time curve from time zero to time infinity; CL, time-averaged total body clearance; CLNR, time-averaged non-renal clearance; CLR, time-averaged renal clearance; MRT, mean residence time.
Data are expressed as mean±s.d. (MCC, n=6; OPC, DXC, INC, INT and DXT, n=8; MCT and OPT, n=9). Significant difference from respective control, *P<0.05 and ***P<0.001.
In rats pretreated with 3-methylcholanthrene (MCT), orphenadrine (OPT) and isoniazid (INT), the pharmacokinetic parameters of metformin listed in Table 1 were comparable to those in their respective control rats. However, in rats pretreated with dexamethasone (DXT), the AUC was significantly smaller (27.6% decrease), CL and CLNR were significantly faster (39.6 and 57.0% increase, respectively) and MRT was significantly shorter (29.4% decrease) than in the controls. Note that the body weight decreased significantly in the rats with OPT, INT and DXT compared with the controls.
Pharmacokinetics of metformin in rats pretreated with various enzyme inhibitors
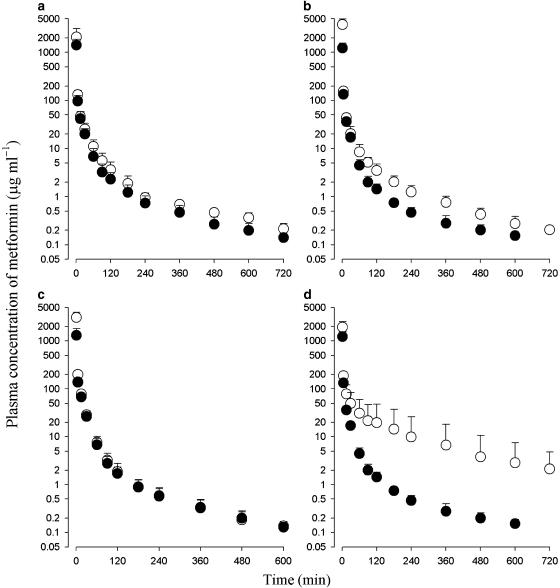
The mean arterial plasma concentration–time profiles of metformin after 1 min i.v. administration at a dose of 100 mg kg−1 to rats pretreated with SKF 525-A, sulfaphenazole, quinine or troleandomycin and to their respective control rats are shown in Figure 2, and some relevant pharmacokinetic parameters are listed in Table 2. After i.v. administration, the plasma concentrations of metformin also declined in a polyexponential manner for all groups of rats.
Figure 2.
Arterial plasma concentration–time profiles of metformin after 1-min i.v. administration at a dose of 100 mg kg−1 to rats pretreated with various enzyme inhibitors (open circles), SKF525-A (a), sulfaphenazole (b), quinine (c) or troleandomycin (d), and their respective control rats (filled circles). Data are presented as mean±s.d.
Table 2.
Pharmacokinetic parameters of metformin after i.v. administration at a dose of 100 mg kg−1 to rats pretreated with various enzyme inhibitors, SKF525-A (SKT), quinine (QNT), sulfaphenazole (SPT) and troleandomycin (TMT), and their respective control rats (SKC, QNC, SPC and TMC)
| Parameter | SKC | SKT | QNC | QNT | SPC, TMC | SPT | TMT |
|---|---|---|---|---|---|---|---|
| Final body weight (g) | 260±10.0 | 266±9.04 | 283±10.7 | 261±5.63** | 308±9.35 | 310±8.45 | 308±9.34 |
| AUC (μg min ml−1) | 4650±1003 | 6640±1500** | 5220±945 | 8760±1610** | 4350±634 | 8960±1650** | 12700±10400* |
| t1/2 (min) | 173±22.6 | 234±55.6 | 176±67.8 | 147±30.5 | 164±51.0 | 146±33.5 | 135±51.2 |
| MRT (min) | 36.7±9.46 | 52.8±19.8 | 28.9±8.56 | 22.9±4.37 | 27.7±9.21 | 23..8±10.2 | 70.2±71.6 |
| Vss (ml kg−1) | 844±367 | 568±434 | 642±256 | 184±86.7** | 566±307 | 225±192*** | 441±180 |
| CL (ml min−1 kg−1) | 21.5±4.75 | 14.6±3.30** | 18.8±2.77 | 11.4±12.6** | 23.0±3.48 | 11.2±2.42** | 7.87±6.61** |
| CLR (ml min−1 kg−1) | 13.4±2.96 | 8.48±2.33** | 11.8±2.09 | 7.81±1.96** | 12.8±3.02 | 6.96±2.38** | 5.13±4.34*** |
| CLNR (ml min−1 kg−1) | 7.68±2.46 | 5.81±1.85* | 6.53±2.03 | 1.46±2.96** | 9.61±2.07 | 3.57±1.29*** | 2.05±2.97*** |
| Ae0–24 h (% of i.v. dose) | 62.8±6.88 | 55.3±5.38 | 63.6±7.74 | 70.6±14.3 | 57.0±7.89 | 64.9±11.4 | 65.0±9.82 |
Abbreviations: AUC, total area under the plasma-concentration–time curve from time zero to time infinity; CL, time-averaged total body clearance; CLNR, time-averaged non-renal clearance; CLR, time-averaged renal clearance; MRT, mean residence time.
Data are expressed as mean±s.d. (SKC, QNC and SPT, n=8; QNT, SPC, TMC and TMT, n=9; SKT, n=12). Significant difference from respective control, *P<0.05, **P<0.01 and ***P<0.001.
In rats pretreated with SKF 525-A (SKT), quinine (QNT), sulfaphenazole (SPT) and troleandomycin (TMT), the AUC was significantly greater (42.8, 67.8, 106 and 192% increase for SKT, QNT, SPT and TMT, respectively) than in the controls. The CL (32.1, 39.4, 51.3 and 65.8% decrease, respectively), CLR (36.7, 33.8, 45.6 and 59.9% decrease, respectively) and CLNR (24.3, 77.6, 62.9 and 78.7% decrease, respectively) were significantly slower than in the controls. Note that the contribution of AUC10 or 12 h–∞ to the total AUC was below 5.6% in all the rats studied.
Plasma protein binding of metformin
The plasma protein binding (bound fraction) values of metformin in the rats with QNT and SPT and in the control rats were 18.6±10.1, 11.2±3.90 and 5.96±1.63%, respectively. The values in the rats with QNT and SPT were significantly greater (88 and 211% increase, respectively) than in the controls. The adsorption of metformin to an equilibrium dialysis apparatus, which included the semi-permeable membrane, was almost negligible, and 90.9–109% of the added amounts of metformin were recovered from the plasma and buffer compartments. The binding of metformin to 4% human serum albumin was independent of metformin concentrations ranging from 1 to 200 μg ml−1. The mean value was 10.1% (Choi et al., 2006). Hence, a 10 μg ml−1 concentration of metformin was arbitrarily chosen in this plasma protein binding study.
HPLC analysis of metformin
The retention times of metformin and the internal standard in the plasma samples were approximately 4 and 6.5 min, respectively, and the corresponding values in the urine samples were approximately 12 and 6 min. The quantitation limits of metformin in the rat plasma and urine were 0.05 and 0.1 μg ml−1, respectively. The inter- and intra-day coefficients of variation were below 9.91 and 8.35% for the plasma and urine samples, respectively, in the concentration ranges of 0.05–5000 μg ml−1 and 0.1–1000 μg ml−1 for the plasma and urine samples, respectively.
Discussion
The AUC values of metformin were dose-proportional after both the i.v. and oral dose ranges (50, 100 and 200 mg kg−1) studied (Choi et al., 2006). Hence, a dose of 100 mg kg−1 of metformin was chosen in this study. After i.v. administration, metformin was mainly excreted via the kidney. The total amounts of metformin excreted in 24-h urine as an unchanged drug (Ae0–24 h) were 54–73% of the i.v. doses (Tables 1 and 2). After i.v. administration, the contribution of the gastrointestinal (including biliary) excretion of the unchanged metformin to the CLNR of metformin was small. After i.v. administration of metformin at doses of 50, 100 and 200 mg kg−1, the total amounts of metformin recovered from the entire gastrointestinal tract (including its contents and feces) were less than 1.75% of the i.v. dose (Choi et al., 2006). Note that these small amounts of metformin were not due to the chemical and enzymatic degradation of metformin in the gastrointestinal tract. Rather, metformin has been found to be stable in rat gastric juices, rat bile juices and various buffer solutions with pH values ranging from 1 to 13 (Choi et al., 2006). Moreover, it was found that the percentage of i.v. metformin at a dose of 100 mg kg−1 excreted in 8-h bile as an unchanged drug was less than 0.3% in six rats (Choi et al., 2006). The above data indicate that the CLNR of metformin listed in Tables 1 and 2 could represent the metabolic clearance of metformin. Hence, the changes in the CLNR of metformin with the administration of various enzyme inducers (Table 1) and inhibitors (Table 2) could indicate changes in the metabolism of metformin in rats.
To find out whether or not CYP isozymes are involved in the metabolism of metformin in rats, SKF525-A, a nonspecific inhibitor of CYP isozymes in rats, was administered to the rats. In the rats pretreated with SKF525-A (SKT), the CLNR of metformin was significantly slower than in the controls (Table 2), indicating that metformin is metabolized via CYP isozymes. Hence, various inducers or inhibitors of CYP isozymes were administered to find out which CYP isozymes were involved in the metabolism of metformin in rats. In rats pretreated with 3-methylcholanthrene (MCT), orphenadrine (OPT) and isoniazid (INT), the CLNR values of metformin did not significantly differ from those in the controls (Table 1). However, in those rats pretreated with dexamethasone (DXT), the CLNR of metformin was significantly faster than in the controls (Table 1). The above data suggest that CYP3A1/2 could contribute to the metabolism of metformin in rats. In rats pretreated with sulfaphenazole (SPT), troleandomycin (TMT) and quinine (QNT), the CLNR of metformin was significantly slower than in the controls (Table 2). This suggests that CYP2C11, 3A1/2 and 2D1 could contribute to the metabolism of metformin in rats. Note that the enzyme inducers and inhibitors used in this study had effects on various CYP isozymes, but only those CYP isozymes that significantly changed are mentioned in this study. Hence, the results are confined to the main CYP isozymes changed by the enzyme inducers and inhibitors.
After i.v. administration of metformin to rats pretreated with QNT and SPT, the Vss of metformin turned out to be significantly smaller than in the controls (Table 1). However, this could not be mainly due to a significant increase in the free (unbound to plasma proteins) fractions of metformin in plasma in the rats with QNT and SPT. Although the protein binding values of metformin increased significantly, the free fractions of metformin in the rats with QNT and SPT increased by only 13.4 and 5.5%, respectively, whereas the Vss in the rats with QNT and SPT decreased by 71.3 and 60.2%, respectively, compared with the controls (Table 2). Although the exact reason is not clear, the smaller Vss of metformin in the rats with QNT and SPT could be due to the smaller affinity of rat tissues to metformin with the administration of quinine and sulfaphenazole, respectively.
The present results would predict changes in the CLNR of metformin in rat models of disease in which CYP activities are also altered. These would include rats with protein–calorie malnutrition, who, after having been fed for 4 weeks on a 5% casein diet exhibited lower expressions and mRNA levels of CYP2C11 and 3A1/2 (Cho et al., 1999). Also in rats with acute renal failure induced by uranyl nitrate (U-ARF), CYP2C11 expression and mRNA level are decreased, whereas the expression of CYP3A1 is increased (Moon et al., 2003). Furthermore, in rats with diabetes mellitus induced by alloxan or streptozotocin, the expression and mRNA level of CYP2C11 decreased whereas those of 3A1 increased (Kim et al., 2005). Because several CYP isozymes are involved in metformin metabolism, there may be many more conditions where the relevant CYP isozymes and hence metformin pharmacokinetics are changed.
In conclusion, our results show that metformin was metabolized mainly via CYP2C11, 2D1 and 3A1/2 in rats. It would clearly be of importance to determine the CYP isozymes involved in metformin pharmacokinetics in humans.
Acknowledgments
This work was supported in part by a grant from the 2006 BK21 Project for Applied Pharmaceutical Life Sciences.
Abbreviations
- Ae0–24 h
total amount excreted in 24-h urine
- AUC
total area under the plasma-concentration–time curve from time zero to time infinity
- CL
time-averaged total body clearance
- CLNR
time-averaged non-renal clearance
- CLR
time-averaged renal clearance
- DX
dexamethasone
- IN
isoniazid
- 3-MC
3-methylcholanthrene
- MRT
mean residence time
- OP
orphenadrine
- QN
quinine
- SK
SKF 525-A
- SP
sulfaphenazole
- TM
troleandomycin
- Vss
apparent volume of distribution at a steady state
Conflict of interest
The authors state no conflict of interest.
References
- Arlotto MP, Sonderfan AJ, Klaassen CD, Pakinson A. Studies on the pregnenolone-16-carbonitrile-inducible form of rat liver microsomal cytochrome P-450 and UDP-glucuronosyl transferase. Biochem Pharmacol. 1987;36:3859–3866. doi: 10.1016/0006-2952(87)90450-3. [DOI] [PubMed] [Google Scholar]
- Bae SK, Lee DY, Lee AK, Kwon JW, Lee I, Chung S-J, et al. Effects of cysteine on the pharmacokinetics of intravenous torasemide in rats with protein–calorie malnutrition. J Pharm Sci. 2004;93:2388–2398. doi: 10.1002/jps.20151. [DOI] [PubMed] [Google Scholar]
- Chiou WL. Critical evaluation of the potential error in pharmacokinetic studies using the linear trapezoidal rule method for the calculation of the area under the plasma level–time curve. J Pharmacokinet Biopharm. 1978;6:539–546. doi: 10.1007/BF01062108. [DOI] [PubMed] [Google Scholar]
- Chiou WL. New calculation method for mean apparent drug volume of distribution and application to rational dosage regimen. J Pharm Sci. 1979;68:1067–1069. doi: 10.1002/jps.2600680843. [DOI] [PubMed] [Google Scholar]
- Chiou WL. New calculation method of mean total body clearance of drugs and its application to rational dosage regimens. J Pharm Sci. 1980;69:90–91. doi: 10.1002/jps.2600690125. [DOI] [PubMed] [Google Scholar]
- Cho MK, Kim YG, Lee MG, Kim SG. Suppression of rat hepatic cytochrome P450s by protein−calorie malnutrition: complete or partial restoration by cysteine or methionine supplementation. Arch Biochem Biophys. 1999;372:150–158. doi: 10.1006/abbi.1999.1482. [DOI] [PubMed] [Google Scholar]
- Choi YM, Kim SH, Lee MG. Effect of phenobarbital and 3-methylcholanthrene pretreatment on the pharmacokinetics and pharmacodynamics of furosemide in rats. J Pharm Sci. 1991;80:638–642. doi: 10.1002/jps.2600800705. [DOI] [PubMed] [Google Scholar]
- Choi YH, Kim SG, Lee MG.Dose-independent pharmacokinetics of metformin in rats: hepatic and gastrointestinal first-pass effects J Pharm Sci 2006(in press) [DOI] [PubMed]
- Conney AH.Environmental factors influencing drug metabolism Fundamentals of Drug Metabolism and Drug Disposition 1971Williams & Wilkins Company: Baltimore; 269In: La du BN, Mandel HG, Way EL (eds)p [Google Scholar]
- Correia MA.Appendix. B Rat and human liver cytochrome P450. Substrate and inhibitor specificities and functional markers Cytochrome P450. Structure, Mechanism, and Biochemistry 1995Plenum Press: New York and London; 607–630.In: Oritz de Montellano PR (ed)2nd edn [Google Scholar]
- Eatman FB, Colburn WA, Boxenbaum HG, Posmanter HN, Weinfeld RE, Ronfeld R, et al. Pharmacokinetics of diazepam following multiple dose oral administration to healthy human subjects. J Pharmacokinet Biopharm. 1977;5:481–494. doi: 10.1007/BF01061729. [DOI] [PubMed] [Google Scholar]
- Gibaldi M, Perrier D. Pharmacokinetics 1982Marcel-Dekker: New York; 2nd edn [Google Scholar]
- Hale TW, Kristensen JH, Hackett LP, Kohan R, Ilett KF. Transfer of metformin into human milk. Diabetologia. 2002;45:1509–1514. doi: 10.1007/s00125-002-0939-x. [DOI] [PubMed] [Google Scholar]
- Kim SH, Choi YM, Lee MG. Pharmacokinetics and pharmacodynamics of furosemide in protein–calorie malnutrition. J Pharmacokinet Biopharm. 1993;21:1–17. doi: 10.1007/BF01061772. [DOI] [PubMed] [Google Scholar]
- Kim YC, Lee AK, Lee JH, Lee I, Lee DC, Kim SH, et al. Pharmacokinetics of theophylline in diabetes mellitus rats: induction of CYP1A2 and CYP2E1 on 1,3-dimethyluric acid formation. Eur J Pharm Sci. 2005;26:114–123. doi: 10.1016/j.ejps.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Moon YJ, Lee AK, Chung HC, Kim EJ, Kim SH, Lee DC, et al. Effects of acute renal failure on the pharmacokinetics of chlorzoxazone in rats. Drug Metab Dispos. 2003;31:776–784. doi: 10.1124/dmd.31.6.776. [DOI] [PubMed] [Google Scholar]
- Murray M, Fiala-Beer E, Sutton D. Upregulation of cytochromes P450 2B in rat liver by orphenadrine. Br J Pharmacol. 2003;139:787–796. doi: 10.1038/sj.bjp.0705305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiso T, Iwaki M, Tanaka H, Kobayashi E, Tanino T, Sawada A, et al. Pharmacokinetic drug interactions between ampiroxicam and sulfaphenazole in rats. Biol Pharm Bull. 1999;22:196–199. doi: 10.1248/bpb.22.191. [DOI] [PubMed] [Google Scholar]
- Pentikäinen PJ, Neuvonen PJ, Penttilä A. Pharmacokinetics of metformin after intravenous and oral administration to man. Eur J Clin Pharmacol. 1979;16:195–202. doi: 10.1007/BF00562061. [DOI] [PubMed] [Google Scholar]
- Ross PH, Ciosk BG, Kallweit P, Kauczinski D, Hanstein WG. Formation of ligand and metabolite complexes as a means for selective quantitation of cytochrome P450 isozymes. Biochem Pharmacol. 1993;45:2239–2250. doi: 10.1016/0006-2952(93)90195-3. [DOI] [PubMed] [Google Scholar]
- Ryan DE, Ramanathan L, Iida S, Thomas PE. Characterization of a major form of rat hepatic microsomal cytochrome P-450 induced by isoniazid. J Biol Chem. 1985;260:6385–6393. [PubMed] [Google Scholar]
- Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 1996;30:359–371. doi: 10.2165/00003088-199630050-00003. [DOI] [PubMed] [Google Scholar]
- Shim HJ, Lee EJ, Kim SH, Kim SH, You M, Kwon JW, et al. Factors influencing the protein binding of a new phosphodiesterase V inhibitor, DA-8159, using an equilibrium dialysis technique. Biopharm Drug Dispos. 2000;21:285–291. doi: 10.1002/bdd.238. [DOI] [PubMed] [Google Scholar]
- Sinclair JF, Szakacs JG, Wood SG, Kostrubsky VE, Jeffery EH, Wrighton SA, et al. Acetaminophen hepatotoxicity precipitated by short-term treatment of rats with ethanol and isopentanol: protection by triacetyloleandomycin. Biochem Pharmacol. 2000;59:445–454. doi: 10.1016/s0006-2952(99)00349-4. [DOI] [PubMed] [Google Scholar]
- Sirtori CR, Franceschini G, Galli-kienle M, Cighetti G, Galli G, Bondioli A, et al. Disposition of metformin (N,N-dimethylbiguanide) in man. Clin Pharmacol Ther. 1978;24:683–693. doi: 10.1002/cpt1978246683. [DOI] [PubMed] [Google Scholar]
- Tomkins DM, Otton SV, Joharchi N, Li N-Y, Balster RF, Tyndale RF, et al. Effects of cytochrome P450 2D1 inhibition on hydrocodone metabolism and its behavioral consequences in rats. J Pharmacol Exp Ther. 1997;280:1374–1382. [PubMed] [Google Scholar]
- Tucker GT, Casey C, Phillips PJ, Connor H, Ward JD, Woods HF. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br J Clin Pharmacol. 1981;12:235–246. doi: 10.1111/j.1365-2125.1981.tb01206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyndale RF, Li Y, Li N-Y, Messina E, Miksys S, Sellers EM. Characterization of cytochrome P-450 2D1 activity in rat brain: high-affinity kinetics for dextromethorphan. Drug Metab Dispos. 1999;27:924–930. [PubMed] [Google Scholar]
- Williams JF, Lowitt S, Szentivanyi A. Effect of phenobarbital and 3-methylcholanthrene pretreatment on the plasma half-life and urinary excretion profile of theophylline and its metabolites in rats. Biochem Pharmacol. 1979;28:2935–2940. doi: 10.1016/0006-2952(79)90589-6. [DOI] [PubMed] [Google Scholar]
- Wrighton SA, Maurel P, Schuetz EG, Watkins PB, Young B, Guzelian PS. Identification of the cytochrome P-450 induced by macrolide antibiotics in rat livers as the glucodorticoid responsive cytochrome P450. Biochemistry. 1985;24:2171–2178. doi: 10.1021/bi00330a010. [DOI] [PubMed] [Google Scholar]