Abstract
Background and purpose:
Recently, a number of mimics of the second messenger cyclic ADP-ribose (cADPR) with replacement of adenosine by inosine were introduced. In addition, various alterations in the molecule ranging from substitutions at C8 of the base up to full replacement of the ribose moieties still retained biological activity. However, nothing is known about the metabolic stability and cellular effects of these novel analogues.
Experimental approach:
cADPR and the inosine-based analogues were incubated with CD38, ADP-ribosyl cyclase and NAD-glycohydrolase and metabolism was analysed by RP-HPLC. Furthermore, the effect of the analogues on cytokine expression and proliferation was investigated in primary T-lymphocytes and T-lymphoma cells.
Key results:
Incubation of cADPR with CD38 resulted in degradation to adenosine diphosphoribose. ADP-ribosyl cyclase weakly catabolised cADPR whereas NAD-glycohydrolase showed no such activity. In contrast, N1-cyclic inosine 5′-diphosphoribose (N1-cIDPR) was not hydrolyzed by CD38. Three additional N1-cIDPR analogues showed a similar stability. Proliferation of Jurkat T-lymphoma cells was inhibited by N1-cIDPR, N1-[(phosphoryl-O-ethoxy)-methyl]-N9-[(phosphoryl-O-ethoxy)-methyl]-hypoxanthine-cyclic pyrophosphate (N1-cIDP-DE) and N1-ethoxymethyl-cIDPR (N1-cIDPRE). In contrast, in primary T cells neither proliferation nor cytokine expression was affected by these compounds.
Conclusions and Implications:
The metabolic stability of N1-cIDPR and its analogues provides an advantage for the development of novel pharmaceutical compounds interfering with cADPR mediated Ca2+ signalling pathways. The differential effects of N1-cIDPR and N1-cIDPRE on proliferation and cytokine expression in primary T cells versus T-lymphoma cells may constitute a starting point for novel anti-tumor drugs.
Keywords: cyclic ADP-ribose, ADP-ribosyl cyclase, CD38, T-lymphoma cell, primary T cell, stable analogues, calcium signalling, proliferation, cytokine expression, signal transduction
Introduction
Cyclic ADP-ribose (cADPR) is a Ca2+-mobilizing cyclic nucleotide active in many cell types and tissues, both in animals, protists and plants (reviewed by Galinoe and Churchill, 2002; De Flora et al., 2004; Guse, 2004a; Lee, 2004). The role of cADPR as an important signal transducer in human cell types implies that analogues of the molecule may become relevant as therapeutical agents in the future. Accordingly, the biological activity and metabolical stability of a number of such analogues have been studied (reviewed by Guse, 2004b; Potter and Walseth, 2004; Shuto and Matsuda, 2004). Alterations in the adenine base resulted in metabolically stable analogues: 3-deaza-cADPR is a potent and hydrolysis-resistant agonist (Wong et al., 1999), whereas 7-deaza-cADPR is also resistant to hydrolysis, but only a partial agonist (Sethi et al., 1997). Modifications of the 8-position of adenine usually resulted in antagonists (Walseth and Lee, 1993); thus, further substitution of 7-deaza-cADPR at the 8-position converted the partial agonist 7-deaza-cADPR into the membrane-permeant, hydrolysis-resistant antagonist 7-deaza-8-Br-cADPR (Sethi et al., 1997; Guse et al., 1999; Schoettelndreier et al., 2001). Metabolic stability was also achieved by converting either the ‘southern' or the ‘northern' ribose into carbocyclic moieties; the resulting analogues cyclic aristeromycin diphosphoribose and cyclic adenosine diphospho-carbocyclic-ribose were biologically active, although at different magnitude (Guse et al., 2002), and hydrolysis-resistant (Bailey et al., 1996; Shuto et al., 2001). Likewise, 2″-amino-cADPR, modified in the ‘northern' ribose, was a potent and hydrolysis-resistant agonist (Guse et al., 2002).
Despite the wide acceptance of cADPR as a second messenger, its metabolism is still an enigma as the only well-described mammalian enzymes, CD38 and CD157 (reviewed by Schuber and Lund, 2004), catalyse both the formation and breakdown of cADPR. In addition, the active sites of CD38 and CD157 are localized in the extracellular space (or in intracellular vesicles), making a direct involvement of these enzymes in the intracellular metabolism of cADPR difficult. However, to solve this topological paradox, transport systems for the export of nicotinamide adenine dinucleotide (NAD)+ and the import of cADPR have been described in some cell types (Bruzzone et al., 2001; Guida et al., 2002; Guida et al., 2004).
Recently, we have introduced N1-cyclic inosine diphosphoribose (N1-cIDPR) and a number of its derivatives as mimics of cADPR (Wagner et al., 2003; Gu et al., 2004; Guse et al., 2005; Wagner et al., 2005). In the present study, the metabolic stability and the effects of N1-cIDPR and some of its derivatives on Ca2+ mobilization, cytokine expression and cell proliferation were analysed.
Materials and methods
Drugs and materials
cADPR was obtained from Biolog (Bremen, Germany). NAD was supplied by Roche Diagnostics (Mannheim, Germany). N1-cIDPR, 8-bromo-cyclic inosine 5′-diphosphoribose (8-Br-N1-cIDPR), N1-ethoxymethyl-cIDPR (N1-cIDPRE) and N1-[(phosphoryl-O-ethoxy)-methyl]-N9-[(phosphoryl-O-ethoxy)-methyl]-hypoxanthine-cyclic pyrophosphate (N1-cIDP-DE) were synthesized and characterized spectroscopically as detailed previously (Wagner et al., 2003, 2005; Gu et al., 2004; Guse et al., 2005). ADP-ribosyl cyclase from Aplysia californica and NAD glycohydrolase (NADase) from Neurospora crassa were purchased from Sigma-Aldrich (Deisenhofen, Germany). NADase was purified as described previously (Gasser et al., 2006). All other chemicals used were of the highest purity grade and purchased from Merck (Darmstadt, Germany) or Fluka (Buchs, Switzerland). Culture medium reagents were supplied by Sigma-Aldrich and Gibco-Invitrogen (Auckland, New Zealand).
Jurkat T-lymphocytes cell culture
Jurkat T-lymphocytes (subclone JMP) were cultured as described previously (Guse et al., 1993) at 37°C in the presence of 5% CO2 in Roswell Park Memorial Institute (RPMI) 1640 medium containing Glutamax I and N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulphonic acid] (HEPES) (25 mM) and supplemented with 7.5% newborn calf serum, 100 U ml−1 penicillin and 50 μg ml−1 streptomycin.
Experiments
Metabolic assay and high-performance liquid chromatography analysis of nucleotide degradation
Cyclic nucleotides (50 μM) were incubated with either native CD38 on intact Jurkat T cells (1 × 107, or 2 × 107, or 4 × 107 cells ml−1) in buffer A (20 mM HEPES, 140 mM NaCl, 5 mM KCl, 1 mM MgSO4, 1 mM CaCl2, 1 mM NaH2PO4, 5 mM D-glucose, pH 7.4), or recombinant soluble mouse CD38 (0.75 μg ml−1) in buffer A, or with NADase (180 μg ml−1) in buffer B (1 mM Tris, 2.5 mM MgCl2, pH 7.3) or with ADP-ribosyl cyclase (100 ng ml−1) in buffer C (10 mM NaH2PO4, pH 7.3), respectively. Incubation was carried out as indicated at room temperature (RT) under continuous shaking. To control for any background signals in high-performance liquid chromatography (HPLC), intact cells and enzymes were also incubated in the absence of nucleotides under identical conditions. The incubation was stopped by placing the samples into an ice-salt bath. Samples containing intact Jurkat T–lymphocytes were placed into an ice bath, and the cells were rapidly removed by centrifugation for 2 min at 13 700 g and 4°C (Heraeus Biofuge fresco). Enzymes were removed from all samples by centrifugation for 30 min at 3000 g and 4°C by use of centrifugal filter devices type Centricon YM-10 (10 kDa MW cut-off; Millipore, Bedford, USA).
Reversed Phase-HPLC analysis of nucleotides was performed on a 250 × 4.6 mm Multohyp BDS C18-5μ column (CS Chromatographic Service, Langerwehe, Germany) equipped with a 17 × 4.6 mm guard column filled with the same column material or with a 4.0 × 3.0 guard cartridge containing a C18 (ODS) filter element (Phenomenex, Aschaffenburg, Germany). The separation was performed as described previously (Schweitzer et al., 2001) at a flow rate of 1 ml min−1 with RP-HPLC buffer (20 mM KH2PO4, 5 mM tetrabutylammonium dihydrogen phosphate, pH 6) containing increasing amounts of methanol. The gradient used for separation was (% methanol) 0 min (6.5), 3.5 min (7.5), 5.5 min (16), 8 min (25), 18 min (6.5) and 27 min (6.5). Nucleotides were detected using an UV detector (HPLC detector 432, Kontron Instruments, Neufahru, Germany) at 270 nm for cADPR and at 250 nm for N1-cIDPR, 8-Br-N1-cIDPR, N1-cIDPRE and N1-cIDP-DE because of the different absorption maxima of the nucleotides. Integration of peaks was performed with the data–acquisition system MT2 from Kontron Instruments.
Proliferation assay
Jurkat T–lymphocytes (1 × 105 ml−1) were incubated with increasing concentrations of cADPR, N1-cIDPR, 8-Br-N1-cIDPR, N1-cIDPRE or N1-cIDP-DE or vehicle in RPMI 1640 medium containing Glutamax I, HEPES (25 mM), 100 U ml−1 penicillin and 50 μg ml−1 streptomycin at 37°C in the presence of 5% CO2 for 2 h. Then, the medium was supplemented with 7.5% newborn calf serum and the incubation was continued. Cell density was determined after 24, 48, 72 and 96 h with CASY Model DT cell counter (Schärfe System, Reutligen, Germany).
Quantitative PCR
mRNA was extracted using standard protocols (Sigma-Aldrich, Deisenhofen, Germany) and reversed to cDNA (Invitrogen, Auckland, New Zealand). Taqman analysis was performed as reported (Flügel et al., 2001) using ABI Prim 7700 Sequence Detector ‘Taqman' (PE Applied Biosystems, Foster City, USA). The expression of a housekeeping gene (β-actin) was set into relation to the specific mRNA. Data were obtained by independent duplicate measurements. The CT value of the individual measurements did not exceed 0.5 amplification cycles.
In vitro rat cell reactivity assay
Myelin-basic protein-specific rat T cells retrovirally transfected with green fluorescent protein (GFP) (TMBP−GFP cells) were co-cultured for 48 h in 96-well plates (in Dulbecco's modified Eagle's medium (DMEM) 1% rat serum) with antigen-presenting cells in the presence or absence of specific antigen (10 μg ml−1 myelin basic protein (MBP)). Amplification of TMBP−GFP cells was measured by cytofluorometry as described (Kawakami et al., 2005). Their numbers were determined in relation to a known absolute number of added phycoerythrin-labelled plastic beads (Becton-Dickinson, Franklin Lakes, USA). The amplification rate was calculated in relation to the GFP+ T-cell numbers at day 0.
In vitro human cell reactivity assay
Human T cells specific for myelin-basic protein were cultured in 96-well plates (in DMEM 7.5% calf serum) in the presence of specific antigen (peptides 139–152 of human myelin-basic protein), or with an irrelevant antigen (tetanus toxoid). [3H] desoxythymin (2 Ci/mmol; Amersham-Buchler, Braunschweig, Germany) was added to the cultures after 24 h. The radioactive label present in the different cultures was determined as described (Flügel et al., 2001).
Results
Effects of N1-cIDPR and its N1-coupled cyclic inosine diphosphoribose analogues on cellular Ca2+ concentration
N1-cIDPR has recently been shown to mobilize Ca2+ from intracellular stores with an effector concentration for half-maximum response (EC50) of approximately 33 μM (Table 1; Wagner et al., 2003). The natural nucleotide cADPR gave almost identical results (Table 1; Guse et al., 2005; Wagner et al., 2005), indicating that the replacement of the imino–group at C6 by an oxo–group does not change binding and interaction properties of the nucleotide at its receptor protein. As both compounds are very polar, only few experiments were conducted with intact cells; as expected, no Ca2+ mobilization was observed. 8-Br-N1-cIDPR induced a transient Ca2+ mobilization in intact cells (Table 1; Wagner et al., 2003); however, its Ca2+-releasing activity in permeabilized cells was very low. The latter may indicate that 8-Br-N1-cIDPR acts via a mechanism distinct from the one induced by cADPR and is presently under investigation. The compounds N1-cIDPRE and N1-cIDP-DE were less effective in permeabilized cells as compared to the maximal Ca2+ release obtained with cADPR (Table 1; Gu et al., 2004; Guse et al., 2005). Concentrations of 100 μM for N1-cIDPRE and 500 μM for N1-cIDP-DE did not even reach the magnitude of Ca2+ release obtained by cADPR (Table 1; Gu et al., 2004; Guse et al., 2005). In intact cells, the EC50 for N1-cIDPRE was approximately 500 μM (Gu et al., 2004), while an exact EC50 for N1-cIDP-DE could not be determined since at 1 mM extracellular concentration there was still no saturation of the effect (Guse et al., 2005); however, it did not appear reasonable to further increase the concentration. Antagonistic effects were not observed with any inosine-based analogues of cADPR (not shown).
Table 1.
Effects of N1-cIDPR and some of its derivatives on Ca2+ mobilization in Jurkat T cells
| Compound |
EC50 (μM) |
Reference | |
|---|---|---|---|
| Permeabilized cell | Intact cell | ||
| cADPR | 33 | ND | Wagner et al. (2005); Guse et al. (2005) |
| N1-cIDPR | 33 | ND | Wagner et al. (2005) |
| 8-Br-N1-cIDPR | ND | Active | Wagner et al. (2003) |
| N1-cIDPRE | >100 | Approx. 500 | Gu et al. (2004) |
| N1-cIDP-DE | >500 | >500 | Guse et al. (2005) |
Abbreviations: 8-Br-N1-cIDPR, 8-bromo-cyclic inosine 5′-diphosphoribose; cADPR, cyclic adenosine 5′-diphosphoribose; N1-cIDP-DE, N1-[(phosphoryl-O-ethoxy)-methyl]-N9-[(phosphoryl-O-ethoxy)-methyl]-hypoxanthine-cyclic pyrophosphate; N1-cIDPR, N1-cyclic inosine 5′-diphosphoribose; N1-cIDPRE, N1-ethoxymethyl-cIDPR; ND, not determined.
Permeabilized cells: Jurkat T cells were transferred to an intracellular buffer and permeabilized using saponin as described by Guse et al. (2002, 2005) and Gu et al. (2004). [Ca2+] was determined ratiometrically in a Hitachi F-2000 fluorimeter in the presence of 1 μM fura2/free acid, ATP (1 mM) and an ATP-regenerating system consisting of creatin kinase and creatin phosphate.
Intact cells: Jurkat T cells were loaded with fura2/AM and analysed by ratiometric fluorimetry in a Hitachi F-2000 fluorimeter as described by Guse et al. (2002, 2005) and Gu et al. (2004). Extracellular Ca2+ was present at 1 mM throughout the experiments.
Metabolic stability of cADPR against CD38, ADP-ribosyl cyclase and NADase
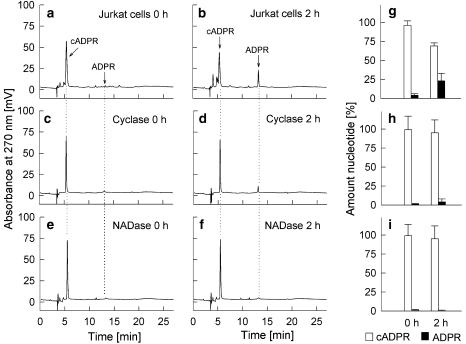
To study the metabolism of cADPR by CD38, the nucleotide was incubated with intact Jurkat T–lymphocytes. These cells express CD38 as an integral type 2 membrane protein with the active site located in the extracellular space (Da Silva et al., 1998). The well-described cADPR-hydrolase activity of CD38 resulted in a slow decrease of cADPR with a concomitant increase in the hydrolysis product adenosine 5′-diphosphoribose (ADPR) (Figure 1a, b and g). ADP-ribosyl cyclase from A. californica displayed only a minor degradation of cADPR (Figure 1c, d and h), although complete cyclization of NAD to cADPR was achieved under the same experimental conditions (data not shown). NADase from N. crassa did not metabolize any cADPR under identical conditions (Figure 1e, f and i).
Figure 1.
Metabolism of cADPR by CD38, ADP-ribosyl cyclase and NADase. cADPR (50 μM) was incubated with Jurkat T-lymphocytes (1 × 107 ml−1) (a, b, g), ADP-ribosyl cyclase (100 ng ml−1) (c, d, h) and NADase (180 μg ml−1) (e, f, i) for 120 min at RT (n=4–5 each condition). Aliquots were taken at 0 or 120 min and were analysed by RP-HPLC. Characteristic chromatograms are shown. Data in panels g, h and i are mean±s.d. (n=4–5).
Metabolic stability of N1-cIDPR against CD38
As N1-cIDPR and some of its analogues displayed Ca2+-mobilizing properties in mammalian cells (Table 1), these compounds are well suited as novel tools for signal–transduction research and may further serve as starting material for the synthesis of novel pharmaceutical compounds. The latter ideally are metabolically stable to maintain a constant effective concentration during therapy.
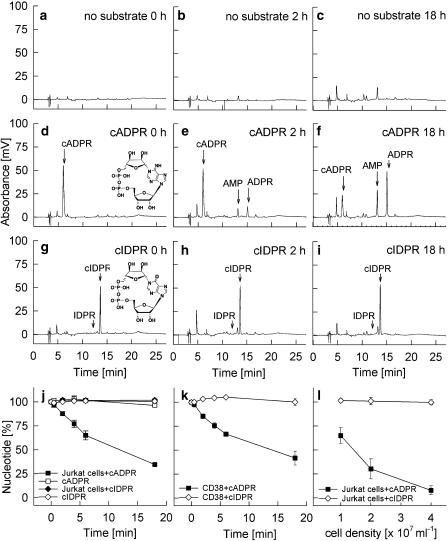
The stability of N1-cIDPR was studied under identical conditions as above for cADPR. As CD38 was the only enzyme capable of hydrolysing cADPR to ADPR to some extent (Figure 1a, b and g), the cyclic inosine nucleotides were incubated with intact Jurkat T cells in a similar manner as for cADPR. Control incubations without substrate were carried out to analyse production and/or release of endogenous nucleotides by intact Jurkat T-lymphocytes (Figure 2a–c). Although slight increases in some compounds were observed, for example, an unknown peak eluting at about 4.5 min and a small amount of adenosine monophosphate, the background produced by the cells during the 18h-incubation period was negligible. The metabolism of cADPR and N1-cIDPR, a cyclic nucleotide with a very similar structure (Figure 2d and g) and biological activity as compared to cADPR (Wagner et al., 2005), was analysed during incubations with CD38 on intact cells for up to 18 h. Although about 60% of cADPR was metabolized to ADPR within 18 h at cell density of 1 × 107 cells ml−1 (Figure 2d–f and j), there was no decrease in the peak area of N1-cIDPR (Figure 2g–i and j). Likewise, incubation of cADPR or N1-cIDPR for 18 h at room temperature in the absence of intact cells did not result in any significant metabolism (Figure 2j). These experiments in which CD38 expressed on Jurkat T cells was used were confirmed by replacement of the Jurkat cells by mouse recombinant soluble CD38 (Figure 2k). Our data confirm the idea that substitution of the amino/imino–group at C6 by an oxo-group, thus converting the bond between N1 and C″1 at the northern ribose into a much more chemically stable, amide bond, produces compounds that are also biologically stable. Further evidence for the biological stability towards CD38 was obtained by increasing the cell density, and thereby the CD38 concentration, during the incubations: while approximately 85% of cADPR was degraded within 6 h when the cell number was increased fourfold, almost no metabolism of N1-cIDPR was observed (Figure 2l).
Figure 2.
Metabolism of cADPR and N1-cIDPR by CD38. Vehicle (no substrate), cADPR (50 μM) or N1-cIDPR (50 μM) were incubated at RT either with 1 × 107 ml−1 Jurkat T-lymphocytes for the times indicated (a–i, j), with recombinant soluble mouse CD38 (0.75 μg ml−1) (k) or with increasing numbers of Jurkat T-lymphocytes for 6 h (l). Aliquots were taken at the time points indicated and were analysed by RP-HPLC. The detection was performed at 270 nm for cADPR and at 250 nm for N1-cIDPR. Data are presented as mean±s.d. (n=3). Note that at some time points s.d. values are smaller than symbols and thus cannot be seen properly.
If the amide-like structure of N1-cIDPR vs the natural structure of cADPR is indeed the reason for its metabolic stability, one would expect other cyclic inosine derivatives to be similarly stable towards CD38.
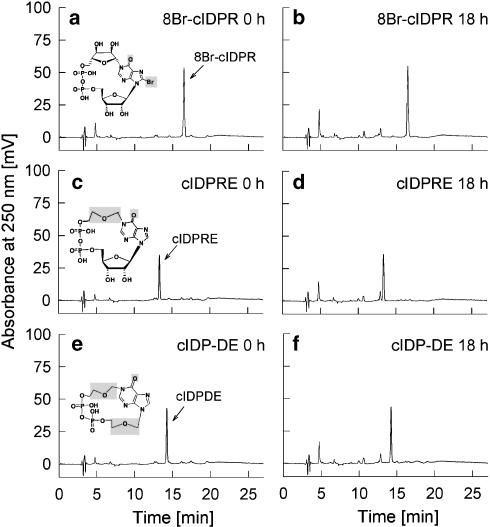
Metabolic stability of 8-Br-N1-cIDPR, N1-cIDPRE and N1-cIDP-DE
Thus, we next analysed the metabolic stability of 8-Br-N1-cIDPR, N1-cIDPRE and N1-cIDP-DE. These compounds all have an identical amide-like structure as in N1-cIDPR, but other parts of the molecule are different, for example, a substitution at the 8-position of the base hypoxanthine, or replacement of the northern ribose or both ribose residues by ether bridges (Figure 3). None of the three compounds 8-Br-N1-cIDPR, N1-cIDPRE and N1-cIDP-DE, was metabolized by surface CD38 on intact Jurkat T cells during an 18 h incubation period (Figure 3), suggesting that indeed the amide-like structure present in all inosine-based cyclic analogues of cADPR analysed here is the reason for their stability towards CD38.
Figure 3.
Metabolism of 8-Br-N1-cIDPR, N1-cIDPRE or N1-cIDP-DE by CD38. Jurkat T-lymphocytes (1 × 107 ml−1) were incubated with each 50 μM 8-Br-N1-cIDPR (a, b), N1-cIDPRE (c, d) or N1-cIDP-DE (e, f) for 18 h at RT (n=2). Aliquots were taken at 0 min and 18 h and were analysed by RP-HPLC (detection at 250 nm).
All inosine-based cyclic analogues of cADPR described above were also incubated with ADP-ribosyl cyclase (ADPRC) from A. californica and NADase from N. crassa; however, no degradation of any of the compounds was observed (data not shown).
Cellular effects of cADPR, N1-cIDPR, 8-Br-N1-cIDPR, N1-cIDPRE and N1-cIDP-DE on T cells
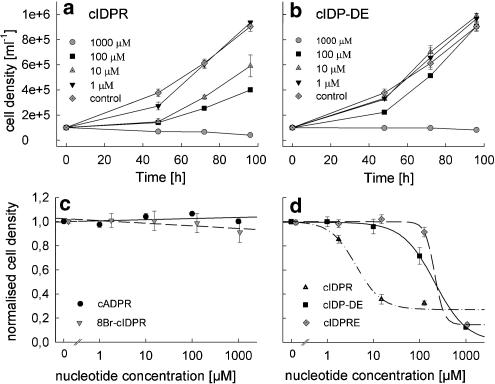
Finally, cADPR, N1-cIDPR and the analogues were incubated with the autonomously proliferating Jurkat T-lymphoma cells. Whereas cADPR and 8-Br-N1-cIDPR had no effect on proliferation (Figure 4c), there was a dose-dependent inhibition of proliferation by N1-cIDPR (Figure 4a and d), N1-cIDP-DE (Figure 4b and d) and N1-cIDPRE (Figure 4d).
Figure 4.
Proliferation of Jurkat T-lymphocytes in the presence of cADPR, N1-cIDPR, 8-Br-N1-cIDPR, N1-cIDPRE or N1-cIDP-DE. Jurkat T-lymphocytes (1 × 105 ml−1) were incubated with increasing concentrations of cADPR (c), 8-Br-N1-cIDPR (c), N1-cIDPR (a, d), N1-cIDPRE (d) or N1-cIDP-DE (b, d) at 37°C/5% CO2. Cell density was determined after 48, 72 and 96 h. (a, b) Cell density plotted against incubation time and concentration of N1-cIDPR (a) and N1-cIDP-DE (b); data are presented as mean±s.d. (n=3–9). (c, d) Concentration–response relationship; data are presented as mean±s.e. (n=2–3).
These antiproliferative effects of N1-cIDPR and N1-cIDPRE were further studied in primary T cells. Surprisingly, only a very modest and statistically not significant effect on proliferation induced by myelin-basic protein in primary rat T cells was observed (Table 2). Similarly, expression of none of the cytokines, interferon-γ (IFN-γ), transforming growth factor-β, interleukin-2 (IL-2) or the IL-2 receptor, was affected by N1-cIDPR or N1-cIDPRE, as analysed by quantitative PCR in relation to mRNA of the housekeeping gene β-actin (Table 2). To find out whether the difference observed was due to the different species involved (human Jurkat T cells vs primary rat T cells), a similar experiment was carried out using human myelin-basic protein-specific T cells. Again, at the concentrations of N1-cIDPR and N1-cIDPRE almost completely blocking autonomous proliferation of Jurkat T-lymphoma cells, no effect on the proliferation and a slightly stimulatory effect on IFN-γ was observed in the human primary T cells (data not shown).
Table 2.
Effects of N1-cIDPR and N1-cIDPRE on cytokine expression and proliferation in rat primary T cells
| IFN-γ (rel. copy no.) | TGF-β (rel. copy no.) | IL-2 (rel. copy no.) | IL-2 receptor (rel. copy no.) | Proliferation (fold increase) | |
|---|---|---|---|---|---|
| Control | 0.0006±0.0002 | 0.010±0.0004 | 0.003±0.0002 | 0.032±0.025 | 6.41 |
| MBP alone | 0.0019±0.0003 | 0.014±0.0026 | 0.005±0.0011 | 0.046±0.020 | 20.34 |
| N1-cIDPR/MBP | 0.0015±0.0005 | 0.015±0.0016 | 0.006±0.0006 | 0.043±0.032 | 19.75 |
| N1-cIDPRE/MBP | 0.0017±0.0003 | 0.011±0.0028 | 0.004±0.0020 | 0.058±0.036 | 18.13 |
Abbreviations: MBP, myelin basic protein; N1-cIDPR, N1-cyclic inosine 5′-diphosphoribose; N1-cIDPRE, N1-ethoxymethyl-cIDPR.
MBP-specific rat T cells were incubated with N1-cIDPR (25 μM) or N1-cIDPRE (1 mM) in the absence of serum for 2 h. Then, serum and antigen-presenting cells pulsed with MBP were added and the cells were cultured for 48 h. At the 48 h time point, cells were harvested, mRNA was prepared and the number of mRNA copies in relation to the number of mRNA copies of the housekeeping gene β-actin was determined by quantitative PCR. Proliferation was also determined 48 h after stimulation and is expressed as the multiplication factor between time point 0 and 48 h. Data are mean±s.e.m. from two to three independent experiments.
Discussion
In this report, we describe cellular effects and metabolic stability of the cADPR mimics N1-cIDPR, 8-Br-N1-cIDPR, N1-cIDPRE and N1-cIDP-DE.
Jacobson and co-workers were the first to purify a mammalian membrane-bound multifunctional enzyme catalysing both formation of cADPR from NAD, and conversion of cADPR to ADPR (Kim et al., 1993). This enzyme was isolated from canine spleen, indicating that leucocytes might express such enzymes. Indeed, the sequence homology between the lymphocyte surface antigen CD38 and ADP-ribosyl cyclase from A. californica was first described by States et al. (1992); briefly thereafter, Howard et al. (1993) described the ADP-ribosyl cyclase and cADPR-hydrolase activity of CD38. However, membrane–bound cADPR-hydrolysing activities were also found in tissue extracts from various invertebrate and vertebrate animals (Lee and Aarhus, 1993), and in human erythrocyte ghosts (Lee et al., 1993). Calf spleen NADase was another enzyme displaying cADPR-hydrolase activity (Muller-Steffner et al., 1994); interestingly, this enzyme was later identified as bovine CD38 (Augustin et al., 2000). Thus, our results using either intact Jurkat T cells or recombinant soluble CD38 confirm the cADPR-degrading activity of CD38 found on the surface of lymphocytes.
Schuber and co-workers described that high concentrations of ADP-ribosyl cyclase from A. californica hydrolyzed cADPR to ADPR (Cakir-Kiefer et al., 2000); however, as the specificity ratio V(max)/K(m) was 10 000-fold higher for NAD as compared to cADPR, it is clear that even under our experimental conditions, where complete conversion of NAD to cADPR was observed, only a modest hydrolysis of cADPR was obtained (Figure 1c, d and h).
NADase from N. crassa did not metabolize cADPR or any of the N1-cIDPR analogues. This finding is consistent with earlier data showing that this enzyme does neither convert NAD to cADPR nor nicotinamide guanine dinucleotide (NGD) to cyclic guanosine 5′-diphosphoribose (cGDPR) (Graeff et al., 1994). Thus, it is very unlikely that the N. crassa NADase would bind or convert cyclic nucleotides.
The first cyclic inosine derivative published was N7-cIDPR (Graeff et al., 1996). In contrast to the findings in our current report – metabolic stability of N1-cIDPR and its analogues – N7-cIDPR was readily hydrolyzed to IDPR by the multifunctional enzyme CD38 (Graeff et al., 1996). In addition to N7-cIDPR also N7-cGDPR was metabolized by CD38, albeit at a lower rate (Moreau et al., 2006). The synthesis of the N7-cyclized products N7-cIDPR and N7-cGDPR from NHD and NGD has been explained by a reduced reactivity of N1 in case of substitution of the amino/imino-group at C6 by an oxo-group, as exemplified in NHD and NGD. It was proposed that via free rotation of the base around the C1′N9 axis from syn- to anti-position, the more reactive N7 was cyclized with C1″ of the northern ribose (Graeff et al., 1996). This largely reduced reactivity of the C″1-N1 bond in N1-cIDPR and its analogues 8-Br-N1-cIDPR, N1-cIDPRE and N1-cIDP-DE very well explains the metabolic stability of these compounds vs CD38-type ADPRC.
For N1-cIDPR and its analogues, our previous study (Wagner et al., 2005) confirmed that these novel compounds, which are the closest relatives to the natural cADPR in structure, are chemically very stable. We demonstrated that 8-Br-N1-cIDPR is very stable to chemical hydrolysis at normal pH, even at higher temperature. This is likely to be typical of this general class of compound and, unusually, this allowed the direct chemical modification of the 8-position to be undertaken without damage to the rest of the molecule. Moreover, in a recent study we were able to demonstrate that, chemically, the N1-amide bond of an 8-halo N1-cIDPR is very stable even at low acidic pH, whereas the N9-ribosyl system is degraded giving unusually the N1-inosine-5′-diphosphate ribose derivative that is then further degraded to the mononucleotide (Moreau et al., 2006). This is the reverse of what is normally observed for cADPR.
Regarding the effect of inosine-based derivatives on proliferation of Jurkat T cells, some of the results are unexpected. The lack of effect of the membrane-impermeant cADPR was anticipated in Jurkat T cells, although some other cell types have been shown to express transporters for cADPR (Bruzzone et al., 2001; Guida et al., 2002; Guida et al., 2004). Similarly, 8-Br-cIDPR was without effect, although Ca2+ mobilization in intact T cells was observed (Wagner et al., 2003); however, the fact that the Ca2+ signals were only transient in these experiments well explains their lack on proliferation. Both N1-cIDPRE and N1-cIDP-DE have been shown to evoke large biphasic Ca2+ signals in intact Jurkat T cells (Gu et al., 2004, Guse et al., 2005). In the same concentration range, inhibition of proliferation was observed (Figure 4d), indicating that prolonged Ca2+ signalling alone results in cell death. Indeed, initial experiments showed the presence of apoptotic cells in these experiments. This was unexpected as both N1-cIDPRE- and N1-cIDP-DE-induced Ca2+ signalling was in their kinetics similar to Ca2+ signalling induced by ligation of the T-cell receptor/CD3 complex (Gu et al., 2004; Guse et al., 2005). However, it is conceivable that without parallel activation of additional signal–transduction pathways, such as the ras/mitogen-activated protein kinase pathway or the phosphatidylinositol 3-kinase pathway, a situation normally seen upon T-cell receptor/CD3 complex stimulation, Ca2+ signalling induced in such an artificial way turns into a death signal. Nevertheless, additional long-term effects of N1-cIDPRE and N1-cIDP-DE cannot be excluded. Experiments in which 8-Br-cADPR, an established membrane-permeant antagonist of cADPR, was used to revert the inhibitory effects of N1-cIDPRE were without success (data not shown). This may, on the one hand, be due to the weak antagonistic effect of 8-Br-cADPR in Jurkat T cells, for example, less than 50% inhibition of cADPR-induced Ca2+ release by 100 μM 8-Br-cADPR, or may indicate that the inhibitory effect of N1-cIDPRE and N1-cIDP-DE on proliferation are mechanistically not linked with Ca2+ signalling.
However, the most unexpected result was the strong inhibitory effect of N1-cIDPR on Jurkat T-lymphoma cell proliferation (Figure 4a and d). We reasoned that inhibition of ecto-CD38 enzymatic activity by N1-cIDPR might be the cause, but initial experiments showed no blockade of the enzymatic activity. Perhaps, N1-cIDPR blocks nucleotide pyrophosphatase activity or metabolism of purine monophosphates to the corresponding nucleosides on the surface of the T-lymphoma cells, thereby limiting supply of adenosine and guanosine for the salvage pathway. Whether this indeed is the case will be subject of further investigations. The situation is even more complex as this inhibitory effect of N1-cIDPR on autonomously proliferating T-lymphoma cells was not observed in antigen-stimulated primary rat and human T cells (Table 2). Although the underlying mechanism is unclear at present, these results may indicate that N1-cIDPR may be used to target specifically T-lymphoma cells without much side effects on primary T cells, even if the latter are proliferating.
In conclusion, although the endogenous second messenger cADPR was degraded by CD38-type ADPRC, N1-cIDPR and its analogues were metabolically stable. Along with the proven Ca2+-mobilizing activity of these compounds and the newly discovered inhibitory effects on proliferation of T-lymphoma cells, but not on primary T cells, their metabolic stability is another advantage for the development of new tools for signal-transduction research and potentially also for the design of novel pharmaceutical compounds.
Acknowledgments
This study was supported by the Deutsche Foschungsgemeinschaft (Grant Nos. GU 360/9-1 and 9-2 to AHG), the Deutsche Akademische Austauschdienst (GrantNo. 423/PPP-vrc-sr to AHG, jointly with LHZ), the Gemeinnützige Hertie-Stiftung (Grant No. 1.01.1/04/010 to AHG), the Wellcome Trust (Biomedical Research Collaboration Grant No. 068065 to AHG and BVLP) and the National Natural Sciences Foundation of China (Grant No. SFCBIC 20320130046 and 20132030 to LHZ).
Abbreviations
- 8-Br-N1-cIDPR
8-bromo-cyclic inosine 5′-diphosphoribose
- ADPR
adenosine 5′-diphosphoribose
- ADPRC
ADP-ribosyl cyclase
- cADPR
cyclic adenosine 5′-diphosphoribose
- cGDPR
cyclic guanosine 5′-diphosphoribose
- EC50
effector concentration for half-maximum response
- GFP
green fluorescent protein
- HEPES
N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulphonic acid]
- HPLC
high-performance liquid chromatography
- IDPR
inosine 5′-diphosphoribose
- MBP
myelin basic protein
- N1-cIDP-DE
N1-[(phosphoryl-O-ethoxy)-methyl]-N9-[(phosphoryl-O-ethoxy)-methyl]-hypoxanthine-cyclic pyrophosphate
- N1-cIDPR
N1-cyclic inosine 5′-diphosphoribose
- N1-cIDPRE
N1-ethoxymethyl-cIDPR
- NAD
nicotinamide adenine dinucleotide
- NADase
NAD glycohydrolase
- NGD
nicotinamide guanine dinucleotide
- RT
room temperature
Conflict of interest
The authors have an interest in potential commercial use of the data contained in this publication; thus, a conflict of interest exists.
References
- Augustin A, Muller-Steffner H, Schuber F. Molecular cloning and functional expression of bovine spleen ecto-NAD+ glycohydrolase: structural identity with human CD38. Biochem J. 2000;345:43–52. doi: 10.1042/bj3450043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey VC, Fortt SM, Summerhill RJ, Galione A, Potter BVL. Cyclic aristeromycin diphosphate ribose: a potent and poorly hydrolysable Ca(2+)-mobilising mimic of cyclic adenosine diphosphate ribose. FEBS Lett. 1996;379:227–230. doi: 10.1016/0014-5793(95)01515-9. [DOI] [PubMed] [Google Scholar]
- Bruzzone S, Guida L, Zocchi E, Franco L, De Flora A. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 2001;15:10–12. doi: 10.1096/fj.00-0566fje. [DOI] [PubMed] [Google Scholar]
- Cakir-Kiefer C, Muller-Steffner H, Schuber F. Unifying mechanism for Aplysia ADP-ribosyl cyclase and CD38/NAD(+) glycohydrolases. Biochem J. 2000;349:203–210. doi: 10.1042/0264-6021:3490203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva CP, Schweitzer K, Heyer P, Malavasi F, Mayr GW, Guse AH. Ectocellular CD38-catalyzed synthesis and intracellular Ca2+-signalling activity of cyclic ADP-ribose in T-lymphocytes are not functionally related. FEBS Lett. 1998;439:291–296. doi: 10.1016/s0014-5793(98)01396-9. [DOI] [PubMed] [Google Scholar]
- De Flora A, Zocchi E, Guida L, Franco L, Buzzone S. Autocrine and paracrine calcium signalling by the CD38/NAD+/cyclic ADP-ribose system. Ann NY Acad Sci. 2004;1028:176–191. doi: 10.1196/annals.1322.021. [DOI] [PubMed] [Google Scholar]
- Flügel A, Berkowicz T, Ritter T, Labeur M, Jenne DE, Li Z, et al. Migratory activity and functional changes of green fluorescent effector cells before and during experimental autoimmune encephalomyelitis. Immunity. 2001;14:547–560. doi: 10.1016/s1074-7613(01)00143-1. [DOI] [PubMed] [Google Scholar]
- Galinoe A, Churchill GC. Interactions between calcium release pathways: multiple messengers and multiple stores. Cell Calcium. 2002;32:343–354. doi: 10.1016/s0143416002001902. [DOI] [PubMed] [Google Scholar]
- Gasser A, Bruhn S, Guse AH. Second messenger function of nicotinic acid adenine dinucleotide phosphate (NAADP) revealed by an improved enzymatic cycling assay. J Biol Chem. 2006;281:16906–16913. doi: 10.1074/jbc.M601347200. [DOI] [PubMed] [Google Scholar]
- Graeff RM, Walseth TF, Fryxell K, Branton WD, Lee HC. Enzymatic synthesis and characterizations of cyclic GDP-ribose. A procedure for distinguishing enzymes with ADP-ribosyl cyclase activity. J Biol Chem. 1994;269:30260–30267. [PubMed] [Google Scholar]
- Graeff RM, Walseth TF, Hill HK, Lee HC. Fluorescent analogs of cyclic ADP-ribose: synthesis, spectral characterization, and use. Biochemistry. 1996;35:379–386. doi: 10.1021/bi952083f. [DOI] [PubMed] [Google Scholar]
- Gu X, Yang Z, Zhang L, Kunerth S, Fliegert R, Weber K, et al. Synthesis and biological evaluation of novel membrane-permeant cyclic ADP-ribose mimics: N1-[(5″-O-phosphorylethoxy)methyl]-5′-O-phosphorylinosine 5′,5″-cyclicpyro-phosphate (cIDPRE) and 8-substituted derivatives. J Med Chem. 2004;47:5674–5682. doi: 10.1021/jm040092t. [DOI] [PubMed] [Google Scholar]
- Guida L, Bruzzone S, Sturla L, Franco L, Zocchi E, De Flora A. Equilibrative and concentrative nucleoside transporters mediate influx of extracellular cyclic ADP-ribose into 3T3 murine fibroblasts. J Biol Chem. 2002;277:47097–47105. doi: 10.1074/jbc.M207793200. [DOI] [PubMed] [Google Scholar]
- Guida L, Franco L, Bruzzone S, Sturla L, Zocchi E, Basile G, et al. Concentrative influx of functionally active cyclic ADP-ribose in dimethyl sulfoxide-differentiated HL-60 cells. J Biol Chem. 2004;279:22066–22075. doi: 10.1074/jbc.M314137200. [DOI] [PubMed] [Google Scholar]
- Guse AH. Regulation of calcium signalling by the second messenger cyclic adenosine diphosphoribose (cADPR) Curr Mol Med. 2004a;4:239–248. doi: 10.2174/1566524043360771. [DOI] [PubMed] [Google Scholar]
- Guse AH. Biochemistry, biology, and pharmacology of cyclic adenosine diphosphoribose (cADPR) Curr Med Chem. 2004b;11:847–855. doi: 10.2174/0929867043455602. [DOI] [PubMed] [Google Scholar]
- Guse AH, Cakir-Kiefer C, Fukuoka M, Shuto S, Weber K, Bailey VC, et al. Novel hydrolysis-resistant analogues of cyclic ADP-ribose: modification of the ‘northern' ribose and calcium release activity. Biochemistry. 2002;41:6744–6751. doi: 10.1021/bi020171b. [DOI] [PubMed] [Google Scholar]
- Guse AH, Da Silva CP, Berg I, Weber K, Heyer P, Hohenegger M, et al. Regulation of calcium signalling in T lymphocytes by the second messenger cyclic ADP-ribose. Nature. 1999;398:70–73. doi: 10.1038/18024. [DOI] [PubMed] [Google Scholar]
- Guse AH, Gu X, Zhang L, Weber K, Kramer E, Yang Z, et al. A minimal structural analogue of cyclic ADP-ribose: synthesis and calcium release activity in mammalian cells. J Biol Chem. 2005;280:15952–15959. doi: 10.1074/jbc.M414032200. [DOI] [PubMed] [Google Scholar]
- Guse AH, Roth E, Emmrich F. Intracellular Ca2+ pools in Jurkat T-lymphocytes. Biochem J. 1993;291:447–451. doi: 10.1042/bj2910447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M, Grimaldi JC, Bazan JF, Lund FE, Santos-Argumedo L, Parkhouse RM, et al. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science. 1993;262:1056–1059. doi: 10.1126/science.8235624. [DOI] [PubMed] [Google Scholar]
- Kawakami N, Nagerl UV, Odoardi F, Bonhoeffer T, Wekerle H, Flügel A. Live imaging of effector cell trafficking and autoantigen recognition within the unfolding autoimmune encephalomyelitis lesion. J Exp Med. 2005;201:1805–1814. doi: 10.1084/jem.20050011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Jacobson EL, Jacobson MK. Synthesis and degradation of cyclic ADP-ribose by NAD glycohydrolases. Science. 1993;261:1330–1333. doi: 10.1126/science.8395705. [DOI] [PubMed] [Google Scholar]
- Lee HC. Multiplicity of Ca2+ messengers and Ca2+ stores: a perspective from cyclic ADP-ribose and NAADP. Curr Mol Med. 2004;4:227–237. doi: 10.2174/1566524043360753. [DOI] [PubMed] [Google Scholar]
- Lee HC, Aarhus R. Wide distribution of an enzyme that catalyzes the hydrolysis of cyclic ADP-ribose. Biochim Biophys Acta. 1993;1164:68–74. doi: 10.1016/0167-4838(93)90113-6. [DOI] [PubMed] [Google Scholar]
- Lee HC, Zocchi E, Guida L, Franco L, Benatti U, De Flora A. Production and hydrolysis of cyclic ADP-ribose at the outer surface of human erythrocytes. Biochem Biophys Res Commun. 1993;191:639–645. doi: 10.1006/bbrc.1993.1265. [DOI] [PubMed] [Google Scholar]
- Moreau C, Woodman TJ, Potter BVL. Unusual entry to the novel 8-halo-N1-ribosyl hypoxanthine system by degradation of a cyclic adenosine-5′-diphosphate ribose analogue. Chem Commun. 2006;14:1127–1129. doi: 10.1039/b517916e. [DOI] [PubMed] [Google Scholar]
- Muller-Steffner H, Muzard M, Oppenheimer N, Schuber F. Mechanistic implications of cyclic ADP-ribose hydrolysis and methanolysis catalyzed by calf spleen NAD+ glycohydrolase. Biochem Biophys Res Commun. 1994;204:1279–1285. doi: 10.1006/bbrc.1994.2601. [DOI] [PubMed] [Google Scholar]
- Potter BVL, Walseth TF. Medicinal chemistry and pharmacology of cyclic ADP-ribose. Curr Mol Med. 2004;4:303–311. doi: 10.2174/1566524043360744. [DOI] [PubMed] [Google Scholar]
- Schoettelndreier H, Potter BVL, Mayr GW, Guse AH. Mechanisms involved in alpha6beta1-integrin-mediated Ca(2+) signaling. Cell Signal. 2001;13:895–899. doi: 10.1016/s0898-6568(01)00225-x. [DOI] [PubMed] [Google Scholar]
- Schuber F, Lund FE. Structure and enzymology of ADP-ribosyl cyclases: conserved enzymes that produce multiple calcium mobilizing metabolites. Curr Mol Med. 2004;4:249–261. doi: 10.2174/1566524043360708. [DOI] [PubMed] [Google Scholar]
- Schweitzer K, Mayr GW, Guse AH. Assay for ADP-ribosyl cyclase by reverse-phase high-performance liquid chromatography. Anal Biochem. 2001;299:218–226. doi: 10.1006/abio.2001.5419. [DOI] [PubMed] [Google Scholar]
- Sethi JK, Empson RM, Bailey VC, Potter BVL, Galione A. 7-Deaza-8-bromo-cyclic ADP-ribose, the first membrane-permeant, hydrolysis-resistant cyclic ADP-ribose antagonist. J Biol Chem. 1997;272:16358T–16363T. doi: 10.1074/jbc.272.26.16358. [DOI] [PubMed] [Google Scholar]
- Shuto S, Fukuoka M, Manikowsky A, Ueno Y, Nakano T, Kuroda R, et al. Total synthesis of cyclic ADP-carbocyclic-ribose, a stable mimic of Ca2+-mobilizing second messenger cyclic ADP-ribose. J Am Chem Soc. 2001;123:8750–8759. doi: 10.1021/ja010756d. [DOI] [PubMed] [Google Scholar]
- Shuto S, Matsuda A. Chemistry of cyclic ADP-ribose and its analogs. Curr Med Chem. 2004;11:827–845. doi: 10.2174/0929867043455639. [DOI] [PubMed] [Google Scholar]
- States DJ, Walseth TH, Lee HC. Similarities in amino acid sequences of Aplysia ADP-ribosyl cyclase and human lymphocyte antigen CD38. Trends Biochem Sci. 1992;17:495. doi: 10.1016/0968-0004(92)90337-9. [DOI] [PubMed] [Google Scholar]
- Wagner GK, Black S, Guse AH, Potter BVL. First enzymatic synthesis of an N1-cyclised cADPR (cyclic-ADP ribose) analogue with a hypoxanthine partial structure: discovery of a membrane permeant cADPR agonist. Chem Commun. 2003;15:1944–1945. doi: 10.1039/b305660k. [DOI] [PubMed] [Google Scholar]
- Wagner GK, Guse AH, Potter BVL. Rapid synthetic route toward structurally modified derivatives of cyclic adenosine 5′-diphosphate ribose. J Org Chem. 2005;70:4810–4819. doi: 10.1021/jo050085s. [DOI] [PubMed] [Google Scholar]
- Walseth F, Lee HC. Synthesis and characterization of antagonists of cyclic-ADP-ribose-induced Ca2+ release. Biochim Biophys Acta. 1993;1178:235–242. doi: 10.1016/0167-4889(93)90199-y. [DOI] [PubMed] [Google Scholar]
- Wong L, Aarhus R, Lee HC, Walseth TF. Cyclic 3-deaza-adenosine diphosphoribose: a potent and stable analog of cyclic ADP-ribose. Biochim Biophys Acta. 1999;1472:555–564. doi: 10.1016/s0304-4165(99)00161-0. [DOI] [PubMed] [Google Scholar]