Abstract
Background and purpose:
Macrophages release cytokines that may contribute to pulmonary inflammation in conditions such as chronic obstructive pulmonary disease. Thus, inhibition of macrophage cytokine production may have therapeutic benefit. p38 MAPK may regulate cytokine production, therefore, the effect of two p38 MAPK inhibitors, SB239063 and SD-282, on the release of TNF-α, GM-CSF and IL-8 from human macrophages was investigated.
Experimental approach:
Cytokine release was measured by ELISA. Immunoblots and mRNA expression studies were performed to confirm p38 MAPK isoform expression and activity. Macrophages were isolated from lung tissue of current smokers, ex-smokers and emphysema patients and exposed to lipopolysaccharide. These cells then released cytokines in a concentration-dependent manner.
Key results:
SB239063 only inhibited TNF-α release (EC50 0.3±0.1 μM). Disease status had no effect on the efficacy of SB239063. SD-282 inhibited both TNF-α and GM-CSF release from macrophages (EC50 6.1±1.4 nM and 1.8±0.6 μM respectively) but had no effect on IL-8 release. In contrast, both inhibitors suppressed cytokine production in monocytes.
Conclusions and Implications:
The differential effects of p38 MAPK inhibitors between macrophages and monocytes could not be explained by differences in p38 MAPK isoform expression or activity. However, the stability of TNF-α mRNA was significantly increased in macrophages compared to monocytes. These data suggest a differential involvement for p38 MAPK in macrophage cytokine production compared with monocytes. These effects are not due to lack of p38 activation or p38α expression in macrophages but may reflect differential effects on the stability of cytokine mRNA.
Keywords: TNF-α, MAPKs, mRNA stability, macrophage, monocyte, human
Introduction
Lung inflammation is an important feature of chronic obstructive pulmonary disease (COPD) and is associated with increased numbers of infiltrating macrophages, CD8+ T lymphocytes and neutrophils, together with elevated levels of cytokines, including tumour necrosis factor (TNF)-α, interleukin (IL)-8 and granulocyte macrophage-colony stimulating factor (GM-CSF) (Barnes, 2004a). Moreover, macrophages can account for many of the pathophysiological features associated with COPD (Barnes, 2004b). There are no pharmacological interventions available currently that have been shown to slow the progression of COPD or that effectively suppress the underlying inflammation in the lung parenchyma, indicating a need for alternative anti-inflammatory strategies.
Several new approaches that target the inflammation associated with COPD are in clinical development, including mitogen-activated protein kinase (MAPK) inhibitors (Kumar et al., 2003). Of the three main MAPK pathways, p38 and JNK MAPK are implicated in chronic inflammation (Johnson and Lapadat, 2002). p38 MAPK inhibitors have been shown to suppress lung inflammation in in vivo models of disease (Underwood et al., 2000a; Haddad et al., 2001). For example, SB239063 reduced neutrophil accumulation in the lungs of rats following inhalation of endotoxin (Underwood et al., 2000a). In addition, inhalation of antisense oligonucleotide of p38α MAPK inhibited bronchial hyperreactivity and reduced the number of inflammatory cells in bronchoalveolar lavage fluid in a murine model of asthma (Duan et al., 2005). p38 MAPK inhibitors also suppress cytokine release from inflammatory cells in vitro (Underwood et al., 2000b; Kumar et al., 2003). Hence this pathway is considered a therapeutic target in inflammatory respiratory diseases and p38 MAPK inhibitors are currently in Phase I and II trials for COPD (Prous, 2005).
There are four p38 isoforms α, β, γ and δ that are encoded by four separate genes, the expression of which appears to be tissue dependent. The relative contribution of each of these isoforms to the inflammatory response is currently unknown due to lack of specific pharmacological tools; however, there is evidence that they have different substrates. For example, p38α and p38β phosphorylate MAPKAPK2, but this protein is not phosphorylated by p38γ or p38δ. This would suggest that different p38 MAPK isoforms may have differential effects in inflammation. As a nonsteroidal anti-inflammatory therapy p38 MAPK inhibitors could be of potential use in COPD, as glucocorticosteroids are largely ineffective in inhibiting the inflammatory response both in vivo and in vitro (Burge et al., 2000; Hattotuwa et al., 2002; Russell et al., 2002; Culpitt et al., 2003).
The aim of this study was to determine the importance of the p38 MAPK pathway on cytokine release from lung tissue macrophages with a view to identifying novel targets for pharmaceutical intervention. Consequently, we examined the effects of a novel p38 MAPK inhibitor, SD-282, as this compound is highly potent for selectively inhibiting p38α (EC50 1.6 nM) and p38β (EC50 23.0 nM), without affecting either p38γ or p38δ (Lim et al., 2004). Furthermore, SD-282 has been shown to reduce mRNA levels of proinflammatory cytokines in a murine model of collagen-induced arthritis (Medicherla et al., 2006).
We describe the pharmacological activity of SD-282 in comparison with a reference p38 MAPK inhibitor, SB239063 which inhibits p38α and p38β with equal potency (EC50 44 nM), without affecting either p38γ or p38δ (Underwood et al., 2000b).
Methods
Subjects
The Ethics Committee of the Brompton and Harefield NHS Trust and NHLI approved this study and all subjects gave informed written consent. All subjects had a smoking history of >15 pack years. Ex-smokers had ceased smoking for >6 months. The emphysema subjects were all undergoing lung transplants. There was no difference in the age of the three subject groups (Table 1).
Table 1.
Clinical characteristics of lung tissue donors
| Smokers | Ex-smokers | Emphysema lung transplant | |
|---|---|---|---|
| Age (years) | 55.5±3.2 | 52.3±3.7 | 52±0.8 |
| Sex (M:F) | 3:3 | 3:4 | 3:1 |
| FEV1 (% predicted) | 87.9±6.1 | 83.2±3.5 | NA |
| FEV1/FVC ratio | 77.2±6.3 | 73.4±2.9 | NA |
| Smoking history (pack years) | 34.3±8.4 | 41.0±16.3 | NA |
Data are presented as mean±s.e. NA indicates data are not available.
Isolation and culture of human macrophages
Macrophages were isolated from macroscopically normal lung tissue, obtained from patients undergoing surgical resection for either carcinoma or lung transplant as described previously (Smith et al., 2004). The macrophage preparations used were greater than 95% purity, as determined by Kimura staining and confirmed by immunocytochemistry with labelling using an anti-CD68 antibody.
Cytokine measurements
Cell-free supernatant was removed 20 h post-stimulation with lipopolysaccharide (LPS) (Salmonella enteriditis) and TNF-α, GM-CSF and IL-8 measured by enzyme-linked immunosorbant assay (ELISA) (R & D Systems Europe, Abingdon, UK) according to the manufacturer's instructions. The detection limits of the IL-8 detection assay was 31 pg ml−1 and the detection limit for the GM-CSF and TNF-α assays were 15.5 pg ml−1.
Western immunoblot analysis
Cells were prepared for Western blotting as described previously (Smith et al., 2004). Cell lysates were prepared, insoluble proteins were removed and 20 μg of soluble extract were denatured and subjected to electrophoresis on 10% (wt/vol) sodium dodecyl sulphate (SDS) polyacrylamide gels.
Cell viability
Cell viability was determined colourimetrically by measuring the reduction of the tetrazolium salt, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT), to formazan.
Monocyte isolation
Monocytes were isolated from peripheral blood using discontinuous Percoll gradients followed by adherence to tissue culture plastic (Seldon et al., 1995). This method generated adherent monocyte preparations with purity greater than 97%, as determined by Kimura staining.
Expression of p38 MAPK isoforms
cDNAs encoding α, β, γ and δ p38 MAPK isoforms were amplified by polymerase chain reaction (PCR) techniques and subcloned into an untagged mammalian expression vector, pShuttle (BD Biosciences/Clontech, CA, USA). Clones were sequenced to confirm their respective accessions: L35264, NM_002751, NM_002969 & NM_002754. Subconfluent HeLa cells were transfected with 2 μg of DNA per well using Lipofectamine 2000 (Invitrogen, CA, USA). Cells were harvested 48 h post-transfection and lysed in RIPA buffer (Upstate Biotech, Milton Keynes, UK) supplemented with a protease inhibitor Complete cocktail (Roche, Lewes, UK).
Real-time PCR (Taqman)
Monocytes and lung macrophages were treated with either 3 or 10 ng ml−1 LPS, for 4 h before the addition of 5 μg ml−1 of actinomycin D (Sigma-Aldrich, Poole, UK). At various times points indicated, cells were lysed for RNA extraction. p38 MAPK inhibitors, these were added simultaneously with the actinomycin D and the cells harvested 60 min later. RNA was extracted using RNeasy (Qiagen, Crawley, UK) and quantified spectrophotometrically. Total RNA (0.5 μg) was reverse transcribed as previously described (Smith et al., 2003). Real-time PCR was performed using the equivalent of 100 ng RNA in 25 μl for TNF-α, GM-CSF, IL-8 and HPRT1 for 45 cycles. Analysis used the δδCT method, whereby the TNF-α, GM-CSF and IL-8 mRNA levels were expressed relative to the control gene expression and subsequently analysed by relative quantification (RQ) to mRNA levels in unstimulated cells. The results were then normalized for LPS-stimulated cells (100% of cytokine expression).
Statistical analyses
Data and statistical analyses: data points, and values are presented as mean±s.e. of ‘n' independent determinations using cells from different donors. Concentration–response curves were analysed by the ‘GraphPad PRISM' curve fitting program (GraphPad software, San Diego, CA, USA) and EC50 values were subsequently interpolated from curves of best-fit. Where appropriate, data were analysed statistically using Student's paired t-test or by ANOVA with the Dunn test. The null hypothesis was rejected when P<0.05.
Materials
The p38 MAPK inhibitor SB239063 was a gift from GlaxoSmithKline, Philadelphia, USA and the inhibitor SD-282 was a gift from Scios Inc. (Fremont, CA, USA). The anti-p38α and anti-p38δ antibodies were purchased from Autogen Bioclear (Calne, Wiltshire, UK). The anti-p38β antibody was purchased from Zymed (Invitrogen, Paisley, UK). The anti-p38γ antibody was purchased from Upstate Biotech (Chandlers Ford, Hampshire, UK). The anti-p38 antibody, the anti-phosphorylated p38, the anti-phosphorylated heat shock protein (HSP)27 and anti-total HSP27 were purchased from New England Biolabs (Hertfordshire, UK). The gene expression assays used for analysis of TNF-α (HS99999043_m1), GM-CSF (HS00171266_m1), IL-8 (HS99174103_m1) and the control gene hypoxanthine phosphoribosyltransferase-1 (HPRT1) (HS99999909_m1) by RT–PCR were purchased from Applied Biosystems (Warrington, UK).
Results
Effect of LPS on the release of cytokines from human lung macrophages
There was no detectable basal release of TNF-α from macrophages harvested from lung parenchyma of subjects who were cigarette smokers (n=6) (Figure 1a), whereas macrophages from emphysema subjects released 0.02±0.01 ng ml−1 TNF-α (n=4) (Figure 1a). LPS increased TNF-α release by macrophages from smokers and emphysema subjects in a concentration-dependent manner with EC50 values of 2.15±0.76 and 1.49±0.30 ng ml−1, respectively.
Figure 1.
Effect of LPS on cytokine release by lung macrophages. Macrophages from smokers (open circles) (n=4–6) and emphysema patients (solid circles) (n=3–4) were stimulated with increasing concentrations of LPS and supernatants harvested after 20 h. Subsequently, the concentrations of TNF-α (a), GM-CSF (b) and IL-8 (c) were measured by ELISA. Data are presented as mean±s.e.
Lung macrophages from smokers and subjects with emphysema released GM-CSF spontaneously with levels of 19.81±3.2 pg ml−1 (n=4) and 130.8±96.1 pg ml−1 (n=3), respectively (Figure 1b). LPS enhanced GM-CSF release by macrophages from smokers and emphysema subjects in a concentration-dependent manner with EC50 values of 5.14±1.06 and 1.56±0.72 ng ml−1, respectively.
Lung macrophages from smokers and subjects with emphysema, released 44.1±15.4 ng ml−1 (n=5) and 35.6±22.3 ng ml−1 (n=4) of IL-8, respectively under basal conditions (Figure 1c). LPS increased IL-8 release by macrophages from smokers and emphysema subjects in a concentration-dependent manner with EC50 values of 1.4±0.6 and 2.2±1.3 ng ml−1, respectively. There was no significant difference in the basal release or LPS-induced response of any of these cytokines by macrophages from smokers compared to emphysema subjects.
Effect of SB239063 on LPS-stimulated cytokine release
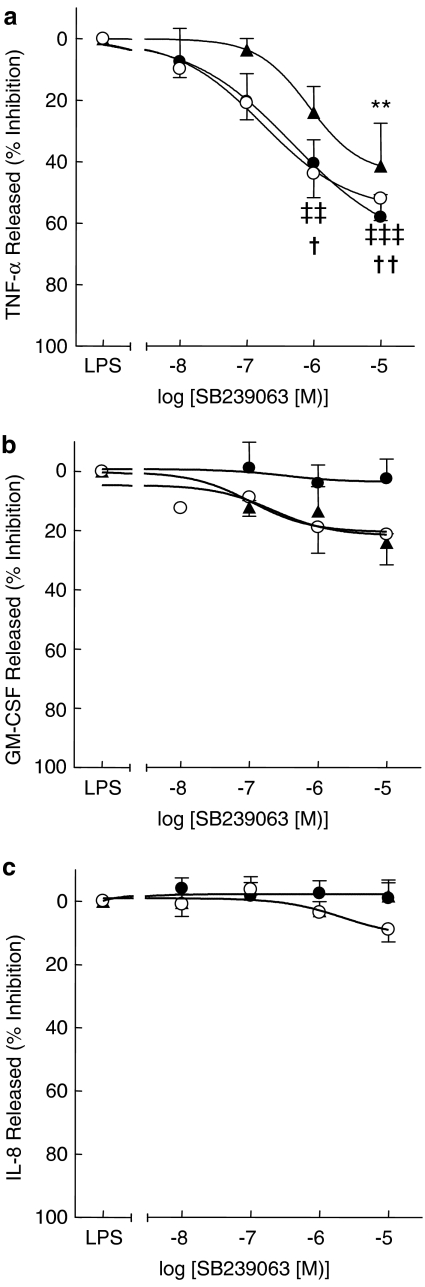
To evaluate the inhibitory activity of p38 MAPK inhibitors on cytokine release by lung macrophages, a submaximal concentration of LPS (10 ng ml−1) was selected that would allow measurement of TNF-α, GM-CSF and IL-8 (Table 2). SB239063 inhibited LPS-induced TNF-α release from macrophages from all subject groups in a concentration-dependent manner (Figure 2a, Table 3). There was no significant difference in the effect of SB239063 on LPS-induced TNF-α release between any of the subject groups (Table 3). The release of either GM-CSF or IL-8 release was not markedly inhibited by SB239063 at any of the concentrations tested (0.01–10 μM) (Figures 2b and c) (Table 3).
Table 2.
Cytokine release from human lung macrophages stimulated with 10 ng ml−1 LPS
| Cytokine | Ex-smokers | Current smokers | Emphysema |
|---|---|---|---|
| TNF-α | 3.8±1.5 | 6.1±2.2 | 2.6±1.2 |
| GM-CSF | 1.8±0.5 | 2.2±0.5 | 2.6±1.2 |
| IL-8 | 251.2±58.7 | 220.8±67.1 | 167.8±49.5 |
Abbreviations: GM-CSF, granulocyte macrophage-colony stimulating factor; IL, interleukin; TNF, tumour necrosis factor.
Data are presented as mean±s.e. cytokine release in ng ml−1, n=3–8.
Figure 2.
Effect of SB239063 on LPS-induced cytokine release by lung macrophages. Macrophages from ex-smokers (open circles) (n=5), current-smokers (closed circles) (n=8) and emphysema patients (solid triangles) (n=4) were treated with increasing concentrations of SB239063 for 30 min before the addition of LPS (10 ng ml−1). Cells were cultured and supernatants were harvested after 20 h and TNF-α (a), GM-CSF (b) and IL-8 (c) were measured by ELISA. Data are presented as mean±s.e. **P<0.01 for emphysema samples compared with control, ‡‡P<0.01, ‡‡‡P<0.001 for smoker samples compared with control and †P<0.05, ††P<0.01 for ex-smoker samples compared with control.
Table 3.
Effect of SB239063 on LPS-stimulated cytokine release from macrophages
|
Ex-smokers |
Current smokers |
Emphysema |
||||
|---|---|---|---|---|---|---|
| Cytokine | EC50 (μM) | % Inhibition at 10 μM | EC50 (μM) | % Inhibition at 10 μM | EC50 (μM) | % Inhibition at 10 μM |
| TNF-α | 0.3±0.1 | 58.0±7.4 | 0.2±0.07 | 52.0±7.2 | 0.8±0.2 | 41.4±13.9 |
| GM-CSF | NI | 2.5±6.6 | NI | 21.3±10.4 | NI | 24.1±3.0 |
| IL-8 | NI | 0.1±4.6 | NI | 8.9±4.0 | NI | 0.0±5.0 |
Abbreviations: GM-CSF, granulocyte macrophage-colony stimulating factor; IL, interleukin; TNF, tumour necrosis factor.
Data are presented as mean±s.e. EC50 values and percentage inhibition at 10 μM, n=4–8. NI indicates that an EC50 value could not be generated from the curve.
Effect of SD-282 on LPS-stimulated cytokine release from human lung macrophages
To investigate further whether the weak effect of SB239063 on LPS-induced GM-CSF and IL-8 release by lung macrophages was due to lack of efficacy of the drug or the lack of involvement of the p38 MAPK pathway, the effect of a structurally dissimilar p38 MAPK inhibitor (SD-282) was also evaluated in parallel with SB239063.
SD-282 suppressed LPS-induced TNF-α release from human lung macrophages (Figure 3a). On a molar basis, SD-282 was 50-times more potent than SB239063 (P<0.002) (Table 4). At the highest concentration studied (10 μM), the maximal inhibition of TNF-α release by SD-282 was not significantly different to that of SB239063 (n=6) (Figure 3a) (Table 4). Consistent with the data shown in Figure 2, SD-282 failed to affect the release of GM-CSF or IL-8 (Figure 3b and c) except at the highest concentration tested where there was a modest inhibitory effect (approximately 35%) on GM-CSF output (n=6) (Figure 3b) (Table 3).
Figure 3.
Comparison of SD282 and SB239063 on LPS-stimulated cytokine release by lung macrophages. Macrophages were treated with increasing concentrations of the p38 MAPK inhibitors SD-282 (solid circles) and SB239063 (open circles) for 30 min before stimulation with LPS (10 ng ml−1). Cells were cultured and supernatants were harvested after 20 h and TNF-α (a), GM-CSF (b) and IL-8 (c) were measured by ELISA. Data are presented as mean±s.e., n=6. *P<0.05, **P<0.01 and ***P<0.001 compared with control.
Table 4.
Comparison of SB239063 with SD-282 on LPS-stimulated cytokine release from macrophages
|
SB239063 |
SD-282 |
|||
|---|---|---|---|---|
| Cytokine | EC50 (nM) | % Inhibition at 10 μM | EC50 (nM) | % Inhibition at 10 μM |
| TNF-α | 307.1±47.0 | 49.0±10.8 | 6.1±1.4 | 71.7±7.7 |
| GM-CSF | NI | 0±20.2 | 1830±600 | 35.4±18.5 |
| IL-8 | NI | 1.9±4.1 | NI | 16.2±6.5 |
Abbreviations: GM-CSF, granulocyte macrophage-colony stimulating factor; IL, interleukin; TNF, tumour necrosis factor.
Data are presented as mean±s.e. EC50 values and percentage inhibition at 10 μM, n=6–8. NI indicates that an EC50 value could not be generated from the curve.
Effect of SD-282 and SB239063 on LPS-induced p38 MAPK activation
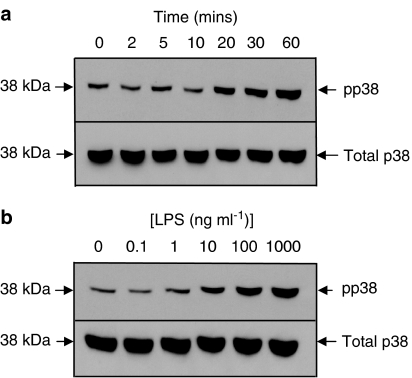
The weak effect of the p38 MAPK inhibitors in this system prompted an assessment of the ability of LPS to activate, and SB239063 and SD-282 to inhibit, the p38 MAPK signalling pathways in macrophages. A fraction of the total p38 MAPK pool was phosphorylated constitutively (activated) in unstimulated macrophages (Figure 4a), however, LPS stimulation (10 ng ml−1), after a lag of approximately 10 min, enhanced the basal phosphorylation of p38 MAPK in a time-dependent manner. By 30 min, the effect of LPS was concentration-dependent with maximal phosphorylation seen at approximately 1 μg ml−1 LPS (Figure 4b).
Figure 4.
Western blot analysis of total and phosphorylated p38 MAPK expression. Lung macrophages were either treated with LPS (10 ng ml−1) for up to 60 min (panel a) or treated with increasing concentrations of LPS for 30 min (panel b). Cell lysates were prepared, insoluble proteins were removed and 20 μg of soluble extract were denatured and subjected to electrophoresis on 10% (wt/vol) SDS polyacrylamide gels. Proteins were transferred to nitrocellulose and probed with either an anti-p38 antibody or anti-phosphorylated p38 antibody. The primary antibodies were detected with peroxidase-conjugated secondary antibodies, and labelled proteins were detected by enhanced chemiluminescence. The blot is representative of four experiments and pp38 indicates phosphorylated p38.
Having established that the p38 MAPK pathway is activated by LPS treatment in these cells, the ability of SB239063 and SD-282 to inhibit p38 activity was assessed. The phosphorylation of HSP27 was used as a measure of p38 MAPK activation. LPS-induced phosphorylation of HSP27, which was inhibited in a concentration-dependent manner by both SD-282 and SB239063 (Figure 5a and b). In both cases, at the highest concentration of p38 MAPK inhibitor tested (10 μM) the level of phospho-HSP27 was similar to that found in unstimulated cells. In contrast, expression of total-HSP27 was unaffected by incubation of the macrophages with LPS, SD-282 or SB239063 (Figure 5). Therefore, both p38 MAPK inhibitors are effective at blocking the downstream signalling events leading from p38 MAPK activation in human lung macrophages.
Figure 5.
Western blot analysis of total and phosphorylated HSP27 expression in lung macrophages following treatment with p38 MAPK inhibitors. Lung macrophages were treated in the absence or presence of SD-282 (a) and SB239063 (b) for 30 min before the addition of a submaximal concentration of LPS (10 ng ml−1) for 30 min. Cell lysates were prepared, insoluble proteins were removed and 20 μg of soluble extract were denatured and subjected to electrophoresis on 10% (wt/vol) SDS polyacrylamide gels. Proteins were transferred to nitrocellulose and probed with either an anti-HSP27 antibody or anti-phosphorylated HSP27 antibody. The primary antibodies were detected with peroxidase-conjugated secondary antibodies, and labelled proteins were detected by enhanced chemiluminescence. The blot is representative of five experiments and pHSP27 indicates phosphorylated HSP27.
Effect of SD-282 and SB239063 on LPS-stimulated cytokine release from human peripheral blood monocytes
As the effects of both p38 MAPK inhibitors on lung macrophages was rather weak, a control system was tested to ensure that these drugs were effective at inhibiting known p38 MAPK-dependent cytokine production. To this end, monocytes were exposed to LPS (3 ng ml−1) a submaximal concentration required to evoke the release of inflammatory cytokines as reported previously (Meja et al., 2000). LPS-stimulated monocytes released TNF-α, GM-CSF and IL-8 at concentrations of 3.4±0.5 ng ml−1, 704.5±165.6 pg ml−1 and 132.7±18.0 ng ml−1, respectively. Pretreatment (30 min) of cells with SD-282 and SB239063 inhibited LPS-stimulated TNF-α and GM-CSF release in a concentration-dependent manner (Figure 6a and b) (Table 5). In contrast to the output of TNF-α and GM-CSF, that of IL-8 induced by LPS was relatively resistant to both SD-282 and SB239063 (Figure 6c), however, SD-282 suppressed IL-8 release from monocytes in a concentration-dependent manner (Figure 6c).
Figure 6.
Comparison of SD282 and SB239063 on LPS-stimulated cytokine release by monocytes. Monocytes were treated with increasing concentrations of the p38 MAPK inhibitors SD-282 (solid circles) and SB239063 (open circles) for 30 min before stimulation with LPS (3 ng ml−1). Cells were cultured and supernatants were harvested after 20 h and TNF-α (a), GM-CSF (b) and IL-8 (c) were measured by ELISA. Data are presented as mean±s.e., n=5.
Table 5.
Comparison of SB239063 with SD-282 on LPS-stimulated cytokine release from peripheral blood monocytes
|
SB239063 |
SD-282 |
|||
|---|---|---|---|---|
| Cytokine | EC50 (nM) | % Inhibition at 10 μM | EC50 (nM) | % Inhibition at 10 μM |
| TNF-α | 101.8±31.4 | 70.4±5.1 | 4.8±1.3 | 99.1±0.9 |
| GM-CSF | 32.6±4.6 | 88.0±2.4 | 1.0±0.2 | 98.5±1.1 |
| IL-8 | NI | 5.0±10.4 | 3200±100 | 46.3±9.3 |
Abbreviations: GM-CSF, granulocyte macrophage-colony stimulating factor; IL, interleukin; TNF, tumour necrosis factor.
Data are presented as mean±s.e. EC50 values and percentage inhibition at 10 μM, n=5. NI indicates that an EC50 value could not be generated from the curve.
Expression of the p38 MAPK isoforms by human lung macrophages and monocytes
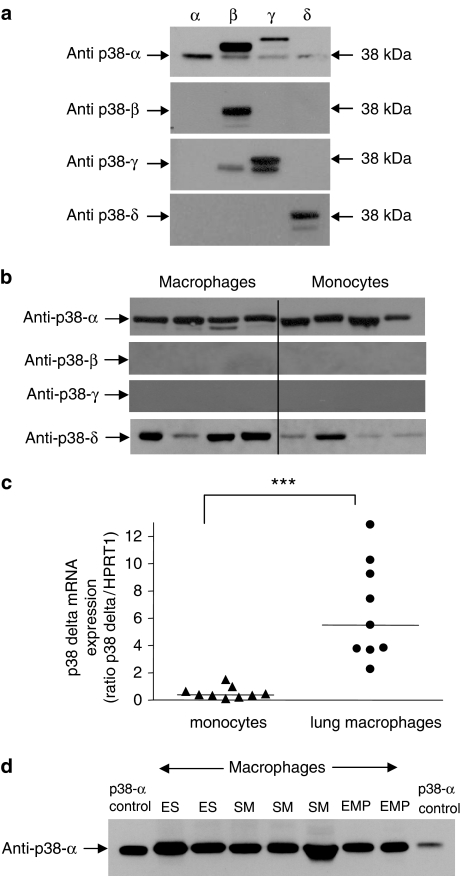
As p38 MAPK inhibition was effective at reducing cytokine production by monocytes, the differential effects of the inhibitors on the two cell types might be explained by a variation in p38 MAPK isoform expression between monocytes and macrophages. Therefore, the expression of the different isoforms of p38 MAPK was examined. Before analyzing the macrophage and monocytes lysates, the specificity of commercially available antibodies used to detect the different p38 MAPK isoforms by Western blotting was evaluated using HeLa cells that had been transfected to express each individual p38 MAPK isoform. The anti-p38α antibody detected an immunoreactive protein at 38 kDa, which was identical in size to a commercially available purified extract of p38α. The antibody also labelled immunoreactive bands from the HeLa cell lysates expressing p38β, p38γ and p38δ and as some of these bands migrated at the same mobilities they therefore, could not be excluded from the subsequent analyses (Figure 7a).
Figure 7.
Western blot analysis of p38 MAPK isoforms. The specificities of the antibodies used in this study were determined using HeLa cell lysates containing each of the individual p38 MAPK isoforms expressed in HeLa cells (a). Blots are representative of five experiments. Highly purified macrophages and monocytes were lysed, insoluble proteins were removed and 20 μg of soluble extract were denatured and subject to electrophoresis on 10% (wt/vol) SDS polyacrylamide gels. Proteins were transferred to nitrocellulose and probed with anti-p38 isoform antibodies. The primary antibodies were detected with peroxidase-conjugated secondary antibodies, and labelled proteins were detected by enhanced chemiluminescence (b). Blots are representative of four experiments. To determine the levels of p38δ mRNA Total RNA was extracted from monocytes (solid triangles) (n=9) and lung macrophages (solid circles) (n=9) and reverse transcribed using Avian myeloblastosis virus (AMV) reverse transcriptase. Taqman real-time PCR analysis was performed for p38δ and HPRT1 (c) ***P<0.001. Western blot analysis of p38-α MAPK expression in human lung macrophages from current smokers (SM), ex-smokers (ES) and patients with emphysema (EMP) (panel d). Blots are representative of 3 experiments.
The antibodies that recognized p38β and p38δ exhibited greater specificity for these proteins as they each detected only their specific p38 MAPK isoform 38 kDa and no other immunoreactive bands were observed on these blots (Figure 7a). The antibody recognizing p38γ detected an immunoreactive protein at 38 kDa, however, it also detected an immunoreactive band in the p38β preparation. There was an additional immunoreactive band detected at a different mobility to the 38 kDa p38 isoform and this was excluded from the analyses (Figure 7a).
Using these characterized antibodies, lung macrophages expressed p38α predominantly, but also expressed p38δ. These cells did not express p38β or p38γ as determined by Western blotting (Figure 7b). The p38 MAPK isoforms expressed by peripheral blood monocytes followed the same pattern of expression as observed in the lung macrophages (Figure 7b). The data from the samples analysed suggest that macrophages express increased levels of p38δ compared to monocytes. Moreover, there was found to be significantly higher levels of p38δ mRNA in lung macrophages compared to monocytes (Figure 7c). There were no differences in the expression profile of the p38 MAPK isoforms from macrophages of smokers with normal lung function compared to those from emphysema patients (Figure 7d).
Effect of SD-282 and SB239063 on TNF-α, GM-CSF and IL-8 mRNA stability by LPS-stimulated human peripheral blood monocytes and macrophages
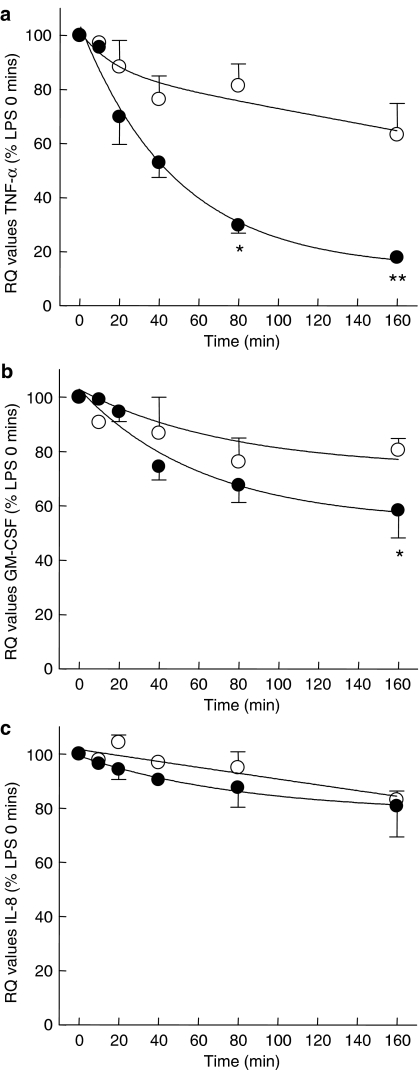
Another possibility that may account for the differential effects of p38 MAPK inhibitors on cytokine production by monocytes and macrophages might be due to effects of p38 MAPK on stability of the mRNA of the cytokine under investigation. In order to investigate this possibility, the stability of TNF-α, GM-CSF and IL-8 mRNA in human monocytes and macrophages was determined following addition of actinomycin D to the cells 4 h after LPS stimulation. The stability of the cytokine mRNA from LPS-stimulated monocytes and lung macrophages decreased, in a time-dependent manner with the stability of TNF-α and GM-CSF mRNA from monocytes being less stable than that derived from lung macrophages. After 4 h post-stimulation with LPS, monocyte TNF-α mRNA had reduced by 82% compared with macrophages where expression was decreased by only 37% (Figure 8). Following 4 h stimulation with LPS, there was no difference between monocyte and macrophage GM-CSF or IL-8 mRNA stability (Figures 8b and c).
Figure 8.
Comparison of TNF-α mRNA stability between monocytes and lung macrophages. Human lung macrophages (open circles) and human peripheral blood monocytes (solid circles) were stimulated with LPS (10 and 3 ng ml−1, respectively) for 4 h before the addition of 5 μg ml−1 actinomycin D (t=0). Cells were harvested at the times indicated after the addition of actinomycin D. Total RNA was extracted and reverse transcribed using AMV reverse transcriptase. Taqman real-time PCR analysis was performed and the δδCT values for TNF-α and HPRT1 were normalized to relative contribution of 1.0 for untreated cells. Data are presented as the mean±s.e., n=4 for expression of TNF-α (a), GM-CSF (b) and IL-8 mRNA (c) relative to that of HPRT1 mRNA (control gene). Data are expressed as percentage of cytokine/HPRT1 mRNA expression at t=0. *P<0.05 and **P<0.01 compared with control.
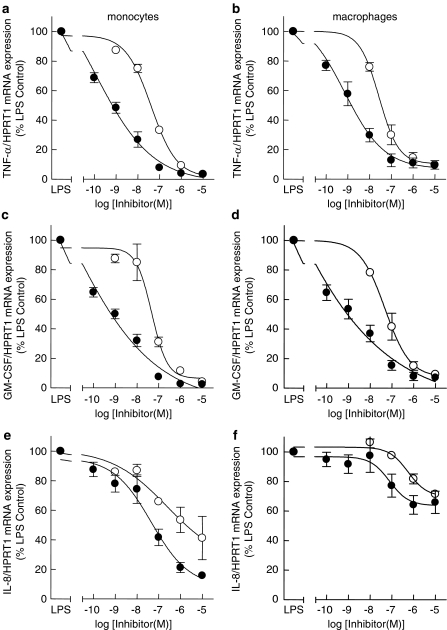
The effectiveness and potency of the p38 MAPK inhibitors in suppressing LPS-induced cytokine production was lower in lung macrophages than in monocytes. Therefore, we examined the effects of the p38 MAPK inhibitors on LPS-induced TNF-α, GM-CSF and IL-8 mRNA stability. The p38 MAPK inhibitors reduced the stability of TNF-α, GM-CSF and IL-8 mRNA in lung macrophages and monocytes in a concentration-dependent manner, although there was no effect on the expression of the control gene HPRT1 (Table 6 and Figure 9a–f). There was no significant difference in the EC50 values for inhibition of cytokine mRNA stability of either SD-282 or SB239063 between monocytes and macrophages (Table 6), although SD-282 was significantly more potent compared to SB239063 (Table 6). At the highest concentration of inhibitors studied (10 μM), the stability of TNF-α and GM-CSF mRNA was reduced in both cell types by >95% compared to LPS-stimulated cells treated with actinomycin D alone (Figure 9a–d). The p38 MAPK inhibitors only partially affected IL-8 mRNA stability. The maximal inhibition values for suppression of IL-8 mRNA stability from monocytes were 84.2±2.4 and 58.3±14.6% inhibition with SD-282 and SB239063, respectively (Figure 9e). The maximal inhibition values for suppression of IL-8 mRNA stability from lung macrophages were 34.1±7.5 and 28.6±1.2% inhibition with SD-282 and SB239063, respectively (Figure 9e).
Table 6.
Comparison of SB239063 with SD-282 on cytokine mRNA stability of peripheral blood monocytes and lung macrophages
|
SB239063 |
SD-282 |
|||
|---|---|---|---|---|
| Cytokine | Monocytes EC50 (nM) | Macrophages EC50 (nM) | Monocytes EC50 (nM) | Macrophages EC50 (nM) |
| TNF-α | 42.6±6.3 | 34.1±7.6 | 0.3±0.1 | 1.7±1.0 |
| GM-CSF | 50.6±15.4 | 65.3±30.0 | 0.2±0.1 | 1.1±0.6 |
| IL-8 | 1830±1160 | 749.0±226.0 | 41.1±19.4 | 98.1±55.5 |
Abbreviations: GM-CSF, granulocyte macrophage-colony stimulating factor; IL, interleukin; TNF, tumour necrosis factor.
Data are presented as mean±s.e. EC50 values n=4.
Figure 9.
Comparison of SD-282 and SB239063 effects on cytokine mRNA stability between monocytes and lung macrophages. The effect of p38 MAPK inhibitors SD-282 (solid circles) and SB239063 (open circles) on LPS-induced TNF-α, GM-CSF and IL-8 mRNA stability at 60 min post addition of 5 μg ml−1 actinomycin D. Total RNA was extracted and reverse transcribed using AMV reverse transcriptase. Taqman real-time PCR analysis was performed and the δδCT values for TNF-α and HPRT1. The effect of the MAPK inhibitors was determined for monocytes (a, c and e) and lung macrophages (b, d and f). Data are expressed as mean±s.e. cytokine/HPRT1 mRNA expression, represented as a percentage of the LPS control (100%), n=4.
Discussion and conclusions
The global importance and prevalence of COPD have been increasing (Pauwels and Rabe, 2004), and it is predicted to be the third most common cause of death by year 2020 (Lopez and Murray, 1998). Since COPD is characterized by inflammation associated with increased numbers of macrophages (Jeffery, 1999), it is of importance to investigate the contribution of inflammatory mediators released from the cells.
In this study, we found that there was no difference in the spontaneous or LPS-induced release of TNF-α, GM-CSF and IL-8 from the human lung-derived macrophages between smokers and patients with emphysema. Previously, it has been reported that alveolar macrophages from smokers release increased levels of TNF-α under both basal and stimulated conditions when compared with cells from non-smokers (Gunella et al., 2006). This study examined the role of the p38 MAPK pathway in the production of inflammatory cytokines from lung macrophages using two structurally dissimilar inhibitors. The p38 MAPK inhibitor SB239063 significantly reduced TNF-α production from LPS-stimulated human lung macrophages, but not GM-CSF or IL-8, suggesting that the mechanism of LPS stimulation for IL-8 and GM-CSF is different from that of TNF-α and is p38 MAPK independent. Furthermore, a second more potent p38 MAPK inhibitor, SD-282, also had a differential effect on inhibition of LPS-induced cytokine release from human lung macrophages. The weak effects of the inhibitors could not be explained by a lack of p38 MAPK activation or lack of efficacy of inhibitors in these cells as phosphorylation of HSP27 was suppressed by >85%. Evidence for a differential effect of p38 MAPK on cytokine release has been shown by Westra et al. (2004) who showed differential effects of the p38 MAPK inhibitor RWJ 67657 on the suppression of TNF-α, IL-8 and IL-6 from LPS-stimulated 7-day monocyte-derived macrophages. We have extended these observations by examining the effects of two different p38 MAPK inhibitors on release of cytokines implicated in lung inflammation by macrophages from patients with emphysema compared to control subjects.
It had previously been found that the selective p38 MAPK inhibitor SB239063 exhibited differential effects on LPS-induced cytokine production from human peripheral blood monocytes (Underwood et al., 2000b). This compound inhibited LPS-induced IL-1 and TNF-α release potently, GM-CSF less so and was unable to inhibit G-CSF release (Underwood et al., 2000b). In this study, we have shown that high concentrations of SD-282, but not SB239063, suppressed GM-CSF release from LPS-stimulated human lung macrophages. In a previous study, LPS-stimulated GM-CSF production from human alveolar macrophages was also not inhibited by the p38 MAPK inhibitor SB203580 (Koch et al., 2004). However, as the potencies of these compounds are less than that of SD-282, with IC50 values of 56 and 44 nM, respectively, for SB203580 and SB239063, this may account for the lack of observed inhibition on GM-CSF release.
The p38 MAPK inhibitor SD-282 suppressed IL-8 release from peripheral blood monocytes, but not from lung macrophages. Moreover, the EC50 values for the suppression of IL-8 in peripheral blood monocytes were higher than those for inhibition of GM-CSF and TNF-α. Similar findings have been reported by Westra et al. (2004) who showed that the IC50 values for inhibition of IL-8 release was higher than that required to suppress TNF-α and IL-1β. In the present study, we have shown that SB239063 suppressed LPS-induced TNF-α and GM-CSF release from monocytes with comparable IC50 values and maximal inhibition to the findings of Underwood et al. (2000b). This data thus validates our experimental system for the evaluation of the novel inhibitor SD-282.
Neither p38 MAPK inhibitor is reported to alter the activity of either p38δ or p38γ (Underwood et al., 2000a), moreover, SD-282 does not cause inhibition of the other MAPKs ERK2 and JNK when tested at 50 μM (Lim et al., 2004). In this study, we have shown that human lung macrophages and monocytes express both p38α and p38δ protein confirming the studies of Hale et al. (1999). Therefore, both inhibitors have the capacity to suppress the p38 MAPK pathway in both monocytes and macrophages. Indeed, p38 MAPK activity is diminished in macrophages in the presence of both inhibitors. Therefore, the relative insensitivity of cytokine release by p38 MAPK inhibitors is not simply due to a lack of p38 MAPK inhibition in macrophages but may be related to a differential regulation of cytokine transcription and translation in these cells. Alternatively, it is possible that the abundance of p38δ in macrophages might also account for the diminished effect of these inhibitors.
We have shown that, in macrophages isolated from human lung tissue, the stability of TNF-α mRNA is significantly greater than in human monocytes. This data is in accordance with the findings of MacKenzie et al. (2002). In addition, the stability of mRNA for other proinflammatory cytokines, such as IL-1β, is also increased in alveolar macrophages compared to monocytes (Herzyk et al., 1992). The reason why the regulation of TNF-α mRNA is altered in the macrophage compared to the monocyte is unclear, but may allow macrophages, as resident tissue cells, to facilitate a more sustained production of cytokines and hence prolong the inflammatory response.
We have found that there is no difference in the efficacy of the two p38 MAPK inhibitors for decreasing TNF-α, GM-CSF or IL-8 mRNA stability with marginal differences in the efficacy of the two p38 MAPK inhibitors on protein release. However, both p38 MAPK inhibitors suppressed TNF-α and GM-CSF mRNA stability at high concentrations, but had only a 50% inhibitory effect on TNF-α protein release and only a weak effect on GM-CSF protein release. Taken together, the data suggest that the effects of the p38 MAPK inhibitors is partly mediated by their effects on mRNA stability, but that p38 MAPK-independent pathways mediate TNF-α and GM-CSF protein release. Other studies have shown in human monocytes that p38 MAPK inhibitors do not regulate the translation for the expression of either TNF-α or COX-2 (Dean et al., 1999). Interestingly, the EC50 values for degradation of TNF-α mRNA are approximately 10-fold lower than those found for the inhibition of TNF-α protein release from lung macrophages, which suggest that other p38 MAPK-dependent mechanisms, such as post-translational modification, may be involved in cytokine release from macrophages.
Inflammation in COPD is associated with an increase in cytokines, including TNF-α, GM-CSF and IL-8. Moreover, there are no current therapies for COPD that suppress the inflammatory response. We report here that p38 MAPK inhibitors targeting the p38α and p38β isoforms were weakly effective in suppressing LPS-induced cytokine production from lung-derived macrophages but the effect was independent of donor status. These findings suggest that p38 MAPK inhibitors may be of little benefit in reducing TNF-α, GM-CSF or IL-8 associated lung inflammation associated with COPD but the development of molecules that target both p38α and p38δ MAPK isoforms may have added value in targeting macrophage-driven inflammation.
Acknowledgments
We thank Mr Peter Goldstraw (Department of Cardiothoracic Surgery, Royal Brompton Hospital) for generously providing human lung specimens for macrophage isolation.
Abbreviations
- COPD
chronic obstructive pulmonary disease
- ELISA
enzyme-linked immunosorbent assay
- GM-CSF
granulocyte-macrophage colony stimulating factor
- HSP
heat shock protein
- IL
interleukin
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- TNF
tumour necrosis factor
Conflict of interest
The authors state no conflict of interest.
References
- Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004a;56:515–548. doi: 10.1124/pr.56.4.2. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Macrophages as orchestrators of COPD. J COPD. 2004b;1:50–70. doi: 10.1081/COPD-120028701. [DOI] [PubMed] [Google Scholar]
- Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320:1297–1303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culpitt SV, Rogers DF, Shah P, De Matos C, Russell RE, Donnelly LE, et al. Impaired inhibition by dexamethasone of cytokine release by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:24–31. doi: 10.1164/rccm.200204-298OC. [DOI] [PubMed] [Google Scholar]
- Dean JL, Brook M, Clark AR, Saklatvala J. p38 mitogen-activated protein kinase regulates cyclooxygenase-2 mRNA stability and transcription in lipopolysaccharide-treated human monocytes. J Biol Chem. 1999;274:264–269. doi: 10.1074/jbc.274.1.264. [DOI] [PubMed] [Google Scholar]
- Duan W, Chan JH, McKay K, Crosby JR, Choo HH, Leung BP, et al. Inhaled p38alpha mitogen-activated protein kinase antisense oligonucleotide attenuates asthma in mice. Am J Respir Crit Care Med. 2005;171:571–578. doi: 10.1164/rccm.200408-1006OC. [DOI] [PubMed] [Google Scholar]
- Gunella G, Bardelli C, Amoruso A, Viano I, Balbo P, Brunelleschi S. Macrophage-stimulating protein differently affects human alveolar macrophages from smoker and non-smoker patients: evaluation of respiratory burst, cytokine release and NF-kappaB pathway. Br J Pharmacol. 2006;148:478–489. doi: 10.1038/sj.bjp.0706751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad EB, Birrell M, McCluskie K, Ling A, Webber SE, Foster ML, et al. Role of p38 MAP kinase in LPS-induced airway inflammation in the rat. Br J Pharmacol. 2001;132:1715–1724. doi: 10.1038/sj.bjp.0704022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale KK, Trollinger D, Rihanek M, Manthey CL. Differential expression and activation of p38 mitogen-activated protein kinase alpha, beta, gamma, and delta in inflammatory cell lineages. J Immunol. 1999;162:4246–4252. [PubMed] [Google Scholar]
- Hattotuwa KL, Gizycki MJ, Ansari TW, Jeffery PK, Barnes NC. The effects of inhaled fluticasone on airway inflammation in chronic obstructive pulmonary disease: a double-blind, placebo-controlled biopsy study. Am J Respir Crit Care Med. 2002;165:1592–1596. doi: 10.1164/rccm.2105025. [DOI] [PubMed] [Google Scholar]
- Herzyk DJ, Allen JN, Marsh CB, Wewers MD. Macrophage and monocyte IL-1 beta regulation differs at multiple sites. Messenger RNA expression, translation, and post-translational processing. J Immunol. 1992;149:3052–3058. [PubMed] [Google Scholar]
- Jeffery PK. Differences and similarities between chronic obstructive pulmonary disease and asthma. Clin Exp Allergy. 1999;29 Suppl 2:14–26. [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Koch A, Giembycz M, Ito K, Lim S, Jazrawi E, Barnes PJ, et al. Mitogen-activated protein kinase modulation of nuclear factor-kappaB-induced granulocyte macrophage-colony-stimulating factor release from human alveolar macrophages. Am J Respir Cell Mol Biol. 2004;30:342–349. doi: 10.1165/rcmb.2003-0122OC. [DOI] [PubMed] [Google Scholar]
- Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- Lim MY, Wang H, Kapoun AM, O'connell M, O'Young G, Brauer HA, et al. p38 Inhibition attenuates the pro-inflammatory response to C-reactive protein by human peripheral blood mononuclear cells. J Mol Cell Cardiol. 2004;37:1111–1114. doi: 10.1016/j.yjmcc.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Murray CC. The global burden of disease, 1990–2020. Nat Med. 1998;4:1241–1243. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- MacKenzie S, Fernandez-Troy N, Espel E. Post-transcriptional regulation of TNF-alpha during in vitro differentiation of human monocytes/macrophages in primary culture. J Leukoc Biol. 2002;71:1026–1032. [PubMed] [Google Scholar]
- Medicherla S, Ma JY, Mangadu R, Jiang Y, Zhao JJ, Almirez R, et al. A selective p38{alpha} mitogen-activated protein kinase inhibitor reverses cartilage and bone destruction in mice with collagen-induced arthritis. J Pharmacol Exp Ther. 2006;318:132–141. doi: 10.1124/jpet.105.098020. [DOI] [PubMed] [Google Scholar]
- Meja KK, Seldon PM, Nasuhara Y, Ito K, Barnes PJ, Lindsay MA, et al. p38 MAP kinase and MKK-1 co-operate in the generation of GM-CSF from LPS-stimulated human monocytes by an NF-kappa B-independent mechanism. Br J Pharmacol. 2000;131:1143–1153. doi: 10.1038/sj.bjp.0703684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD) Lancet. 2004;364:613–620. doi: 10.1016/S0140-6736(04)16855-4. [DOI] [PubMed] [Google Scholar]
- Prous JR. Annual update 2004/2005 – treatment of respiratory disorders. Drugs of the Future. 2005;30:51–112. [Google Scholar]
- Russell RE, Culpitt SV, DeMatos C, Donnelly L, Smith M, Wiggins J, et al. Release and activity of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2002;26:602–609. doi: 10.1165/ajrcmb.26.5.4685. [DOI] [PubMed] [Google Scholar]
- Seldon PM, Barnes PJ, Meja K, Giembycz MA. Suppression of lipopolysaccharide-induced tumor necrosis factor-alpha generation from human peripheral blood monocytes by inhibitors of phosphodiesterase 4: interaction with stimulants of adenylyl cyclase. Mol Pharmacol. 1995;48:747–757. [PubMed] [Google Scholar]
- Smith SJ, Brookes-Fazakerley S, Donnelly LE, Barnes PJ, Barnette MS, Giembycz MA. Ubiquitous expression of phosphodiesterase 7A in human proinflammatory and immune cells. Am J Physiol Lung Cell Mol Physiol. 2003;284:L279–L289. doi: 10.1152/ajplung.00170.2002. [DOI] [PubMed] [Google Scholar]
- Smith SJ, Cieslinski LB, Newton R, Donnelly LE, Fenwick PS, Nicholson AG, et al. Discovery of BRL 50481, a selective inhibitor of phosphodiesterase 7: in vitro studies in human monocytes, lung macrophages and CD8+ T-lymphocytes. Mol Pharmacol. 2004;66:1679–1689. doi: 10.1124/mol.104.002246. [DOI] [PubMed] [Google Scholar]
- Underwood DC, Osborn RR, Bochnowicz S, Webb EF, Rieman DJ, Lee JC, et al. SB 239063, a p38 MAPK inhibitor, reduces neutrophilia, inflammatory cytokines, MMP-9, and fibrosis in lung. Am J Physiol Lung Cell Mol Physiol. 2000a;279:L895–L902. doi: 10.1152/ajplung.2000.279.5.L895. [DOI] [PubMed] [Google Scholar]
- Underwood DC, Osborn RR, Kotzer CJ, Adams JL, Lee JC, Webb EF, et al. SB 239063, a potent p38 MAP kinase inhibitor, reduces inflammatory cytokine production, airways eosinophil infiltration, and persistence. J Pharmacol Exp Ther. 2000b;293:281–288. [PubMed] [Google Scholar]
- Westra J, Limburg PC, De Boer P, Van Rijswijk MH. Effects of RWJ 67657, a p38 mitogen activated protein kinase (MAPK) inhibitor, on the production of inflammatory mediators by rheumatoid synovial fibroblasts. Ann Rheum Dis. 2004;63:1453–1459. doi: 10.1136/ard.2003.013011. [DOI] [PMC free article] [PubMed] [Google Scholar]