Abstract
Background and Purpose:
The biosynthesis of leukotrienes (LT) and platelet-activating factor (PAF) involves the release of their respective precursors, arachidonic acid (AA) and lyso-PAF by the group IVA PLA2 (cPLA2α). This paper aims at characterizing the inhibitory properties of the cPLA2α inhibitor pyrrophenone on eicosanoids and PAF in human neutrophils (PMN).
Experimental Approach:
Freshly isolated human PMN were activated with physiological and pharmacological agents (fMLP, PAF, exogenous AA, A23187 and thapsigargin) in presence and absence of the cPLA2α inhibitor pyrrophenone and biosynthesis of LT, PAF, and PGE2 was measured.
Key Results:
Pyrrophenone potently inhibited LT, PGE2 and PAF biosynthesis in PMN with IC50s in the range of 1–20 nM. These inhibitory effects of pyrrophenone were specific (the consequence of substrate deprivation), as shown by the reversal of inhibition by exogenous AA and lyso-PAF. Comparative assessment of pyrrophenone, methyl-arachidonoyl-fluoro-phosphonate (MAFP) and arachidonoyl-trifluoromethylketone (AACOCF3) demonstrated that pyrrophenone was more specific and 100-fold more potent than MAFP and AACOCF3 for the inhibition of LT biosynthesis in A23187-activated PMN. The inhibitory effect of pyrrophenone on LT biosynthesis was reversible as LT biosynthesis was recovered when pyrrophenone-treated PMN were washed with autologous plasma. No alteration of phospholipase D (PLD) activity in fMLP-activated PMN was observed with up to 10 μM pyrrophenone, suggesting that the cPLA2α inhibitor does not directly inhibit PLD.
Conclusions and Implications:
Pyrrophenone is a more potent and specific cPLA2α inhibitor than MAFP and AACOCF3 and represents an excellent pharmacological tool to investigate the biosynthesis and the biological roles of eicosanoids and PAF.
Keywords: Leukotriene, platelet-activating factor, prostaglandin, neutrophil, leukocyte, phospholipase A2, lipid mediator, pyrrophenone, pyrrolidine-1, cPLA2α
Introduction
Lipid mediators such as prostaglandins (PGs), leukotrienes (LTs), lipoxins and platelet-activating factor (PAF) are important in the development and resolution of the inflammatory response. The biosynthesis of the lipid mediators of inflammation involves the activation of a phospholipase A2 (PLA2) and the subsequent release of arachidonic acid (AA) and lyso-PAF. The free AA is then oxygenated by the lipoxygenases or the cyclooxygenases to yield eicosanoids, whereas lyso-PAF is acetylated to PAF by the acetyl-CoA/lyso-PAF acetyltransferase. Among the PLA2 family, the group IVA phospholipase (cPLA2α) is recognized as the most important PLA2 activity implicated in the biosynthesis of PG, LT and PAF. Indeed, experiments with cPLA2α-deficient mice demonstrated that this enzyme was essential for the biosynthesis of eicosanoids and PAF in vivo (Bonventre et al., 1997; Uozumi et al., 1997; Fujishima et al., 1999; Shindou et al., 2000).
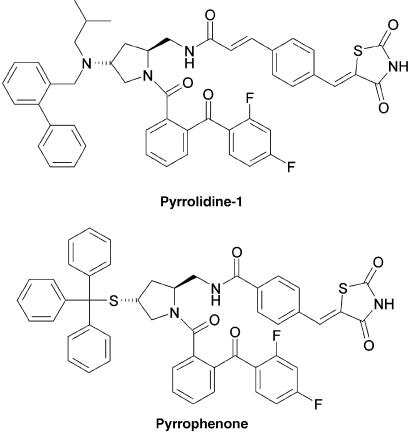
Early pharmacological studies demonstrated that the inhibition of PLA2 activity by p-bromophenacyl bromide, mepacrine, arachidonoyl-trifluoromethylketone (AACOCF3) and methyl-arachidonoyl-fluoro-phosphonate (MAFP) resulted in the inhibition of LT biosynthesis in activated polymorphonuclear neutrophil (PMN) and other leukocytes. Although effective at blocking the biosynthesis of LT, these inhibitors demonstrated a lack of specificity. For example, MAFP, one of the most commonly used cPLA2α inhibitors, also binds to the cannabinoid receptor 1 and inhibits the group VIA of PLA2, fatty acid amide hydrolase and PAF acetylhydrolase (Balsinde and Dennis, 1996; Lio et al., 1996; Deutsch et al., 1997; Kell et al., 2003). The isolation and molecular characterization of cPLA2α led to the development of more potent and specific cPLA2α inhibitors. Among these new cPLA2α inhibitors, pyrrolidine-1 and pyrrophenone (Figure 1) developed by Shionogi Research Laboratories (Seno et al., 2000, 2001; Ono et al., 2002) represent a class of promising pharmacological tools to investigate the mechanisms of lipid mediator biosynthesis and to assess the role of these mediators in physiological and pathological processes such as host defense and inflammatory diseases.
Figure 1.
Structures of the cPLA2α inhibitors pyrrolidine-1 and pyrrophenone.
Although pyrrophenone and pyrrolidine-1 have recently been used successfully in a limited number of studies, their inhibition profile and relative potencies and specificities versus other currently used cPLA2α inhibitors have not been thoroughly investigated. In this study, we characterized the effects of the cPLA2α inhibitor pyrrophenone on LT, prostaglandin E2 (PGE2) and PAF biosynthesis in human PMN stimulated under various experimental conditions and compared the potency and specificity of pyrrophenone with those of the currently used PLA2 inhibitors, MAFP and AACOCF3.
Methods
Isolation of human PMN
Venous blood from healthy donors was collected in 10 ml tubes containing 143 USP units of heparin and PMN were isolated as described previously (Boyum, 1968). Briefly, following centrifugation of blood, the platelet-rich plasma was discarded and erythrocytes were removed by dextran sedimentation. Mononuclear cells were then separated from the granulocytes by centrifugation on Ficoll–Paque cushions and a hypotonic lysis was performed on the granulocyte cell pellets to remove the remaining erythrocytes. The granulocyte suspensions contained mainly PMN (⩾95%) and cell viability was always greater than 98% as measured by Trypan blue exclusion. PMN were finally re-suspended in Hank's balanced salt solution (HBSS) containing 1.6 mM CaCl2 at 5 or 10 × 106 cells ml−1, as indicated. In all experimental settings, incubation volume was 1 ml.
Stimulation of LT and PAF biosynthesis
(A) In experiments involving stimulation with PAF or N-formyl-methionyl-leucyl-phenylalanine (fMLP), PMN suspensions in HBSS containing 1.6 mM CaCl2 (37°C, 5 × 106 cells ml−1) were primed with 1.5 nM tumor necrosis factor-α (TNF-α), 700 pM granulocyte macrophage colony-stimulating factor (GM-CSF) and 10 μM cytochalasin B for 30 min, then stimulated with 300 nM of either fMLP or PAF for 10 min. (B) In experiments involving stimulation with thapsigargin, AA and A23187, unprimed PMN suspensions in HBSS containing 1.6 mM CaCl2 (37°C, 5 × 106 cells ml−1) were stimulated with either thapsigargin (100 nM, 10 min), AA (3 μM, 5 min) or A23187 (100 nM, 5 min). In these experimental settings (A and B), 0.3 U ml−1 adenosine deaminase (ADA) was added to incubation media 5 min prior stimulation to eliminate the inhibitory constraint exerted by extracellular adenosine (Krump et al., 1997). (C) In experiments performed with human blood, freshly drawn human blood samples containing ∼14 U ml−1 heparin were incubated at 37°C in the presence of increasing concentrations of pyrrophenone, then stimulated with 10 μM ionomycin for 20 min. In all experimental settings, the PLA2 inhibitors MAFP, AACOCF3 and pyrrophenone were always added 10 min before stimulation with AA, A23187, thapsigargin, PAF or fMLP. Inhibitors and stimulatory agents were solubilized in ethanol (EtOH) or dimethylsulfoxide (DMSO) for addition to PMN suspensions. The final concentration of these solvents never exceeded 0.2% in incubation media.
5-LO product and AA analysis
For the determination of 5-LO product biosynthesis, cell incubations were stopped by the addition of 0.5. volume of cold (4°C) methanol/acetonitrile (MeOH/MeCN) (1/1, v/v) containing 12.5 ng of each of prostaglandin B2 (PGB2) and 19-OH-PGB2 as internal standards. The denatured samples were centrifuged (600 g, 10 min at 22°C) and the supernatants were analyzed by reverse phase-high-performance liquid chromatography (RP-HPLC) using an online extraction procedure as described previously (Borgeat et al., 1990). In experiments with whole human blood, incubations were stopped by placing the samples in an ice-water bath. The plasma samples obtained by centrifugation (300 g, 20 min at 4°C) were denatured with 10 volumes of a cold stop solution (4°C, MeOH/MeCN, 1/1 (v/v)) containing 12.5 ng of both 19-OH-PGB1 and PGB1 as internal standards. The denatured samples were centrifuged (600 g, 20 min at 4°C) and the supernatants were then evaporated to a volume of ∼1 ml using a stream of nitrogen (in a water bath at 22°C) and analyzed by RP-HPLC as described previously (Surette et al., 1993). LTB4, 20-COOH-LTB4, 20-OH-LTB4, 6(E)-LTB4, 6(E)-12-epi-LTB4 and 5(S)-HETE are collectively referred to as 5-LO products; these six compounds represent the major 5-LO metabolites of AA detectable by RP-HPLC and ultraviolet detection in human PMN. Quantitation of the various metabolites was achieved by using the internal standard PGB2 for normalization and authentic standards of 20-OH-LTB4 (also used for quantitation of 20-COOH-LTB4), LTB4 (also used for quantitation of 6(E)-LTB4 and 6(E)-12-epi-LTB4) and 5-HETE for calibration.
For the analysis of AA, incubations were stopped by the addition of 0.5 volume of a cold (4°C) stop solution (MeOH/MeCN, 1/1, v/v) containing 12.5 ng of both 19-OH-PGB2 and PGB2, and 20 ng of 2H8-AA. The denatured incubation media were centrifuged (600 g, 10 min at 22°C) and the supernatants were analyzed by RP-HPLC. The AA-containing fractions were collected, evaporated to dryness under reduced pressure using a Savant Speed-Vac concentrator model SCIIOA (Thermo Electro Corp., Milford, MA, USA) (drying rate set at ‘low') and redissolved in 50 μl MeCN for analysis by LC/MS using electrospray ionization in the negative mode as described previously (Surette et al., 1993).
PAF and lyso-PAF analysis
For the determination of PAF and lyso-PAF, cell incubations were stopped by the addition of 1 volume of cold (4°C) EtOH containing 5 ng of 2H4-PAF as internal standard. The denatured samples were then centrifuged (600 g, 20 min at 22°C), and PAF and lyso-PAF were recovered from the supernatants and analyzed as described previously (Harrison et al., 1999), with minor modifications. Briefly, the samples were loaded on a 60 mg C18 solid-phase extraction (SPE) cartridge and successively washed with 4 ml water and 2 ml EtOH/water (50/50, v/v). PAF and lyso-PAF were then eluted from the C18 cartridge with 2 ml EtOH/water (98/2, v/v), which were directly loaded onto an EtOH-conditioned 100 mg silica SPE cartridge. The silica cartridge was then washed with 2 ml EtOH and PAF and lyso-PAF were eluted with 1.1 ml MeCN/water (60/40, v/v). Samples were evaporated to dryness under reduced pressure in a Speed-Vac concentrator (drying rate set at ‘low') and re-suspended in 50 μl of the HPLC mobile phase (hexane/isopropanol, 20 mM aqueous ammonium acetate, 3/4/0.7 (v/v/v)). Analysis of PAF and lyso-PAF was then performed by LC-MS/MS by the measurement of the PAF/2H4-PAF ratio ((m/z 508 → 59)/(m/z 512 → 59)) and lyso-PAF/2H4-PAF ratio ((m/z 466 → 377)/(m/z 512 → 59)), respectively. Quantitation was achieved using standard curves generated by analysis (ratio determination) of solutions containing increasing amounts of PAF or lyso-PAF and a fixed amount of 2H4-PAF.
Induction of cyclooxygenase-2 and stimulation of PGE2 biosynthesis
In experiments where PGE2 biosynthesis was investigated, PMN suspensions in HBSS containing 1.6 mM CaCl2 (37°C, 107 cells ml−1) were pre-incubated 4 h with 700 pM GM-CSF, 1.5 nM TNF-α and 10 μM cytochalasin B (to induce cyclooxygenase-2 (COX-2) expression), then stimulated with 100 nM A23187 for 5 min. Incubations were stopped by placing the samples in an ice-water bath. Cell suspensions were immediately centrifuged (600 g, 5 min at 22°C), and PGE2 was measured in the resulting supernatants using a PGE2 ELISA according to the manufacturer's instructions.
Stimulation and analysis of phospholipase D activity
phospholipase D (PLD) activity was measured as described previously (Marcil et al.,. 1999). Briefly, PMN were pre-labeled with 1-O-[3H]alkyl-2-lyso-phosphatidylcholine (2 μCi for 107 PMN) for 90 min at room temperature. The PMN suspensions in HBSS containing 0.8 mM CaCl2 (107 cells ml−1) were warmed up to 37°C, then treated with increasing concentrations of pyrrophenone and 10 μM cytochalasin B for 10 and 5 min, respectively. The suspensions were then stimulated for 10 min with 1 nM fMLP or 100 nM thapsigargin in the presence of 1% EtOH. Incubations were stopped by the addition of 3.6 volumes of a cold (4°C) stop solution (chloroform/MeOH/hydrochloric acid (50/100/1, v/v/v)) containing unlabeled phosphatidylethanol as internal standard. The lipids were extracted (Bligh and Dyer, 1959), the organic phase was evaporated to dryness under a stream of nitrogen and samples were spotted on pre-washed silica gel 60 thin layer chromotography (TLC) plates. Phosphatidylethanol was resolved from the other lipids using the solvent mixture chloroform/MeOH/acetic acid (65/15/2, v/v/v). Lipids were visualized by Coomassie Brilliant Blue staining and the different lipid classes were scraped off the plates. Radioactivity in the phosphatidylethanol fraction was measured by liquid scintillation counting and the results were corrected for background radioactivity and quenching. In all experimental settings, 0.3 U ml−1 ADA was added to the incubation media to eliminate the inhibitory constraint exerted by extracellular adenosine (Krump et al., 1997).
Data analysis
Average results are presented as means±s.e.m.
Materials
2H4-PAF (1-O-(7,7,8,8-2H4)hexadecyl-2-acetyl-glyceryl-3-phosphorylcholine), 2H8-AA ((5,6,8,9,11,12,14,15-2H8)-arachidonic acid), LTB4, 20-OH-LTB4, 5-HETE and the PGE2 ELISA kit were purchased from Cayman Chemical (Ann Arbor, MI, USA). A23187, ionomycin, AA, cytochalasin B, DMSO, PAF, PGB1, PGB2, 19-OH-PGB1 and 19-OH-PGB2 were obtained from the Sigma Chemical Co. (Saint Louis, MO, USA). ADA was purchased from Roche Applied Science (Indianapolis, IN, USA). Thapsigargin was purchased from Research Biochemicals International (Natick, MA, USA). Pyrrophenone was a generous gift from Dr Kaoru Seno (Shionogi Research Laboratories, Osaka, Japan). Ficoll–Paque medium and Trypan blue were purchased from Wisent Laboratories (St-Bruno, Québec, Canada). C18 SPE cartridges (60 mg, 3 ml capacity) were obtained form Waters Corporation (Milford, MA, USA) and Bond Elut SPE silica cartridges (100 mg, 2 ml) were purchased from Varian (Harbor City, CA, USA).
Results
Pyrrophenone inhibits LT, PAF and PGE2 biosynthesis in PMN
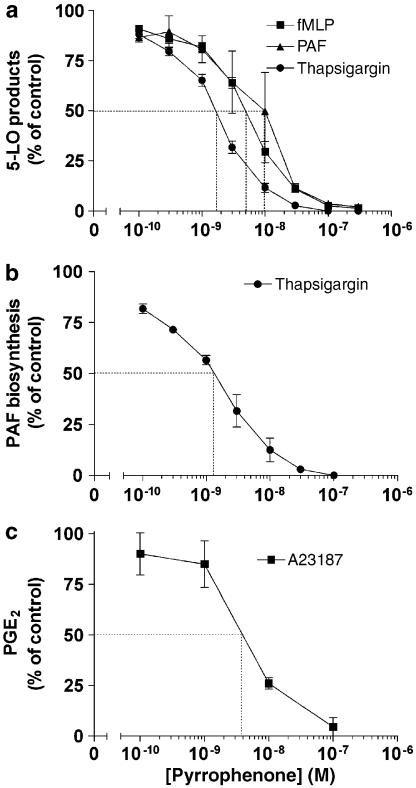
A first series of experiments was undertaken to characterize the inhibitory effect of the cPLA2α inhibitor pyrrophenone on 5-LO products and PAF biosynthesis in PMN activated by either the natural agonists fMLP and PAF or the pharmacological agent thapsigargin. As shown in Figure 2a, pyrrophenone suppressed, in a concentration-dependent manner, the fMLP-, PAF- and thapsigargin-induced biosynthesis of 5-LO products in PMN with IC50s of 1–10 nM. In each experimental condition, the concentration–inhibition curves of the different 5-LO products by pyrrophenone showed similar IC50s (data not shown). Figure 2b shows that thapsigargin-induced PAF biosynthesis is similarly inhibited by pyrrophenone with an IC50 of 1–2 nM. In separate experiments, PMN were exposed to GM-CSF, TNF-α and cytochalasin B for induction of COX-2 expression, and PGE2 synthesis was stimulated with the ionophore A23187; as shown in Figure 2c, pyrrophenone also inhibited PGE2 biosynthesis in PMN with an IC50 of 3–4 nM. In other experiments, pyrrophenone similarly inhibited PGE2 biosynthesis in PMN treated with the priming agents (to induce COX-2 expression) and stimulated with fMLP (data not shown). In the experiments shown in Figure 2, the average amount (±s.e.m.) of PAF biosynthesized following activation with thapsigargin was 5.2 (±0.3) pmol (106 PMN)−1. The average amounts of 5-LO products generated by thapsigargin-, fMLP- and PAF-activated PMN were 114 (±35), 55 (±13), and 41 (±5) pmol (106 PMN)−1, respectively. The average amount of PGE2 formed by A23187-treated PMN was 3.4 (±0.4) pmol (106 PMN)−1. Finally, addition of pyrrophenone 30 min before the stimulation of whole heparinized human blood with 10 μM ionomycin resulted in dose–inhibition curves of the biosynthesis of 5-LO products with IC50s around 1 μM (data not shown).
Figure 2.
Inhibitory effects of pyrrophenone on lipid mediator biosynthesis in activated human PMN. (a) PMN suspensions (37°C, 5 × 106 cells ml−1) were treated with increasing concentrations of pyrrophenone and stimulated with either 300 nM fMLP, 300 nM PAF or 100 nM thapsigargin as described in Methods. Incubations were stopped by the addition of 0.5 volume of a cold (4°C) stop solution (MeOH/MeCN, 1/1) containing 12.5 ng of both 19-OH-PGB2 and PGB2 (as internal standards) and 5-LO products were analyzed by RP-HPLC as described in Methods. (b) PMN suspensions were treated with pyrrophenone and stimulated with 100 nM thapsigargin as described above. Incubations were stopped by the addition of 1 volume of EtOH containing 5 ng of 2H4-PAF. PAF and lyso-PAF were extracted and analyzed by LC/MS/MS as described in Methods. (c) PMN suspensions (37°C, 107 cells ml−1) were treated 4 h with 700 pM GM-CSF, 1.5 nM TNF-α and 10 μM cytochalasin B for optimal COX-2 expression. Cell suspensions were incubated with increasing concentrations of pyrrophenone, then stimulated with 100 nM A23187 for 5 min. Incubations were stopped and PGE2 was analyzed by ELISA in PMN supernatants as described in Methods. In all experimental settings, ADA (0.3 U ml−1) and pyrrophenone were added 10 min before the addition of the agonists. Data represent the mean (±s.e.m.) of three separate experiments, each performed in duplicate.
Inhibition of LT and PAF biosynthesis correlates with the inhibition of AA and lyso-PAF release
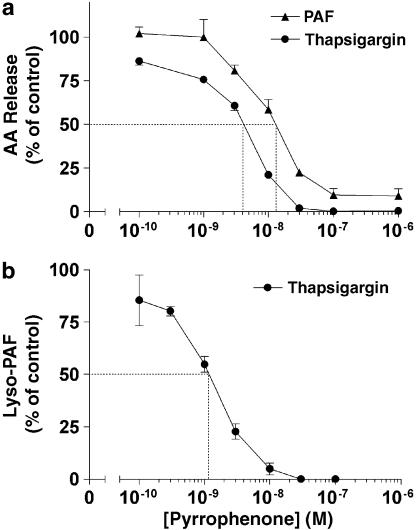
We next directly assessed the effects of pyrrophenone on the release of the products of cPLA2α-mediated phospholipid hydrolysis, namely AA and lyso-PAF. As shown in Figures 3a and b, treatment of PMN with pyrrophenone also led to the concentration-dependent inhibition of both AA and lyso-PAF release. Moreover, the pyrrophenone-mediated inhibition of AA and lyso-PAF release closely paralleled the dose–inhibition curves (similar IC50 values) observed for LT and PAF biosynthesis in both agonist- and thapsigargin-activated PMN (Figure 2), supporting that the inhibition by pyrrophenone of LT and PAF biosynthesis is the consequence of its ability to inhibit cPLA2α catalysis and AA and lyso-PAF release. In these experiments, the average amounts of free AA measured in PAF- and thapsigargin-stimulated PMN suspensions were 110±15 and 338±75 pmol (106 PMN)−1.
Figure 3.
Inhibitory effects of pyrrophenone on AA and lyso-PAF release in activated human PMN. (a) PMN suspensions (37°C, 5 × 106 cells ml−1) were incubated in the presence of increasing concentrations of pyrrophenone, then stimulated with either 100 nM thapsigargin or 300 nM PAF for 5 and 2 min, respectively, as described in Methods. Incubations were stopped by the addition of 0.5 volume of a cold (4°C) stop solution (MeOH/MeCN, 1/1, v/v) containing 12.5 ng of both 19-OH-PGB2 and PGB2, and 20 ng of 2H8-AA as internal standards. Supernatants were collected and analyzed for AA content by LC/MS as described in Methods. (b) PMN suspensions (37°C, 5 × 106 cells ml−1) were stimulated with 100 nM thapsigargin for 10 min as described in Methods. Incubations were stopped by the addition of 1 volume of EtOH containing 5 ng of 2H4-PAF. Supernatants were collected and lyso-PAF was extracted and analyzed as described in Methods. In all experimental settings, 0.3 U ml−1 ADA and pyrrophenone were added 10 min before the addition of the stimuli. Data represent the mean (±s.e.m.) of three separate experiments, each performed in duplicate.
Reversal of the inhibitory effect of pyrrophenone on LT biosynthesis by exogenous AA
The conclusive demonstration that pyrrophenone inhibits LT biosynthesis in activated human PMN through substrate deprivation (rather than through a nonspecific effect on the 5-LO-pathway) implies that LT biosynthesis can be restored by substrate replenishment. As shown in Figure 4, the addition of AA fully restored the biosynthesis of LT in thapsigargin-activated PMN treated with pyrrophenone. Similar results were also obtained with fMLP-activated PMN (data not shown).
Figure 4.
Reversal of the inhibitory effect of pyrrophenone on thapsigargin-induced LT biosynthesis. PMN suspensions (37°C, 5 × 106 cells ml−1) were stimulated with 100 nM thapsigargin for 10 min as described in Methods. AA was added simultaneously with thapsigargin. ADA (0.3 U ml−1) and 100 nM pyrrophenone were added 10 min before the stimulation of the cells with thapsigargin. Incubations were stopped by the addition of 0.5 volume of a cold (4°C) stop solution (MeOH/MeCN, 1/1) containing 12.5 ng of both 19-OH-PGB2 and PGB2 as internal standards and 5-LO products were analyzed as described in Methods. Data represent the mean (± s.e.m.) of three separate experiments, each performed in duplicate.
Pyrrophenone is more potent and specific than MAFP and AACOCF3
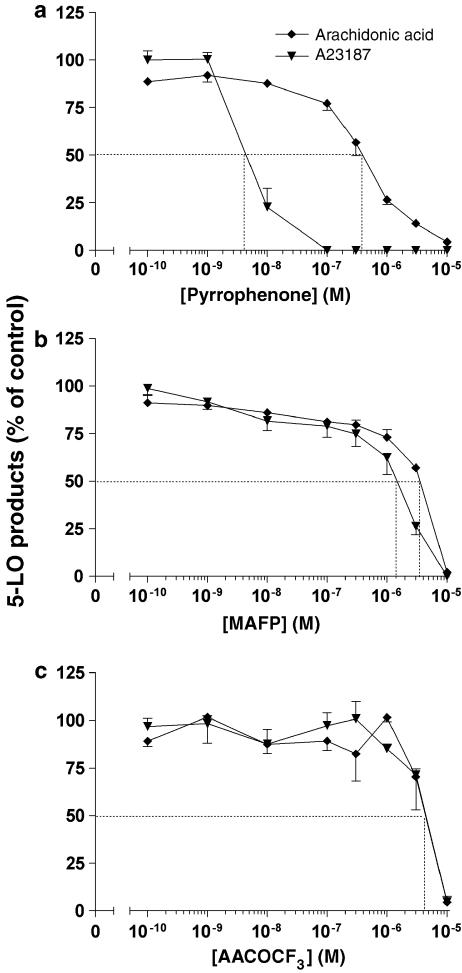
In the next series of experiments, we undertook to compare the ability of pyrrophenone and two other frequently used cPLA2α inhibitors, MAFP and AACOCF3, on LT biosynthesis in PMN. The drugs were tested in two distinct experimental conditions, that is, in A23187-stimulated PMN, in which model AA release and LT biosynthesis involve the cPLA2α; and in AA-stimulated PMN, in which condition LT biosynthesis occurs from exogenous AA and is therefore independent of cPLA2α activity (Surette et al., 1999). In these experiments, as expected, PAF biosynthesis was important in A23187-stimulated PMN and undetectable in AA-stimulated PMN (data not shown), confirming the involvement of cPLA2α in the former but not the latter condition. As shown in Figure 5a, pyrrophenone inhibited 5-LO product biosynthesis in A23187-stimulated PMN with an IC50 (5 nM) similar to those observed with other stimuli of LT biosynthesis (Figure 2a); pyrrophenone also inhibited AA-induced LT biosynthesis, however, at a 100-fold higher concentration, clearly indicating that pyrrophenone also exerts unspecific inhibitory effects on the 5-LO pathway when used at concentrations greater than 100 nM. In contrast, both MAFP and AACOCF3 proved to be much less potent than pyrrophenone (IC50s ⩾1 μM) at inhibiting A23187-induced LT biosynthesis. The most striking and unexpected observation was, however, that MAFP and AACOCF3 showed little if any difference in their abilities to inhibit LT biosynthesis in the two experimental conditions tested, clearly demonstrating that these two inhibitors, at the concentrations required to inhibit AA release by the cPLA2α also exerted unspecific effects on the 5-LO pathway (possibly by a direct inhibition of the 5-LO and/or FLAP).
Figure 5.
Comparative effects of (a) pyrrophenone, (b) MAFP and (c) AACOCF3 on A23187- and AA-induced 5-LO product biosynthesis in human PMN. PMN suspensions (37°C, 5 × 106 cells ml−1) were treated with ADA (0.3 U ml−1) and increasing concentrations of cPLA2α inhibitors 10 min before stimulation with either 100 nM A23187 or 2.5 μM AA for 5 min as described in Methods. Incubations were stopped by the addition of 0.5 volume of cold (4°C) stop solution (MeOH/MeCN, 50/50, v/v) containing 12.5 ng of both 19-OH-PGB2 and PGB2 as internal standards. 5-LO products were analyzed as described in Methods. Data represent the mean (±s.e.m.) of three separate experiments, each performed in duplicate.
The inhibitory effect of pyrrophenone on LT biosynthesis is reversible
MAFP has been shown to irreversibly inhibit cPLA2α by forming a stable complex with the enzyme (Ghomashchi et al.,. 1999), whereas AACOCF3 has been shown to slowly but tightly associate with cPLA2α (Trimble et al., 1993). The occupation of the catalytic site of cPLA2α in a reversible manner is the suggested mechanism by which pyrrophenone inhibits AA release (Ono et al., 2002). In order to assess the reversibility of LT biosynthesis inhibition by pyrrophenone in activated PMN, we performed a series of experiments in which pyrrophenone-treated PMN were washed with incubation buffer or with autologous plasma. Table 1 shows that washing of the pyrrophenone-treated PMN with incubation buffer (HBSS) before stimulation with thapsigargin does not restore LT biosynthesis. In contrast, washing of PMN with autologous plasma fully restored the ability of thapsigargin-activated PMN to generate LT upon stimulation with thapsigargin, demonstrating that the pyrrophenone-mediated inhibition of cPLA2α is a reversible process.
Table 1.
Reversibility of pyrrophenone-induced inhibition of 5-LO products biosynthesis in human PMN
|
5-LO products biosynthesis (% of control) |
|||
|---|---|---|---|
| Not washed |
Washing |
||
| HBSS | Plasma | ||
| Thapsigargin | 100 | 75±7 | 76±8 |
| Thapsigargin+Pyrrophenone | 0.8±0.2 | 0.5±0.2 | 75±7 |
Abbreviations: HBSS, Hank's balanced salt solution; PMN, polymorphonuclear neutrophil.
Pre-warmed human PMN suspensions (37°C, 5 × 106 cells ml−1) were incubated 10 min in the presence of 100 nM pyrrophenone. The cell suspensions were then centrifuged (600 g, 2 min) and the cell pellets were re-suspended and incubated with pre-warmed (37°C) HBSS containing 1.6 mM CaCl2 or human plasma for 20 min. All PMN suspensions were then washed once again with HBSS containing 1.6 mM CaCl2 then re-suspended in HBSS containing 1.6 mM CaCl2, 0.3 U ml−1 ADA and 100 nM thapsigargin for 10 min. Incubations were stopped by the addition of 0.5 volume of a cold (4°C) stop solution (MeOH/MeCN, 50/50, v/v) containing 12.5 ng of both 19-OH-PGB2 and PGB2 as internal standards. Supernatants were collected and analyzed for 5-LO products as described in Methods.
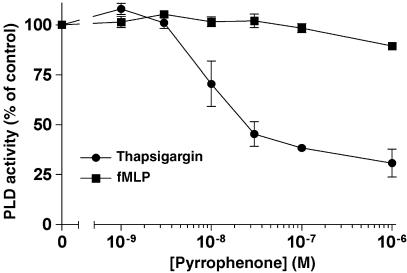
Pyrrophenone does not inhibit PLD activity
In the next experiments, we further assessed the specificity of pyrrophenone by investigating the putative inhibitory effect of the drug in fMLP- and thapsigargin-induced activation of PLD in human PMN. As shown in Figure 6, pyrrophenone did not inhibit fMLP-induced PLD activity at concentrations up to 1 μM. However, pyrrophenone progressively inhibited PLD activity in PMN activated with thapsigargin. The observed inhibition of PLD activity by pyrrophenone in thapsigargin-activated PMN was completely reversed by the addition of AA to the incubation media (data not shown).
Figure 6.
Effect of pyrrophenone on PLD activity in activated PMN. PMN suspensions (37°C, 107 cells ml−1) were labeled with 1-O-[3H]alkyl-2-lyso-phosphatidylcholine. Cells were then stimulated for 10 min with either 1 nM fMLP or 100 nM thapsigargin in the presence of 1% EtOH (final concentration). Incubations were stopped by the addition of 3.6 volumes of a cold (4°C) stop solution (CH3Cl/MeOH/HCl, 50/100/1, v/v/v) containing unlabeled 1-O-alkyl-2-lyso-phosphatidylethanol as internal standard. 1-O-[3H]alkyl-2-lyso-phosphatidylcholine and phosphatidylethanol were separated by TLC and analyzed for radioactivity content as described in Methods. Data represent the mean (±s.e.m.) of three separate experiments.
Discussion and conclusions
Thromboxanes, PG, LT and lipoxins represent families of metabolites of AA implicated in numerous physiological and pathophysiological processes including host defense and inflammatory diseases. Major efforts have therefore been invested in the past three decades to develop PLA2 inhibitors and investigate the mechanisms of lipid mediator biosynthesis and to assess their biological functions. Mepacrine and p-bromophenacyl bromide were precious tools to evaluate the role of eicosanoids in early studies. However, the high IC50 values of these compounds for the inhibition of AA release and their lack of specificity has limited their utilization, and other more potent PLA2 inhibitors such as AACOCF3 and MAFP have been frequently used in eicosanoid research (Meyer et al., 2005).
It has recently been clearly established that cPLA2α is the major PLA2 implicated in the biosynthesis of LT, PG and PAF. Indeed, studies implicating cPLA2α-deficient mice demonstrated an essential role for this enzyme in the biosynthesis of eicosanoids and PAF, as well as in the regulation of the expression of enzymes involved in their biosynthesis (Bonventre et al., 1997; Uozumi et al., 1997; Fujishima et al., 1999; Shindou et al., 2000). Therefore, the availability of potent and selective inhibitors of cPLA2α was critical for future development in lipid mediator research.
A new class of cPLA2α inhibitors has been recently developed, the pyrrolidine inhibitors comprising pyrrolidine-1 and pyrrophenone. These were shown to inhibit cPLA2α in vitro with an important gain of potency compared to AACOCF3 and MAFP (Ono et al., 2002). Moreover, the IC50 of the pyrrolidine inhibitors for cPLA2α was at least 2 orders of magnitude lower than for several other members of the PLA2 family, namely group IB, IIA, IVC, V, VIB and X PLA2 (Seno et al., 2000, 2001; Ghomashchi et al., 2001; Ono et al., 2002), indicating a good level of specificity of pyrrolidine inhibitors for cPLA2α.
In the present study, we characterized the effect of pyrrophenone on LT, PG and PAF biosynthesis in activated PMN, a major source of bioactive lipid mediators in man and animals. As expected, the biosynthesis of eicosanoids and PAF was inhibited by pyrrophenone at low nanomolar concentrations, in agreement with a central role of cPLA2α in the biosynthesis of these lipid mediators in activated-PMN (Syrbu et al., 1999; Degousee et al., 2002). Our results show that pyrrophenone potently inhibits three different classes of lipid mediators of inflammation in PMN, activated by their natural ligands fMLP and PAF as well as by the commonly used pharmacological agents A23187, ionomycin and thapsigargin and are in agreement with the reported inhibitory effects of pyrrolidine-1 on fMLP- and zymosan-induced AA release and PAF biosynthesis in human PMN (Rubin et al., 2005). The IC50s obtained for inhibition of LT and PGE2 biosynthesis are very consistent with those also obtained with pyrrophenone for inhibition of the A23187-induced LTC4 and PGE2 biosynthesis in THP-1 (Seno et al., 2001; Ono et al., 2002). It is very likely that the much increased IC50 observed for inhibition of 5-LO product biosynthesis in whole blood as opposed to isolated PMN in a protein-free buffer is the consequence of binding of the lipophilic drug to plasma proteins, albumin in particular. The complete reversal of LT biosynthesis inhibition by exogenous AA in pyrrophenone-treated, thapsigargin-activated PMN is in agreement with the effect of exogenous lyso-PAF on PAF biosynthesis described earlier using the same experimental conditions (Flamand et al., 2006a), suggesting that a submicromolar concentration of pyrrophenone results in the specific inhibition of both AA and Lyso-PAF release in PMN.
An important result of the present study is the observation that in contrast to pyrrophenone, MAFP and AACOCF3 inhibited both the A23187- and the AA-stimulated LT biosynthesis with similar IC50. Considering that the cPLA2α is not involved in LT biosynthesis induced by exogenous AA, these data clearly demonstrate that in addition to their ability to inhibit the cPLA2α, MAFP and AACOCF3 exert nonspecific effects on the 5-LO pathway resulting in inhibition of LT biosynthesis in intact PMN. This is in agreement with a recent study that showed an inhibitory effect of AACOCF3 on 5-LO activity in a broken cell assay (Fonteh, 2002) and with another study which demonstrated that both MAFP and AACOCF3 bound to a binding site for AA on the 5-LO-activating protein with an affinity similar to that of AA itself (Charleson et al., 1994). It is noteworthy that pyrrophenone also appeared to exert an effect distinct from PLA2 inhibition as it also blocked AA-induced LT biosynthesis; however, this ‘non-PLA2' inhibition by pyrrophenone occurred at a concentration 100-fold greater than that required for inhibition of the A23187-induced LT biosynthesis (IC50 ∼5 nM). Taken together, these comparative data clearly emphasize that pyrrophenone is a more potent and selective inhibitor of cPLA2α than MAFP and AACOCF3 and is therefore a more reliable pharmacological tool for investigation of eicosanoid and PAF biosynthesis and action in intact cells and tissues. Our recent report that AA regulates 5-LO translocation to nuclear structures in activated PMN (Flamand et al., 2006b) provides an example of the usefulness of pyrrophenone in eicosanoid research. Indeed, pyrrophenone efficiently inhibited 5-LO translocation induced by all PMN stimuli tested and its effects were prevented by exogenous AA; in these experiments pyrrophenone turned out to be the key pharmacological tool in defining this novel regulatory mechanism of 5-LO activation.
The present study also demonstrated that the inhibitory effect of pyrrophenone on cPLA2α was reversible in intact PMN. Indeed, lipid mediator biosynthesis was recovered when pyrrophenone-treated human PMN were washed with autologous plasma before stimulation, in agreement with the initial report of the reversibility of the pyrrophenone-mediated inhibition of AA release in a dilution system using the phosphatidylcholine/Triton X-100 micelle cPLA2α assay (Ono et al., 2002). Our data, however, emphasized that pyrrophenone is not easily washed out from whole cells, previously exposed to the drug, with buffered salt solutions and that recovery of cPLA2α activity requires washing in the presence of plasma, enabling the trapping of the lipophilic drug by plasma proteins (albumin in particular).
Finally, the effect of pyrrophenone on PLD activity was also investigated in thapsigargin- and fMLP-activated PMN in order to assess the activity of the drug on this particular PL. PLD is an important enzyme in the regulation of PMN functions implicating vesicular transport such as phagocytosis and degranulation. PLD activity was not inhibited by pyrrophenone in fMLP-activated PMN. In contrast, the data presented herein showed that pyrrophenone inhibited PLD activity in thapsigargin-activated PMN, an effect that could be attributed to inhibition of cPLA2α and biosynthesis of LTB4, which acts as the direct activator of PLD in these experimental conditions (Grenier et al., 2003). These results demonstrate that pyrrophenone has no direct inhibitory effect on PLD.
In conclusion, the results presented herein demonstrate that pyrrophenone is a very potent and specific inhibitor of cPLA2α and consequently of the biosynthesis of lipid mediators in human PMN (and whole blood). This compound provides a useful pharmacological tool to investigate the biosynthesis of lipid mediators in whole cells and tissues, and to assess their roles in biological processes.
Acknowledgments
We thank Dr Kaoru Seno for providing the cPLA2α inhibitor pyrrophenone. This work was supported by The Arthritis Society of Canada and the Canadian Institutes of Health Research. Nicolas Flamand is the recipient of a postdoctoral award from the CIHR.
Abbreviations
- AA
arachidonic acid
- AACOCF3
arachidonoyl-trifluoromethylketone
- ADA
adenosine deaminase
- cPLA2α
group IVA phospholipase A2
- EtOH
ethanol
- LT
leukotriene
- MAFP
methyl-arachidonoyl-fluoro-phosphonate
- MeCN
acetonitrile
- MeOH
methanol
- PAF
platelet-activating factor
- PG
prostaglandin
- PLD
phospholipase D
- RP
reverse phase
- SPE
solid-phase extraction
Conflict of interest
The authors state no conflict of interest.
References
- Balsinde J, Dennis EA. Distinct roles in signal transduction for each of the phospholipase A2 enzymes present in P388D1 macrophages. J Biol Chem. 1996;271:6758–6765. doi: 10.1074/jbc.271.12.6758. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bonventre JV, Huang Z, Taheri MR, O'Leary E, Li E, Moskowitz MA, et al. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- Borgeat P, Picard S, Vallerand P, Bourgoin S, Odeimat A, Sirois P, et al. Automated on-line extraction and profiling of lipoxygenase products of arachidonic acid by high-performance liquid chromatography. Methods Enzymol. 1990;187:98–116. doi: 10.1016/0076-6879(90)87014-t. [DOI] [PubMed] [Google Scholar]
- Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Charleson S, Evans JF, Leger S, Perrier H, Prasit P, Wang Z, et al. Structural requirements for the binding of fatty acids to 5-lipoxygenase-activating protein. Eur J Pharmacol. 1994;267:275–280. doi: 10.1016/0922-4106(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Degousee N, Ghomashchi F, Stefanski E, Singer A, Smart BP, Borregaard N, et al. Groups IV, V, and X phospholipases A2s in human neutrophils: role in eicosanoid production and gram-negative bacterial phospholipid hydrolysis. J Biol Chem. 2002;277:5061–5073. doi: 10.1074/jbc.M109083200. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Omeir R, Arreaza G, Salehani D, Prestwich GD, Huang Z, et al. Methyl arachidonyl fluorophosphonate: a potent irreversible inhibitor of anandamide amidase. Biochem Pharmacol. 1997;53:255–260. doi: 10.1016/s0006-2952(96)00830-1. [DOI] [PubMed] [Google Scholar]
- Flamand N, Lefebvre J, Lapointe G, Picard S, Lemieux L, Bourgoin SG, et al. Inhibition of platelet-activating factor biosynthesis by adenosine and histamine in human neutrophils: involvement of cPLA2α and reversal by lyso-PAF. J Leukocyte Biol. 2006a;79:1043–1051. doi: 10.1189/jlb.1005614. [DOI] [PubMed] [Google Scholar]
- Flamand N, Lefebvre J, Surette ME, Picard S, Borgeat P. Arachidonic acid regulates the translocation of 5-lipoxygenase to the nuclear membranes in human neutrophils. J Biol Chem. 2006b;281:129–136. doi: 10.1074/jbc.M506513200. [DOI] [PubMed] [Google Scholar]
- Fonteh AN. Differential effects of arachidonoyl trifluoromethyl ketone on arachidonic acid release and lipid mediator biosynthesis by human neutrophils. Evidence for different arachidonate pools. Eur J Biochem. 2002;269:3760–3770. doi: 10.1046/j.1432-1033.2002.03070.x. [DOI] [PubMed] [Google Scholar]
- Fujishima H, Sanchez Mejia RO, Bingham CO, III, Lam BK, Sapirstein A, Bonventre JV, et al. Cytosolic phospholipase A2 is essential for both the immediate and the delayed phases of eicosanoid generation in mouse bone marrow-derived mast cells. Proc Natl Acad Sci USA. 1999;96:4803–4807. doi: 10.1073/pnas.96.9.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghomashchi F, Loo R, Balsinde J, Bartoli F, Apitz-Castro R, Clark JD, et al. Trifluoromethyl ketones and methyl fluorophosphonates as inhibitors of group IV and VI phospholipases A2: structure–function studies with vesicle, micelle, and membrane assays. Biochim Biophys Acta. 1999;1420:45–56. doi: 10.1016/s0005-2736(99)00056-5. [DOI] [PubMed] [Google Scholar]
- Ghomashchi F, Stewart A, Hefner Y, Ramanadham S, Turk J, Leslie CC, et al. A pyrrolidine-based specific inhibitor of cytosolic phospholipase A2 blocks arachidonic acid release in a variety of mammalian cells. Biochim Biophys Acta. 2001;1513:160–166. doi: 10.1016/s0005-2736(01)00349-2. [DOI] [PubMed] [Google Scholar]
- Grenier S, Flamand N, Pelletier J, Naccache PH, Borgeat P, Bourgoin SG. Arachidonic acid activates phospholipase D in human neutrophils. Essential role of endogenous leukotriene B4 and inhibition by adenosine A2A receptor engagement. J Leukocyte Biol. 2003;73:111–222. doi: 10.1189/jlb.0702371. [DOI] [PubMed] [Google Scholar]
- Harrison KA, Clay KL, Murphy RC. Negative ion electrospray and tandem mass spectrometric analysis of platelet-activating factor (PAF) (1-hexadecyl-2-acetyl-glycerophosphocholine) J Mass Spectrom. 1999;34:330–335. doi: 10.1002/(SICI)1096-9888(199904)34:4<330::AID-JMS798>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Kell PJ, Creer MH, Crown KN, Wirsig K, McHowat J. Inhibition of platelet-activating factor (PAF) acetylhydrolase by methyl arachidonyl fluorophosphonate potentiates PAF synthesis in thrombin-stimulated human coronary artery endothelial cells. J Pharmacol Exp Ther. 2003;307:1163–1170. doi: 10.1124/jpet.103.055392. [DOI] [PubMed] [Google Scholar]
- Krump E, Picard S, Mancini JA, Borgeat P. Suppression of leukotriene B4 biosynthesis by endogenous adenosine in ligand-activated human neutrophils. J Exp Med. 1997;186:1401–1406. doi: 10.1084/jem.186.8.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lio YC, Reynolds LJ, Balsinde J, Dennis EA. Irreversible inhibition of Ca2+-independent phospholipase A2 by methyl arachidonyl fluorophosphonate. Biochim Biophys Acta. 1996;1302:55–60. doi: 10.1016/0005-2760(96)00002-1. [DOI] [PubMed] [Google Scholar]
- Marcil J, Harbour D, Houle MG, Naccache PH, Bourgoin S. Monosodium urate-crystal-stimulated phospholipase D in human neutrophils. Biochem J. 1999;337:185–192. [PMC free article] [PubMed] [Google Scholar]
- Meyer MC, Rastogi P, Beckett CS, McHowat J. Phospholipase A2 inhibitors as potential anti-inflammatory agents. Curr Pharm Des. 2005;11:1301–1312. doi: 10.2174/1381612053507521. [DOI] [PubMed] [Google Scholar]
- Ono T, Yamada K, Chikazawa Y, Ueno M, Nakamoto S, Okuno T, et al. Characterization of a novel inhibitor of cytosolic phospholipase A2, pyrrophenone. Biochem J. 2002;363:727–735. doi: 10.1042/0264-6021:3630727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BB, Downey GP, Koh A, Degousee N, Ghomashchi F, Nallan L, et al. Cytosolic phospholipase A2α is necessary for platelet-activating factor biosynthesis, efficient neutrophil-mediated bacterial killing, and the innate immune response to pulmonary infection: cPLA2α does not regulate neutrophil NADPH oxidase activity. J Biol Chem. 2005;280:7519–7529. doi: 10.1074/jbc.M407438200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seno K, Okuno T, Nishi K, Murakami Y, Watanabe F, Matsuura T, et al. Pyrrolidine inhibitors of human cytosolic phospholipase A2. J Med Chem. 2000;43:1041–1044. doi: 10.1021/jm9905155. [DOI] [PubMed] [Google Scholar]
- Seno K, Okuno T, Nishi K, Murakami Y, Yamada K, Nakamoto S, et al. Pyrrolidine inhibitors of human cytosolic phospholipase A2. Part 2: synthesis of potent and crystallized 4-triphenylmethylthio derivative ‘pyrrophenone'. Bioorg Med Chem Lett. 2001;11:587–590. doi: 10.1016/s0960-894x(01)00003-8. [DOI] [PubMed] [Google Scholar]
- Shindou H, Ishii S, Uozumi N, Shimizu T. Roles of cytosolic phospholipase A2 and platelet-activating factor receptor in the Ca2+-induced biosynthesis of PAF. Biochem Biophys Res Commun. 2000;271:812–817. doi: 10.1006/bbrc.2000.2723. [DOI] [PubMed] [Google Scholar]
- Surette ME, Krump E, Picard S, Borgeat P. Activation of leukotriene synthesis in human neutrophils by exogenous arachidonic acid: inhibition by adenosine A2A receptor agonist and crucial role of autocrine activation by leukotriene B4. Mol Pharmacol. 1999;56:1055–1062. doi: 10.1124/mol.56.5.1055. [DOI] [PubMed] [Google Scholar]
- Surette ME, Palmantier R, Gosselin J, Borgeat P. Lipopolysaccharides prime whole human blood and isolated neutrophils for the increased synthesis of 5-lipoxygenase products by enhancing arachidonic acid availability: involvement of the CD14 antigen. J Exp Med. 1993;178:1234–1355. doi: 10.1084/jem.178.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrbu SI, Waterman WH, Molski TF, Nagarkatti D, Hajjar JJ, Sha'Afi RI. Phosphorylation of cytosolic phospholipase A2 and the release of arachidonic acid in human neutrophils. J Immunol. 1999;162:2334–2340. [PubMed] [Google Scholar]
- Trimble LA, Street IP, Perrier H, Tremblay NM, Weech PK, Bernstein MA. NMR structural studies of the tight complex between a trifluoromethyl ketone inhibitor and the 85-kDa human phospholipase A2. Biochemistry. 1993;32:12560–12565. doi: 10.1021/bi00210a002. [DOI] [PubMed] [Google Scholar]
- Uozumi N, Kume K, Nagase T, Nakatani N, Ishii S, Tashiro F, et al. Role of cytosolic phospholipase A2 in allergic response and parturition. Nature. 1997;390:618–622. doi: 10.1038/37622. [DOI] [PubMed] [Google Scholar]