Abstract
Studies on the axonal transport of neurofilament proteins in cultured neurons have shown they move at fast rates, but their overall rate of movement is slow because they spend most of their time not moving. Using correlative light and electron microscopy, we have shown that these proteins move in the form of assembled neurofilament polymers. However, the polypeptide composition of these moving polymers is not known. To address this, we visualized neurofilaments in cultured neonatal mouse sympathetic neurons using GFP-tagged neurofilament protein M and performed time-lapse fluorescence microscopy of naturally occurring gaps in the axonal neurofilament array. When neurofilaments entered the gaps, we stopped them in their tracks using a rapid perfusion and permeabilization technique and then processed them for immunofluorescence microscopy. To compare moving neurofilaments to the total neurofilament population, most of which are stationary at any point in time, we also performed immunofluorescence microscopy on neurofilaments in detergent-splayed axonal cytoskeletons. All neurofilaments, both moving and stationary, contained NFL, NFM, peripherin and α-internexin along >85% of their length. NFH was absent due to low expression levels in these neonatal neurons. These data indicate that peripherin and α-internexin are integral and abundant components of neurofilament polymers in these neurons and that both moving and stationary neurofilaments in these neurons are complex heteropolymers of at least four different neuronal intermediate filament proteins.
Keywords: neurofilament, peripherin, internexin, axon, cytoskeleton
INTRODUCTION
Neurofilaments, which are the intermediate filaments of neurons, are remarkable for the diversity of their polypeptide composition. Five different neurofilament proteins are commonly recognized: the low, medium and high molecular weight neurofilament triplet proteins (NFL, NFM and NFH), α-internexin and peripherin, and these proteins can co-assemble with each other in many different combinations [Fliegner and Liem, 1991; Lee and Cleveland, 1996; Leung et al., 1998]. The neurofilament triplet proteins and internexin are members of the Type IV intermediate filament sub-family, which also includes nestin, whereas peripherin is a member of the Type III sub family, which also includes vimentin, desmin and glial fibrillary acidic protein (GFAP).
Most neurons express several different neurofilament proteins, but the identity of these proteins changes in a sequential and overlapping manner during differentiation and development and may also vary with respect to neuronal cell type [Nixon and Shea, 1992]. Neuronal precursor cells express vimentin and nestin but the expression of these proteins declines when these cells exit the cell cycle and differentiate into neurons. In adults, nestin expression is confined to neuronal stem cells [Lendahl et al., 1990] and vimentin expression is retained in only a few unusual neurons [Drager, 1983; Schwob et al., 1986], though it can be upregulated in response to injury [Perlson et al., 2005]. Alpha-internexin, peripherin, NFL and NFM are not expressed until the onset of neuronal differentiation, with α-internexin often preceding the other three proteins [Kaplan et al., 1990; Troy et al., 1990; Fliegner et al., 1994]. Alpha-internexin, NFL and NFM are widely expressed throughout the developing central and peripheral nervous system [Carden et al., 1987; Kaplan et al., 1990; Fliegner et al., 1994] whereas peripherin is expressed almost exclusively in peripheral neurons and certain central neurons with peripheral projections [Greene, 1989; Escurat et al., 1990]. NFH appears late in embryogenesis throughout the developing central and peripheral nervous system and its expression continues to increase postnatally [Shaw and Weber, 1982; Carden et al., 1987]. Alpha-internexin expression continues into adulthood throughout the CNS [Yuan et al., 2006], but declines in the PNS after birth. In adults NFL, NFM and NFH are expressed throughout the central and peripheral nervous systems, whereas α-internexin and peripherin are confined to central and peripheral neurons, respectively. Within the CNS and PNS, the relative expression levels of these proteins varies. In general, the triplet proteins are most abundant in neurons with large axons and are down-regulated in response to nerve injury whereas internexin and peripherin are more abundant in those with smaller axons and are up-regulated in response to injury [Oblinger et al., 1989; Troy et al., 1990; McGraw et al., 2002; Uveges et al., 2002], but the functional significance of this is not well understood.
Much of what we know about the assembly properties of the various neuronal intermediate filament proteins comes from studies using SW13 vim- human adrenal carcinoma cells. These cells are useful for such studies because they lack cytoplasmic intermediate filaments [Sarria et al., 1990; Yamamichi-Nishina et al., 2003]. Transfection of these cells with intermediate filament proteins in various combinations has permitted analysis of the polypeptide requirements for filament formation. Such studies have established that the neurofilament triplet proteins from mice and rats cannot form homopolymers in vivo. NFL can form neurofilaments with NFM and/or NFH, but NFM and NFH cannot form filaments in the absence of NFL [Ching and Liem, 1993; Lee et al., 1993]. In contrast, internexin and peripherin can form homopolymers [Ching and Liem, 1993; Cui et al., 1995] and they can also copolymerize with each of the neurofilament triplet proteins [Parysek et al., 1991; Ching and Liem, 1993; Athlan and Mushynski, 1997; Athlan et al., 1997; Yuan et al., 2006].
Studies on GFP-tagged neurofilament proteins M and H expressed in cultured superior cervical ganglion (SCG) neurons have demonstrated that these proteins are transported rapidly in the form of filamentous structures that spend most of their time pausing [Roy et al., 2000; Wang et al., 2000; Wang and Brown, 2001; Brown et al., 2005]. Using quantitative immunofluorescence microscopy and correlative light and electron microscopy, we have shown that the moving structures are single 10-nm-diameter neurofilament polymers [Yan and Brown, 2005]. However, the composition of these polymers is not known. In the present study, we have characterized the polypeptide composition of moving neurofilaments in cultured SCG neurons by immunofluorescence microscopy using specific antibodies and compared them with the total axonal neurofilament population, most of which are stationary at any point in time. To analyze moving neurofilaments, we took advantage of a rapid perfusion and permeabilization technique that we developed previously to capture neurofilaments while they are moving through naturally occurring gaps in the neurofilament array. We found that peripherin and α-internexin are integral and abundant components of neurofilament polymers in these neurons and that they colocalize with NFL and NFM along almost the entire length of each neurofilament. Thus both moving and stationary neurofilaments in these neurons are complex heteropolymers of at least four different neuronal intermediate filament proteins.
MATERIALS AND METHODS
Cell Cultures
Neurons dissociated from SCG of neonatal (P0-P1) mice were cultured at low density (0.03 dissociated ganglia per mm2) on glass coverslips coated with poly-d-ly-sine and Matrigel™ (BD Biosciences). The cultures were maintained in Leibovitz's L-15 medium (GIBCO Life Technologies, Phenol Red-free) supplemented with glucose,l-glutamine, nerve growth factor (BD Biosciences), adult rat serum (Harlan Bioproducts), and hydroxypropylmethylcellulose (Methocel™, Dow Corning) at 37°C as described by Brown [2003]. Human adrenal carcinoma SW13 cl.2 vim- cells [Sarria et al., 1990] were obtained from Dr. Robert Evans of the University of Colorado and cultured on glass coverslips at 105 cells per cm2. The cultures were maintained in DMEM/F12 medium (Invitrogen) supplemented with 5% fetal bovine serum (FBS) and 10 μg/ml gentamicin at 37°C in a humidified atmosphere of 5% CO2.
Plasmid Constructs
Mouse neurofilament protein L and M cDNAs were obtained by reverse transcriptase polymerase chain reaction (RT-PCR) using RNA from wild type P24 mouse cerebellum (Genbank Accession Numbers DQ201635 and DQ201636, respectively). For NFM, the forward primer sequence was 5′-AGTAGGATCCGCCTCCAAGATGAGCTACAC and the reverse primer sequence was 5′-GCACGGATCCTGGGAGAACCCATTCTGTTT. These primers introduced BamH I sites at both ends of the amplified DNA. To generate pEGFP-NFM, the PCR product was cut with Bam HI and cloned into the corresponding site of pEGFP-C1 (BD Biosciences). The resulting expression vector encoded the codon-optimized F64L/S65T variant of green fluorescent protein attached to the amino terminus of mouse neurofilament protein M by a 25 amino acid linker. To generate pNFM, the EGFP coding sequence was removed using Age I and BspE I, which generate complementary sticky ends, and then the plasmid was re-ligated. For NFL, the forward primer sequence was 5′-CCTGCTCGAGTTCTCTCTAGGTCCCCCATCTCC-3′ and the reverse primer was 5′-CGTTGAATTCGGAATAGTTGGGAATAGGGC-3′. These primers introduced Xho I and EcoR I sites at the 5′ and 3′ ends of the amplified DNA. To generate pNFL, the PCR product was cut with Xho I and EcoR I and cloned into the corresponding sites of pEGFP-C1 and then the EGFP coding sequence was removed using Age I and BspE I as described above. All three constructs were confirmed by sequencing. The pRSV-α expression vector encoding rat α-internexin [Ching and Liem, 1993] and pCI-NFH expression vector encoding rat NFH [Leung et al., 1999] were provided by Dr. Ronald Liem of Columbia University. The pBluescript-peripherin vector containing the human peripherin gene under the control of a human beta actin promoter [Parysek et al., 1991] was provided by Dr. Linda Parysek of the University of Cincinnati. All plasmids were amplified in Escherichia coli (DH5α) and purified using Qiagen Maxi Prep plasmid purification kits (Qiagen).
Transfection
Cultured neurons were transfected with pEGFP-NFM by nuclear injection two days after plating using an Eppendorf InjectMan™ NI2 micromanipulator and FemtoJet™ microinjector (Brinkman Instruments) as described by Brown [2003]. 1.25 mg/ml (Mw 10,000) tetramethylrhodamine dextran (Sigma) was included in the injection solution to allow visual confirmation of the injection procedure. SW13 cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. After exposure to the Lipofectamine/DNA mixure for 4 h, the cells were rinsed with fresh medium and then maintained for 24 h before fixation.
Live Cell Imaging
Coverslips with transfected neurons on them were mounted in an RC-21B closed bath imaging and perfusion chamber (Warner Instruments) as described previously [Yan and Brown, 2005] and observed by epifluorescence and phase contrast microscopy with a Nikon TE300 inverted microscope 3 days after transfection, which corresponded to 5 days after plating. Image acquisition was performed with a Nikon 100×/1.4NA Plan Apo DM oil immersion objective and an FITC/EGFP filter set (Chroma Technology, HQ 41001). Images were acquired with a MicroMax 512BFT cooled CCD camera (Roper Scientific). The temperature on the microscope stage was maintained at approximately 35°C using a Nicholson ASI-400 Air Stream Incubator (Nevtek). For time-lapse imaging, the epifluorescent illumination was attenuated by 70–90% using neutral density filters, and images were acquired at 4 s intervals with 1 s exposures using Metamorph™ software (Universal Imaging) as described by Brown [2003].
Capture and Fixation of Moving Filaments
To capture moving neurofilaments, gaps in the neurofilament array were observed by time-lapse imaging. When a neurofilament moved into the gap, we immediately flowed permeabilization solution into the imaging chamber under gravity at a rate of 3.8 ml/min for about one minute while simultaneously acquiring time-lapse images of the fluorescence as described previously [Yan and Brown, 2005]. The permeabilization solution consisted of 0.02% saponin (Sigma) in a solution composed of NPHEM (0.19 M NaCl, 60 mM sodium PIPES, 25 mM sodium HEPES, 10 mM sodium EGTA, 2 mM MgCl2, pH 6.9) and a cocktail of protease inhibitors (10 μg/ml Bestatin, 10 μg/ml Leupeptin and 10 μg/ml E64). Five to ten minutes later, the cells were fixed by perfusing 3 ml 4% (w/v) formaldehyde (>10 chamber volumes) into the chamber at a rate of approximately 2 ml/min. Subsequently, the location of the axon of interest was marked on the coverslip using a Leitz diamond scoring object marker. After fixation for 10–20 min, the chamber was removed from the microscope stage, sub-merged in PBS and disassembled. The coverslips were then immersed in fresh fixative for another 20–30 min. After fixation, coverslips were rinsed with PBS and the cells were demembranated with PBSNT (1% Triton X-100 + 0.3 M NaCl in PBS) for 15 min [Brown, 1997]. For experiments using the peripherin antibody, we used 2% formaldehyde instead of 4% and we did not immerse the cells in fresh fixative after disassembling the chamber because the epitope of this antibody appeared to be sensitive to over-fixation.
Fixation of SW13 Cells and Splayed Axonal Cytoskeletons
SW13 cells were rinsed twice in PBS and then fixed with 4% (w/v) formaldehyde in PBS for 15–20 min. After the fixation, the cells were rinsed with PBS and then treated with 2 ml 0.25% Triton X-100 in PBS at room temperature for 15 min to ensure complete demembranation of the cells prior to immunostaining. To generate splayed axonal cytoskeletons, neurons on coverslips were treated with 0.5% Triton X-100 (Sigma) at room temperature and then fixed with 4% formaldehyde in PBS as described previously [Brown, 1997; Yan and Brown, 2005].
Immunofluorescence Microscopy
After fixation, the cells were rinsed with PBS and then “blocked” with 4% normal goat serum (Jackson Immunoresearch) in PBS (“blocking solution”). All antibody incubations were performed for 45 min at 37°C. NFL was visualized using rabbit polyclonal antiserum NFLAS (gift of Dr. Virginia Lee of the University of Pennsylvania) [Trojanowski et al., 1989]. NFM was visualized using mouse monoclonal antibody RMO270 (Zymed) [Lee et al., 1987]. NFH was visualized using rabbit polyclonal antibody AB1989 (Chemicon). Peripherin was visualized using mouse monoclonal antibody 7C5 (gift of Dr. Gerry Shaw of the University of Florida). α-internexin was visualized using rabbit polyclonal antibody αBB (gift of Dr. Ronald Liem of Columbia University) [Ching et al., 1999]. All the primary mouse monoclonal antibodies were visualized using goat anti-mouse secondary antibody conjugated to Alexa 647 (Molecular Probes), diluted 1:200 in blocking solution, and all the primary rabbit polyclonal antibodies were visualized using goat anti-rabbit secondary antibody conjugated to Rhodamine Red-X™ (Jackson Immunoresearch), diluted 1:200 in blocking solution. Coverslips were mounted on glass slides using Prolong Gold antifade reagent (Molecular Probes). The stained cells were observed by epifluorescence microscopy using a Nikon TE 300 inverted microscope and images were acquired with a Quantix KAF1400 cooled CCD camera (Roper Scientific).
Analysis of Fluorescence Along Captured and Splayed Neurofilaments
To quantify the distribution of different polypeptides along captured and splayed filaments, SCG neurons were processed for double-labeling immunofluorescence microscopy using two different antibodies, one to either NFL, NFM, peripherin or α-internexin, which we refer to as the test antibody, and the other to either NFL or NFM, which we refer to as the reference antibody. If the test antibody was from mouse, then we used NFL antibody as the reference because it is from rabbit. If the test antibody was from rabbit, we used the NFM antibody as the reference because it is from mouse. Our choice of NFL and NFM antibodies as the reference antibodies was based initially on our previous finding that neurofilaments in cultured DRG neurons contain both NFL and NFM along their entire length [Brown, 1998]. The images were analyzed using MetaMorph™ software (Universal Imaging). To measure the relative proportion of the neurofilament length that stained with the test antibody, a line was drawn along the medial axis of each filament in the reference antibody image, and then one or more lines were drawn along the corresponding portion or portions of the filament that stained with the test antibody [Brown, 1998]. For each filament, the total length that was stained with the test antibody was divided by the total length that was stained with the reference antibody.
SDS-PAGE and Western Blotting
Crude preparations of cytoskeletal proteins were obtained from spinal cord and sciatic nerve of adult mice essentially as described by Uchida [Uchida et al., 1999]. Total protein was extracted from cultured SCG neurons by dissolving the cells in SDS sample buffer. Proteins were separated by SDS-PAGE on 7.5% polyacrylamide gels and transferred onto Immobilon-P PVDF membrane (0.2 or 0.45 μm pore size, Millipore) using a mini transblot electrophoretic transfer cell (Bio-Rad Laboratories). To test the dependence of antibody binding on phosphorylation state, neurofilament proteins were dephosphorylated enzymatically by treating the PVDF blots with 3 units/ml alkaline phosphatase (purified from Escherichia coli, Sigma) in dephosphorylation buffer (50 mM Tris-HCl, 138 mM NaCl, 2.7 mM KCl, 1 mM ZnSO4, 1 mM MgCl2, pH 8.0) for at least 18 h at room temperature on a shaker [Sternberger and Sternberger, 1983; Carden et al., 1985; Brown, 1998]. The reaction was terminated by rinsing the blots with 400 mM sodium phosphate, 100 mM NaCl, 50 mM sodium EDTA, pH 7.2. For antibody staining, strips of PVDF membrane were rinsed with Tris buffered saline (TBS; 50 mM Tris-HCl, 138 mM NaCl, 2.7 mM KCl, pH 7.4) and blocked with a 5% (w/v) solution of Carnation brand non-fat dry milk in TBS (“blocking solution”). Primary and secondary antibody incubations were carried out on a shaker at room temperature for one hour. Unbound antibodies were rinsed off with TBS containing 0.1% Tween-20 (Sigma) and then with TBS alone. The secondary antibody was goat anti-mouse IgG or goat anti-rabbit IgG conjugated to horseradish peroxidase or alkaline phosphatase, diluted 1:50,000 in TBS containing 1% normal serum. Antibodies conjugated to horseradish peroxidase were visualized by chemiluminescence using ECL Plus™ Western blotting detection reagent (Amersham Biosciences) whereas antibodies bound to alkaline phosphatase were visualized using BCIP/NBT phosphatase substrate (KPL, Inc.).
RESULTS
To confirm the specificity of the antibodies that we used for this study, we performed Western blotting using cytoskeletal protein fractions prepared from mouse spinal cord and sciatic nerve (Fig. 1). All antibodies bound to a single band of the expected mobility on both untreated and enzymatically dephosphorylated blots. Dephosphorylation had no apparent effect on the staining intensity for the antibodies to NFL and NFM, indicating that these antibodies bind independently of phosphorylation state, but it did result in slightly stronger staining for the NFH and peripherin antibodies and slightly weaker staining for the internexin antibody, indicating that the epitopes of these antibodies display a weak dependence on phosphorylation. Enzymatic dephosphorylation completely abolished staining by RMO55, which is a mouse monoclonal antibody that is specific for phosphorylated NFM [Lee et al., 1987; Brown, 1998], confirming that the dephosphorylation was effective.
Fig. 1.
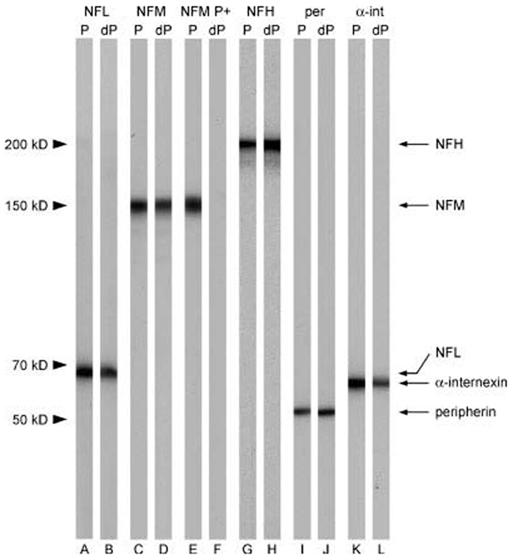
Characterization of antibody specificity by Western blotting. Western blotting of cytoskeletal preparations from mouse sciatic nerve (A–F, I, J) or mouse spinal cord (G, H, K, L). (A, B) NFLAS polyclonal antibody specific for NFL. (C, D) RMO270 monoclonal antibody specific for NFM. (E, F) RMO55 monoclonal antibody specific for phosphorylated NFM (NFM P+). (G, H) AB1989 polyclonal antibody specific for NFH. (I, J) 7C5 monoclonal antibody specific for peripherin (per). (K, L) αBB polyclonal antibody specific for α-internexin (α-int). The blot strips marked dP were treated with alkaline phosphatase to dephosphorylate the neurofilament proteins prior to immunostaining (B, D, F, H, J, L). The blot strips marked P were untreated (A, C, E, G, I, K). Enzymatic dephosphorylation abolished the RMO55 staining, confirming that dephosphorylation had occurred. NFLAS and RMO270 antibodies bind equally well to their cognate proteins in both the native and dephosphorylated form. AB1989, 7C5 and αBB antibodies bind to both the native and dephosphorylated forms of their cognate proteins, though not with identical affinity.
To determine which neurofilament proteins are expressed by cultured neonatal mouse SCG neurons, we performed Western blotting on total protein extracts from 5-day old cultures (Fig. 2). The neurons expressed robust levels of NFL, NFM, peripherin and α-internexin but no detectable NFH, even though NFH was detected with the same antibody in cytoskeletal protein fractions prepared from mouse spinal cord. Thus we focused exclusively on NFL, NFM, peripherin and α-internexin in the remainder of this study. To confirm the specificity of the antibodies to these proteins by immunostaining, we cultured SW13 vim- cells, which lack cytoplasmic intermediate filaments, and transfected them with various combinations of five different neurofilament proteins (NFL, NFM, NFH, peripherin and internexin), systematically omitting a different one of the five in each experiment. After 24 h, the cells were fixed and processed for double-label immunofluorescence microscopy (Fig. 3). The transfection efficiency for NFL, NFM, NFH, peripherin and α-internexin was 44%, 33%, 4%, 2% and 29% respectively. This variation was probably due to differences between the clones and expression vectors that we used for the different proteins (see Methods). To be sure that the low transfection efficiency for the NFH and peripherin constructs did not influence our results adversely, we scanned the entire coverslip (>10,000 cells) at low magnification. For each antibody, we observed many fluorescent cells when the cognate protein was expressed, but none when it was absent. Thus the antibodies are specific as judged by both Western blotting and immunofluorescence microscopy.
Fig. 2.
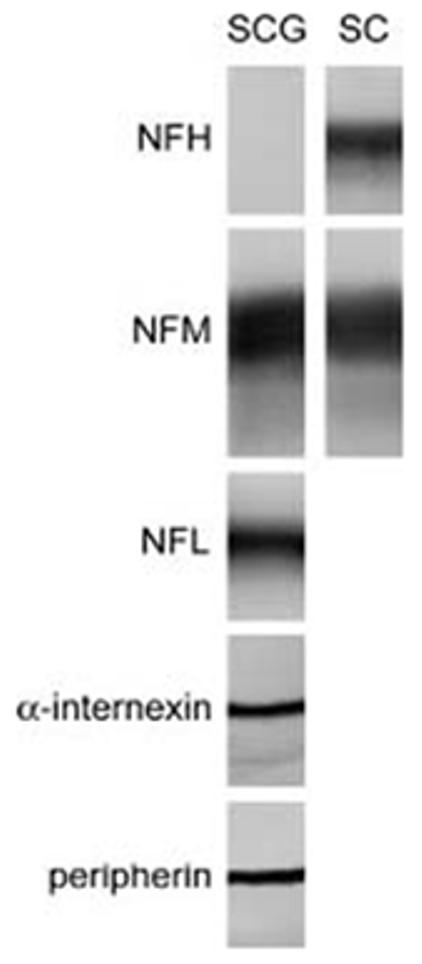
Western blotting of total protein from cultured SCG neurons using antibodies specific for NFH, NFM, NFL, peripherin and α-internexin (left lane; labeled SCG). Note the absence of NFH. The reactivity of the NFH antibody was confirmed by its staining of NFH in cytoskeletal preparations from mouse spinal cord (right lane; labeled SC), using NFM as a loading control.
Fig. 3.
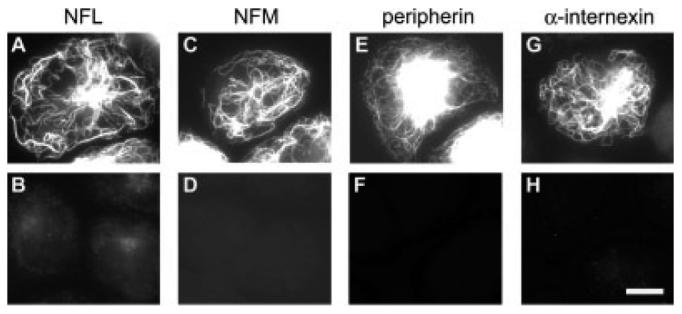
Characterization of antibody specificity by immunostaining. SW13 vim- cells, which have no endogenous cytoplasmic intermediate filaments, were co-transfected with constructs encoding NFL, NFM, NFH, peripherin and α-internexin and then processed for immunofluorescence microscopy using the NFL antibody NFLAS (A,B), the NFM antibody RMO270 (C, D), the peripherin antibody 7C5 (E, F) and the α-internexin antibody αBB (G, H). The cells in the top row (A, C, E, G) were co-transfected with all five constructs the same. The cells in the bottom row (B, D, F, H) were co-transfected with the same constructs except that NFL was omitted in (B), NFM in (D), peripherin in (F) and α-internexin in (H). For each antibody, the images were scaled identically to permit comparison of the staining intensity. Each antibody only stained those cells that expressed that antibody's cognate protein. Scale bar = 15 μm.
In addition to the specificity of the antibodies, we also evaluated their staining quality. Staining quality is determined by the accessibility of the antibody's epitope, which can be influenced by fixation. SW13 vim- cells were transfected with neurofilament proteins either alone or in combination and then processed for immunostaining (Fig. 4). Because mouse NFL and NFM cannot form homopolymers in vivo, we co-transfected cells with these two proteins to generate NFL/NFM heteropolymers. Internexin and peripherin, on the other hand, were transfected alone because they are capable of forming homopolymers. The NFL and NFM antibodies stained NFL/NFM heteropolymers uniformly and continuously along their entire length and peripherin and α-internexin antibodies stained peripherin and α-internexin homopolymers uniformly and continuously also. Thus the epitopes of these antibodies are uniformly accessible along the length of these filaments.
Fig. 4.
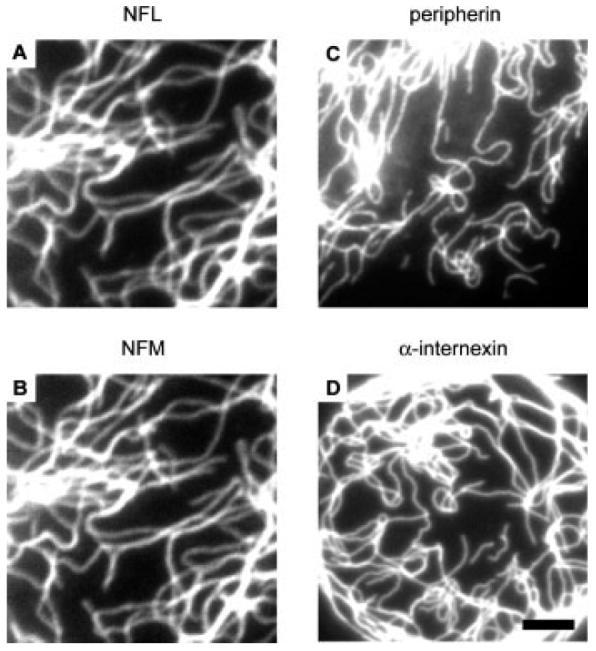
Characterization of antibody staining quality. SW13 vim-cells expressing mouse NFL and NFM were double-stained with the NFL antibody NFLAS (A) and the NFM antibody RMO270 (B). SW13 vim- cells expressing peripherin were stained with peripherin antibody 7C5 (C) and SW13 vim- cells expressing α-internexin were stained with the α-internexin antibody αBB (D). Note the uniformity of the staining for each antibody, which demonstrates that their epitopes are accessible along the entire length of the filaments. Scale bar = 3 μm.
To characterize the polypeptide composition of moving neurofilaments, we took advantage of naturally occurring gaps in the axonal neurofilament array of cultured SCG neurons. Neurons expressing GFP-tagged neurofilament protein M (GFP-NFM) were mounted in an imaging and perfusion chamber and gaps in the GFP fluorescence were observed by time-lapse fluorescence microscopy. When a filament entered the gap, it was stopped in its tracks by rapid perfusion of a permeabilization solution containing 0.02% saponin. The captured filament was then fixed and processed for immunofluorescence microscopy. To compare moving neurofilaments with the total axonal neurofilament population, neurons that were cultured under identical conditions were treated with detergent to induce the neurofilaments to splay apart from each other and then processed for immunostaining under identical conditions.
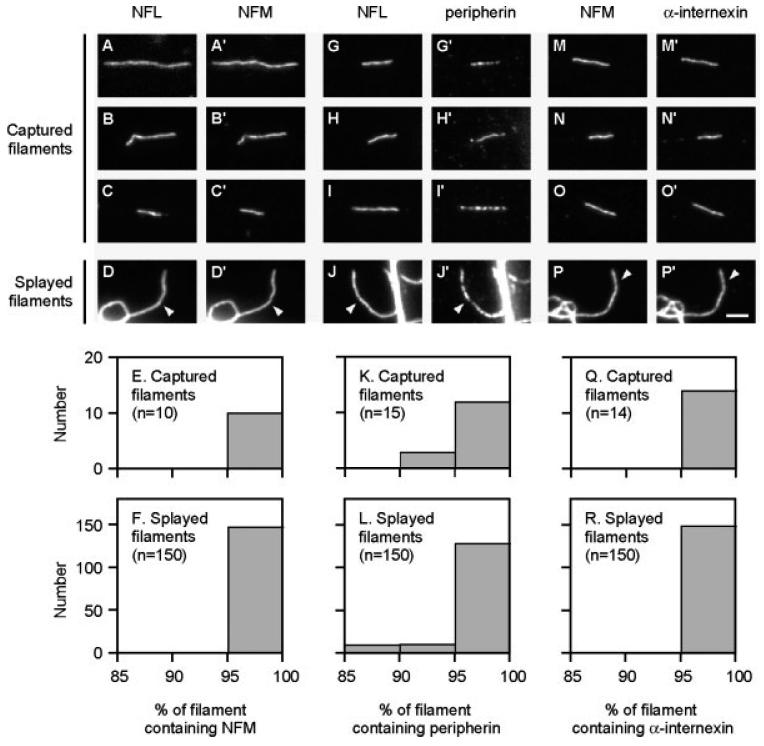
To measure NFM incorporation along neurofilaments, we stained captured and splayed axonal neurofilaments with antibodies specific for NFL and NFM. Both antibodies stained uniformly along the entire length of each captured and splayed filament (Figs. 5A-5D and 5A′-5D′). To quantify this, we measured the proportion of the neurofilament length (defined by staining for NFL) that also stained for NFM. In total, we analyzed 10 captured filaments, six of which were moving anterogradely and four of which were moving retrogradely. Two of these captured filaments stopped before they had moved completely into the gap so it was not possible to measure their length. For these two filaments, we only analyzed the portion that lay within the gap. The average length for the other eight filaments was 12.0 μm (minimum = 3.7 μm, maximum = 21.1 μm; n = 8). On average, the captured filaments had NFM incorporated along 99.8% of their length (minimum = 96.4%, maximum = 100%, n = 10; Fig. 5E). In comparison, neurofilaments in splayed axonal cytoskeletons from untransfected cells had NFM incorporated along 99.2% of their length (minimum = 90.0%, maximum = 100.0%, n = 150; Fig. 5F). To examine the influence of the exogenous GFP-tagged NFM on these measurements, we also analyzed NFM distribution along neurofilaments in splayed axonal cytoskeletons from transfected cells after the same number of days in culture. The average proportion of the splayed filaments that stained for NFM was 99.7% (minimum = 94.4%, maximum = 100.0%, n = 150; data not shown), which is only 0.5% more than for untransfected neurons. This indicates that exogenous GFP-NFM had negligible effect on the proportion of the neurofilament length that contained NFM. Thus both captured and splayed neurofilaments appear to contain NFM along almost the entire length.
Fig. 5.
Characterization of the polypeptide composition of captured and splayed neurofilaments Moving neurofilaments captured in gaps were double-stained with either the NFL antibody NFLAS (A–C) and the NFM antibody RMO270 (A′–C′), the NFL antibody NFLAS (G–I) and the peripherin antibody 7C5 (G′–I′), or the NFM antibody RMO270 (M-O) and the α-internexin antibody αBB (M′–O′). Splayed neurofilaments (see arrowheads) were double-stained with either the NFL antibody NFLAS (D) and the NFM antibody RMO270 (D′), the NFL antibody NFLAS (J) and the peripherin antibody 7C5 (J′), or the NFM antibody RMO270 (P) and the α-internexin antibody αBB (P′). The histograms show the quantification of the proportion of the neurofilament length that contained NFM (E, F), peripherin (K, L) and α-internexin (Q, R) along captured neurofilaments (E, K, Q) and splayed neurofilaments (F, L, R). Each histogram represents combined data from at least three independent experiments. All neurofilaments contained all four polypeptides along at least 85% of their length. Scale bar = 3 μm.
To measure peripherin incorporation along neurofilaments, we stained captured and splayed axonal neurofilaments with antibodies specific for NFL and peripherin. The peripherin staining was continuous along almost the entire length of each filament, though less uniform than for NFL (Figs. 5G-5J and 5G′-5J′). In total we analyzed fifteen captured filaments, eight of which were moving anterogradely and seven of which were moving retrogradely. Portions of three of these filaments lay outside the gap or were folded, precluding us from measuring their length. For the other twelve filaments, the average length was 8.2 μm (minimum = 4.1 μm, maximum = 21.2 μm, n = 12). On average, the captured neurofilaments had peripherin incorporated along 96.8% of their length (minimum = 92.6%, maximum = 100%, n = 15; Fig. 5K). In comparison, neurofilaments in splayed axonal cytoskeletons from untransfected neurons had peripherin incorporated along 97.8% of their length (minimum = 86.3%, maximum = 100%, n = 150; Fig. 5L). Thus both captured and splayed neurofilaments contain peripherin along almost their entire length.
To measure α-internexin incorporation along moving neurofilaments, we stained captured and splayed neurofilaments with antibodies specific for NFM and α-internexin. We used NFM antibody as the reference antibody for these experiments because the NFL antibody was from the same species as the internexin antibody. Both antibodies stained all filaments uniformly and continuously along their entire length (Figs. 5M-5P and 5M′-5P′). In total we analyzed 14 captured filaments, eight of which were moving anterogradely and six of which were moving retrogradely. Portions of five of these filaments lay outside the gap or were folded, precluding us from measuring their length. The average length of the other nine filaments was 9.8 μm (minimum = 3.7 μm, maximum = 14.9 μm, n = 9). On average, the captured neurofilaments had α-internexin incorporated along 99.7% of their length (minimum = 97.9%, maximum = 100%, n = 14; Fig. 5Q) and this value was the same for neurofilaments in splayed axonal cytoskeletons (minimum = 94.6%, maximum = 100%, n 150; Fig. 5R). Since peripherin and NFM were both present along almost the entire length of neurofilaments visualized with NFL antibody, and since α-internexin was present along almost the entire length of the neurofilaments visualized with NFM antibody, it is clear that NFL was also contained throughout these neurofilaments. Thus all four proteins were distributed along essentially the entire length of both captured and splayed neurofilaments.
DISCUSSION
We have characterized the polypeptide composition of neurofilaments in cultured neurons from the superior cervical ganglia of neonatal mice using immunofluorescence microscopy. We found that these neurons express NFL, NFM, peripherin and α-internexin but no detectable NFH. The absence of NFH in these cultures is consistent with previous observations that NFH expression remains at low levels in most neurons until after birth [Shaw and Weber, 1982; Carden et al., 1987] and that this delayed expression in vivo is recapitulated in cell culture [Lee, 1985; Shaw et al., 1985; Foster et al., 1987; Breen and Anderton, 1991; Benson et al., 1996].
To quantify the incorporation of NFL, NFM, peripherin and α-internexin along individual neurofilaments, we measured the relative proportion of the neurofilament length that stained with antibodies specific for these proteins. We analyzed moving neurofilaments, which were captured using a rapid perfusion and permeabilization technique, and also the total axonal neurofilament population, visualized in splayed axonal cytoskeletons. By performing pairwise comparisons using different antibodies, we found that peripherin and α-internexin were co-localized with NFL and NFM along >85% of the length of both captured and splayed neurofilaments. This indicates that peripherin and α-internexin are integral and abundant components of neurofilaments in these neurons. While in principle, the expression of GFP-NFM in these neurons might be expected to skew our measurements of NFM distribution along neurofilaments, our measurements actually indicate that any such effect was minimal, at least at the low expression levels that we use in our studies. Since at any point in time most of the neurofilaments in the axon are not moving [Wang et al., 2000; Brown et al., 2005], we can consider the splayed axonal neurofilaments, which represent the total axonal neurofilament population, to be predominantly stationary. Thus our data indicate that both moving and stationary neurofilaments in these axons are complex heteropolymers of four different neurofilament proteins and that moving and stationary neurofilaments do not differ qualitatively in their subunit composition, at least for the proteins that we have examined.
The expression of α-internexin in these cultured peripheral neurons is consistent with previous reports that α-internexin is widely expressed throughout the central and peripheral nervous systems during development, even though its expression is restricted to the central nervous system in adults (see Introduction), and also with a previous report that α-internexin is expressed in cultured embryonic neurons from dorsal root ganglia [Athlan et al., 1997]. Interestingly, even though both α-internexin and peripherin can self-assemble into homopolymers in vivo [Ching and Liem, 1993; Cui et al., 1995], we didn't observe any neurofilaments that contained only α-internexin or only peripherin in this study. Thus both peripherin and α-internexin prefer to form heteropolymeric interactions with each other and with the neurofilament triplet proteins when these proteins are co-expressed. This is consistent with previous studies using immunogold electron microscopy which have shown that peripherin co-localizes with NFM and/or NFH along neurofilaments in a subset of PC12 cells that co-express these proteins [Parysek et al., 1991] and that α-internexin co-localizes with NFM along neurofilaments in mouse optic nerve [Yuan et al., 2006].
Neurofilaments are thought to move along microtubule tracks, powered by dynein and perhaps kinesin motor proteins [Shah et al., 2000; Xia et al., 2003; Wagner et al., 2004; He et al., 2005]. However, the complexity and interchangeability of the subunit proteins that can comprise neurofilaments raises an interesting question about how these motors interact with the neurofilament polymer. Studies on knockout mice suggest that heteroligomerization of the neurofilament triplet proteins is required for their transport [Yuan et al., 2003] and this is consistent with evidence from live cell imaging and correlative light and electron microscopy of captured neurofilaments, which indicate that neurofilament proteins are transported as assembled neurofilament polymers [Wang et al., 2000; Yan and Brown, 2005]. However, studies on knockout mice also indicate that no single neurofilament protein is required for neurofilament transport into axons. For example, targeted deletion of NFM, NFH or α-internexin does not impair neurofilament transport [Zhu et al., 1998; Jacomy et al., 1999; Rao et al., 2002; Yuan et al., 2003, 2006]. Targeted deletion of NFL results in very low expression levels of NFM and NFH, but nevertheless NFM transport has been detected in NFL/H double knockout mice, presumably because it can form heteropolymers with α-internexin, and this indicates that NFL is also not required for neurofilament transport [Yuan et al., 2003]. Neurofilament transport has not been examined in peripherin knockout mice but it seems clear that neurofilaments must be transported robustly in those mice because the number and caliber of myelinated axons is unchanged, even though there is selective loss of a subpopulation of unmyelinated sensory neurons [Lariviere et al., 2002].
If no single neurofilament subunit protein is critical for neurofilament transport, how do motors interact with the neurofilament polymer? One possibility is that there is an additional neurofilament subunit, as yet unidentified, that interacts with the neurofilament motors and that this subunit is common to all neurofilaments. In fact, recent studies indicate that neurons may also express an alternately spliced isoform of the intermediate filament protein synemin, termed synemin L [Izmiryan et al., 2006], so it is quite possible that we do not yet know the full extent of the complexity of neurofilament composition. However, it seems more likely that the neurofilament motors are capable of interacting with multiple different neurofilament protein subunits. One possible explanation is that they bind directly or indirectly to some as yet unidentified conserved sequence or structural motif. Given that every neurofilament must contain NFL, peripherin or α-internexin, i.e. that at least one of these proteins must be present for neurofilaments to form, any putative motor binding site would have to be shared by at least these three proteins even if it were not shared by NFM and NFH. Since the amino and carboxy terminal domains of neurofilament proteins are so variable, it would seem likely that any such binding site would reside within the rod domain. Alternatively, there could be multiple specific adaptor proteins, enabling the motors to bind indirectly to a different site on each neurofilament polypeptide. To test these ideas, it will be necessary to learn much more about the specific identity of the neurofilament motors and the precise components of the neurofilament-motor interaction complex.
ACKNOWLEDGMENTS
We thank Atsuko Uchida and Gulsen Colakoglu of the Brown lab for their help, Ron Liem and Linda Parysek for antibodies and cDNA expression constructs, Virginia Lee for the NFL antibody, and Robert Evans for the SW13 cell lines.
Contract grant sponsor: NIH.
REFERENCES
- Athlan ES, Mushynski WE. Heterodimeric associations between neuronal intermediate filament proteins. J Biol Chem. 1997;272:31073–31078. doi: 10.1074/jbc.272.49.31073. [DOI] [PubMed] [Google Scholar]
- Athlan ES, Sacher MG, Mushynski WE. Associations between intermediate filament proteins expressed in cultured dorsal root ganglion neurons. J Neurosci Res. 1997;47:300–310. [PubMed] [Google Scholar]
- Benson DL, Mandell JW, Shaw G, Banker G. Compartmentation of α-internexin and neurofilament triplet proteins in cultured hippocampal neurons. J Neurocytol. 1996;25:181–196. doi: 10.1007/BF02284795. [DOI] [PubMed] [Google Scholar]
- Breen KC, Anderton BH. Temporal expression of neurofilament polypeptides in differentiating neuroblastoma cells. Neuroreport. 1991;2:20–24. doi: 10.1097/00001756-199101000-00005. [DOI] [PubMed] [Google Scholar]
- Brown A. Visualization of single neurofilaments by immunofluorescence microscopy of splayed axonal cytoskeletons. Cell Motil Cytoskel. 1997;38:133–145. doi: 10.1002/(SICI)1097-0169(1997)38:2<133::AID-CM3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Brown A. Contiguous phosphorylated and non-phosphorylated domains along axonal neurofilaments. J Cell Sci. 1998;111:455–467. doi: 10.1242/jcs.111.4.455. [DOI] [PubMed] [Google Scholar]
- Brown A. Live-cell imaging of slow axonal transport in cultured neurons. Methods Cell Biol. 2003;71:305–323. doi: 10.1016/s0091-679x(03)01014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A, Wang L, Jung P. Stochastic simulation of neurofilament transport in axons: The “stop-and-go” hypothesis. Mol Biol Cell. 2005;16:4243–4255. doi: 10.1091/mbc.E05-02-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carden MJ, Schlaepfer WW, Lee VM. The structure, biochemical properties, and immunogenicity of neurofilament peripheral regions are determined by phosphorylation state. J Biol Chem. 1985;260:9805–9817. [PubMed] [Google Scholar]
- Carden MJ, Trojanowski JQ, Schlaepfer WW, Lee VM. Two-stage expression of neurofilament polypeptides during rat neurogenesis with early establishment of adult phosphorylation patterns. J Neurosci. 1987;7:3489–3504. doi: 10.1523/JNEUROSCI.07-11-03489.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching GY, Liem RK. Assembly of type IV neuronal intermediate filaments in nonneuronal cells in the absence of preexisting cytoplasmic intermediate filaments. J Cell Biol. 1993;122:1323–1335. doi: 10.1083/jcb.122.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching GY, Chien CL, Flores R, Liem RKH. Overexpression of α-internexin causes abnormal neurofilamentous accumulations and motor coordination deficits in transgenic mice. J Neurosci. 1999;19:2974–2986. doi: 10.1523/JNEUROSCI.19-08-02974.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui CQ, Stambrook PJ, Parysek LM. Peripherin assembles into homopolymers in SW13 cells. J Cell Sci. 1995;108:3279–3284. doi: 10.1242/jcs.108.10.3279. [DOI] [PubMed] [Google Scholar]
- Drager UC. Coexistence of neurofilaments and vimentin in a neurone of adult mouse retina. Nature. 1983;303:169–172. doi: 10.1038/303169a0. [DOI] [PubMed] [Google Scholar]
- Escurat M, Djabali K, Gumpel M, Gros F, Portier MM. Differential expression of two neuronal intermediate-filament proteins, peripherin and the low-molecular-mass neurofilament protein (NF-L), during the development of the rat. J Neurosci. 1990;10:764–784. doi: 10.1523/JNEUROSCI.10-03-00764.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegner KH, Liem RK. Cellular and molecular biology of neuronal intermediate filaments. Int Rev Cytol. 1991;131:109–167. doi: 10.1016/s0074-7696(08)62018-5. [DOI] [PubMed] [Google Scholar]
- Fliegner KH, Kaplan MP, Wood TL, Pintar JE, Liem RKH. Expression of the gene for the neuronal intermediate filament protein α-internexin coincides with the onset of neuronal differentiation in the developing rat nervous system. J Comp Neurol. 1994;342:161–173. doi: 10.1002/cne.903420202. [DOI] [PubMed] [Google Scholar]
- Foster GA, Dahl D, Lee VM. Temporal and topographic relationships between the phosphorylated and nonphosphorylated epitopes of the 200 kDa neurofilament protein during development in vitro. J Neurosci. 1987;7:2651–2663. doi: 10.1523/JNEUROSCI.07-09-02651.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LA. A new neuronal intermediate filament protein. Trends Neurosci. 1989;12:228–230. doi: 10.1016/0166-2236(89)90127-6. [DOI] [PubMed] [Google Scholar]
- He Y, Francis F, Myers KA, Yu W, Black MM, Baas PW. Role of cytoplasmic dynein in the axonal transport of microtubules and neurofilaments. J Cell Biol. 2005;168:697–703. doi: 10.1083/jcb.200407191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izmiryan A, Cheraud Y, Khanamiryan L, Leterrier JF, Federici T, Peltekian E, Moura-Neto V, Paulin D, Li Z, Xue ZG. Different expression of synemin isoforms in glia and neurons during nervous system development. Glia. 2006;54:204–213. doi: 10.1002/glia.20378. [DOI] [PubMed] [Google Scholar]
- Jacomy H, Zhu Q, Couillard-Despres S, Beaulieu JM, Julien JP. Disruption of type IV intermediate filament network in mice lacking the neurofilament medium and heavy subunits. J Neurochem. 1999;73:972–984. doi: 10.1046/j.1471-4159.1999.0730972.x. [DOI] [PubMed] [Google Scholar]
- Kaplan MP, Chin SSM, Fliegner KH, Liem RKH. α-Internexin, a novel neuronal inermediate filament protein, precedes the low molecular weight neurofilament protein (NF-L) in the developing rat brain. J Neurosci. 1990;10:2735–2748. doi: 10.1523/JNEUROSCI.10-08-02735.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariviere RC, Nguyen MD, Ribeiro-da-Silva A, Julien JP. Reduced number of unmyelinated sensory axons in peripherin null mice. J Neurochem. 2002;81:525–532. doi: 10.1046/j.1471-4159.2002.00853.x. [DOI] [PubMed] [Google Scholar]
- Lee MK, Cleveland DW. Neuronal intermediate filaments. Annu Rev Neurosci. 1996;19:187–217. doi: 10.1146/annurev.ne.19.030196.001155. [DOI] [PubMed] [Google Scholar]
- Lee MK, Xu Z, Wong PC, Cleveland DW. Neurofilaments are obligate heteropolymers in vivo. J Cell Biol. 1993;122:1337–1350. doi: 10.1083/jcb.122.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VM. Neurofilament protein abnormalities in PC12 cells: Comparison with neurofilament proteins of normal cultured rat sympathetic neurons. J Neurosci. 1985;5:3039–3046. doi: 10.1523/JNEUROSCI.05-11-03039.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VM, Carden MJ, Schlaepfer WW, Trojanowski JQ. Monoclonal antibodies distinguish several differentially phosphorylated states of the two largest rat neurofilament subunits (NF-H and NF-M) and demonstrate their existence in the normal nervous system of adult rats. J Neurosci. 1987;7:3474–3488. doi: 10.1523/JNEUROSCI.07-11-03474.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Leung CL, Flores RL, Liem RKH. The complexity of intermediate filaments in the nervous system. In: Herrmann H, Harris JR, editors. Intermediate Filaments. Plenum; New York: 1998. pp. 497–526. [PubMed] [Google Scholar]
- Leung CL, Sun D, Liem RK. The intermediate filament protein peripherin is the specific interaction partner of mouse BPAG1-n (dystonin) in neurons. J Cell Biol. 1999;144:435–446. doi: 10.1083/jcb.144.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw TS, Mickle JP, Shaw G, Streit WJ. Axonally transported peripheral signals regulate α-internexin expression in regenerating motoneurons. J Neurosci. 2002;22:4955–4963. doi: 10.1523/JNEUROSCI.22-12-04955.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA, Shea TB. Dynamics of neuronal intermediate filaments: A developmental perspective. Cell Motil Cytoskel. 1992;22:81–91. doi: 10.1002/cm.970220202. [DOI] [PubMed] [Google Scholar]
- Oblinger MM, Wong J, Parysek LM. Axotomy-induced changes in the expression of a type III neuronal intermediate filament gene. J Neurosci. 1989;9:3766–3775. doi: 10.1523/JNEUROSCI.09-11-03766.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parysek LM, Mcreynolds MA, Goldman RD, Ley CA. Some neural intermediate filaments contain both peripherin and the neurofilament proteins. J Neurosci Res. 1991;30:80–91. doi: 10.1002/jnr.490300110. [DOI] [PubMed] [Google Scholar]
- Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, Fain-zilber M. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron. 2005;45:715–726. doi: 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Rao MV, Garcia ML, Miyazaki Y, Gotow T, Yuan A, Mattina S, Ward CM, Calcutt NA, Uchiyama Y, Nixon RA, Cleveland DW. Gene replacement in mice reveals that the heavily phosphorylated tail of neurofilament heavy subunit does not affect axonal caliber or the transit of cargoes in slow axonal transport. J Cell Biol. 2002;158:681–693. doi: 10.1083/jcb.200202037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Coffee P, Smith G, Liem RKH, Brady ST, Black MM. Neurofilaments are transported rapidly but intermittently in axons: Implications for slow axonal transport. J Neurosci. 2000;20:6849–6861. doi: 10.1523/JNEUROSCI.20-18-06849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarria AJ, Nordeen SK, Evans RM. Regulated expression of vimentin cDNA in cells in the presence and absence of a preexisting vimentin filament network. J Cell Biol. 1990;111:553–565. doi: 10.1083/jcb.111.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob JE, Farber NB, Gottlieb DI. Neurons of the olfactory epithelium in adult rats contain vimentin. J Neurosci. 1986;6:208–217. doi: 10.1523/JNEUROSCI.06-01-00208.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah JV, Flanagan LA, Janmey PA, Leterrier J-F. Bidirectional translocation of neurofilaments along microtubules mediated in part by dynein/dynactin. Mol Biol Cell. 2000;11:3495–3508. doi: 10.1091/mbc.11.10.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G, Weber K. Differential expression of neurofilament triplet proteins in brain development. Nature. 1982;298:277–279. doi: 10.1038/298277a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G, Banker GA, Weber K. An immunofluorescence study of neurofilament protein expression by developing hippocampal neurons in tissue culture. Eur J Cell Biol. 1985;39:205–216. [PubMed] [Google Scholar]
- Sternberger LA, Sternberger NH. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci USA. 1983;80:6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski JQ, Kelsten ML, Lee VM-Y. Phosphate-dependent and independent neurofilament protein epitopes are expressed throughout the cell cycle in human medulloblastoma (D283 MED) cells. Am J Pathol. 1989;135:747–758. [PMC free article] [PubMed] [Google Scholar]
- Troy CM, Muma NA, Greene LA, Price DL, Shelanski ML. Regulation of peripherin and neurofilament expression in regenerating rat motor neurons. Brain Res. 1990;529:232–238. doi: 10.1016/0006-8993(90)90832-v. [DOI] [PubMed] [Google Scholar]
- Uchida A, Yorifuji H, Lee VMY, Kishimoto T, Hisanaga S. Neurofilaments of aged rats: The strengthened interneurofilament interaction and the reduced amount of NF-M. J Neurosci Res. 1999;58:337–348. [PubMed] [Google Scholar]
- Uveges TE, Shan Y, Kramer BE, Wight DC, Parysek LM. Intron 1 is required for cell type-specific, but not injury-responsive, peripherin gene expression. J Neurosci. 2002;22:7959–7967. doi: 10.1523/JNEUROSCI.22-18-07959.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner OI, Ascano J, Tokito M, Leterrier JF, Janmey PA, Holzbaur EL. The interaction of neurofilaments with the microtubule motor cytoplasmic dynein. Mol Biol Cell. 2004;15:5092–5100. doi: 10.1091/mbc.E04-05-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Brown A. Rapid intermittent movement of axonal neurofilaments observed by fluorescence photobleaching. Mol Biol Cell. 2001;12:3257–3267. doi: 10.1091/mbc.12.10.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Ho C-L, Sun D, Liem RKH, Brown A. Rapid movement of axonal neurofilaments interrupted by prolonged pauses. Nat Cell Biol. 2000;2:137–141. doi: 10.1038/35004008. [DOI] [PubMed] [Google Scholar]
- Xia CH, Roberts EA, Her LS, Liu X, Williams DS, Cleveland DW, Goldstein LS. Abnormal neurofilament transport caused by targeted disruption of neuronal kinesin heavy chain KIF5A. J Cell Biol. 2003;161:55–66. doi: 10.1083/jcb.200301026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamichi-Nishina M, Ito T, Mizutani T, Yamamichi N, Watanabe H, Iba H. SW13 cells can transition between two distinct subtypes by switching expression of BRG1 and Brm genes at the post-transcriptional level. J Biol Chem. 2003;278:7422–7430. doi: 10.1074/jbc.M208458200. [DOI] [PubMed] [Google Scholar]
- Yan Y, Brown A. Neurofilament polymer transport in axons. J Neurosci. 2005;25:7014–7021. doi: 10.1523/JNEUROSCI.2001-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A, Rao MV, Kumar A, Julien JP, Nixon RA. Neurofilament transport in vivo minimally requires hetero-oligomer formation. J Neurosci. 2003;23:9452–9458. doi: 10.1523/JNEUROSCI.23-28-09452.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A, Rao MV, Sasaki T, Chen Y, Kumar A, Veeranna, Liem RK, Eyer J, Peterson AC, Julien JP, Nixon RA. α-Internexin is structurally and functionally associated with the neurofilament triplet proteins in the mature CNS. J Neurosci. 2006;26:10006–10019. doi: 10.1523/JNEUROSCI.2580-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Lindenbaum M, Levavasseur F, Jacomy H, Julien JP. Disruption of the NF-H gene increases axonal microtubule content and velocity of neurofilament transport: Relief of axonopathy resulting from the toxin β,β′-iminodipropionitrile [see comments] J Cell Biol. 1998;143:183–193. doi: 10.1083/jcb.143.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]