Abstract
Context: Some female athletes may have decreased bone mineral density (BMD), which puts them at higher risk for stress fractures and future osteoporosis.
Objective: To compare site-specific BMD among National Collegiate Athletic Association Division I varsity female athletes and to determine predictor variables of BMD measurements.
Design: Between-groups design.
Setting: University health care system.
Patients or Other Participants: All women varsity athletes were invited to participate in a cross-sectional study. Of 12 sports, we obtained complete data from 99 women (mean age = 20.2 ± 1.3 years) representing gymnastics, softball, cross-country, track, field hockey, soccer, crew, and swimming/diving.
Main Outcome Measure(s): Each participant was weighed, measured, and questioned about her menstrual status. Using dual-energy x-ray absorptiometry, we measured total-body BMD and region-of-interest scores for lumbar spine, pelvis, and average leg (average from right and left leg measurements) BMD. Using analyses of covariance, we compared BMD measurements among sports at each site while controlling for menstrual status and mass, and we performed a stepwise regression analysis to determine significant predictors of BMD at each site.
Results: Twenty-three athletes were oligomenorrheic or amenorrheic. Runners had the lowest total-body (1.079 ± 0.055 g·cm −2) and site-specific ( P < .01) BMD values for every site except average leg score when compared with gymnasts and softball players. Swimmers and divers had significantly lower average leg BMD (1.117 ± 0.086 g·cm −2) than athletes in every other sport except runners and rowers ( P < .01). Regression analysis revealed only mass and sport as significant predictors of total-body BMD.
Conclusions: Runners and swimmers and divers demonstrated some deficits in site-specific BMD values when compared with athletes in other sports. When treating a female varsity athlete, athletic trainers should consider her mass and sport type with regard to her bone health.
Keywords: female athlete triad, bone health, amenorrhea, oligomenorrhea
Key Points
Although bone mineral density values were markedly similar among athletes in various Division I collegiate sports, female runners and swimmers/divers displayed deficits in total-body and site-specific bone mineral density values when compared with other athletes.
Body mass and sport type were important determinants of bone health in Division I female collegiate athletes.
Larger, longitudinal studies are needed to provide information on how bone health of female collegiate athletes may change in response to intense training over time.
Involvement in athletics can promote healthy lifestyle behaviors and decrease the risk for a number of health problems. In particular, weight-bearing exercise in female athletes increases bone mineral density (BMD) and lean body mass, which may help to prevent stress fractures and osteoporosis later in life. 1–5 Unfortunately, involvement in athletics also may be detrimental to some female athletes engaging in extreme amounts of vigorous activity. These young women may be at risk for the female athlete triad, which consists of disordered eating, menstrual disturbance, and decreased BMD. 6 Decreased BMD increases the risk for stress fractures 7 and osteoporosis later in life. 8–10 Stress fractures are a common problem for female athletes, 11 and osteoporosis is reported to affect about 44 million adults, of whom 80% are women. 12 Thus, for the current and future health of female athletes, it is imperative to explore the determinants of BMD.
Several investigators 2, 8–10, 13–16 have evaluated female athletes' BMD in reference to menstrual history. However, these studies have various limitations. Several authors focused on one sport, 10, 14–17 did not specify a sport, 8, 13 or measured limited BMD sites. 9, 15 Also, many authors reported on a wide age range not reflective of the typical college athlete 8, 9, 13, 15 and almost all studied a limited number of subjects. 2, 10, 13–16 Exercise type (ie, impact versus nonimpact) appears to influence the osteogenic response and can lead to either higher or lower values of BMD at different sites. 17 Strength-based and high-impact sports seem to be associated with higher BMD, whereas non–weight-bearing sports have a neutral or negative relationship. 3, 18 To our knowledge, no researchers have compared BMD at a variety of sites among athletes in several collegiate sports. Thus, our purpose was to compare total-body and site-specific BMD values among Division I female athletes to determine which sports, if any, are associated with low BMD, which may lead to stress fractures. A secondary purpose was to determine predictor variables for each BMD measurement.
METHODS
Subjects
We invited all women varsity athletes from a National Collegiate Athletic Association Division I university to participate in the study. Athletes were recruited through the athletic department with the help of team coaches and athletic trainers. The university committee on research involving human subjects approved the study, and written informed consent was obtained from each participant. Athletes were assured that their results would remain confidential unless they chose to release them to medical staff.
The subjects were part of a cross-sectional study on body composition previously reported; data on 99 of the 135 original subjects were used. 19, 20 Of the 36 excluded subjects, 14 were nonvarsity athletes, 15 lacked menstrual history data, 2 volleyball athletes exceeded height limitations for the bone scan, and the remaining 4 volleyball players were excluded because there were too few to adequately represent the sport. Finally, 1 runner was excluded because she was strongly suspected of having an eating disorder. Our previous analysis on these subjects focused on body fatness and did not consider BMD measurements.
Bone Measurements
A dual-energy x-ray absorptiometry (DXA) instrument (model QDR-1000W with software version 6.0; Hologic Inc, Bedford, MA) was used as the criterion measure for BMD in the present investigation. Details of our DXA methods can be found in a previous report. 20 Total-body BMD was measured with a whole-body scan. The lumbar spine, pelvis, and average leg BMD values were ascertained from region-of-interest values reported with the whole body scan.
Menstrual Histories
A single investigator asked each athlete 3 questions regarding her menstrual history. The first question was age at menarche. Gynecologic age was calculated as current age minus age at menarche. The second asked how many menstrual cycles she had in the last year. The third was a yes/no question regarding oral contraceptive use in the past year. Amenorrhea was defined as 0 to 3 menstrual cycles, oligomenorrhea as 4 to 9 menstrual cycles, and eumenorrhea as 10 to 12 menstrual cycles in the past year. This categorization schema was used in a previous study. 15 In regression analyses, menstrual status was classified as a dichotomous variable, with normal menstrual status referring to 10 to 12 cycles in the past year and abnormal menstrual status referring to 0 to 9 cycles in the past year.
Data Collection Procedures
Testing occurred in the Rheumatology Center of Sparrow Healthcare System Pavilion (East Lansing, MI). On arrival, each woman was measured for standing height and mass using a precalibrated stadiometer and beam balance scale. Body mass index (BMI) was calculated as mass in kilograms divided by height in meters squared. A single investigator conducted individual interviews for menstrual history. Then each participant underwent a single DXA analysis conducted by 2 Hologic-certified technicians. The DXA machine was calibrated daily to a lumbar spinal phantom for bone density per protocol. 20
Each athlete wore a sports bra, shorts, and a standard long T-shirt for height and mass measurements. For the DXA scan, only the T-shirt, sports bra, and underwear were worn. Each athlete was given a set of written guidelines, as described previously, to adhere to before her designated testing date. 20 A data printout from the DXA scan provided values for head, left and right arms, left and right ribs, thoracic spine, lumbar spine, pelvis, left and right legs, and total-body BMD for each participant.
For comparison purposes, total-body, lumbar spine, pelvis, and average leg score BMD were evaluated. Average leg score BMD was calculated as the average of the left and right leg BMD measurements. These sites were chosen because they are most sensitive to weight-bearing activity. In addition, the lumbar spine comprises mainly trabecular bone, whereas the leg score reflects cortical bone; thus, the differential effects of training on different bone types could be evaluated.
Statistical Analysis
Statistics were calculated with SPSS (version 13.0 for Windows; SPSS Inc, Chicago, IL). Descriptive statistics on anthropometric characteristics were calculated for the total group and each sport team individually. Total percentage of body fat was obtained from the DXA scan report. To examine the possible effect of menstrual dysfunction on BMD measurements, we conducted 4 t tests to compare the total-body, lumbar spine, pelvis, and average leg BMD values between athletes with normal and abnormal menstrual status. Using analysis of covariance testing, we evaluated differences in the BMD measurements among sports with menstrual status and mass as covariates. Tukey post hoc testing was performed when necessary.
Pearson product moment correlations were calculated for each BMD measurement with age, gynecologic age, height, mass, BMI, and percentage of fat for the total group of athletes. Linear regression analysis was performed to determine predictors of each BMD measurement. Independent variables included age, gynecologic age, height, mass, and BMI as continuous variables and sport and oral contraceptive use as categoric variables. These analyses could not be stratified by sport because of the small number of athletes recruited from some sports. Body mass index was used instead of percentage of fat in these analyses because BMI is easier to measure clinically. Therefore, a practicing athletic trainer might have an easier time determining an athlete's risk for low BMD based on her BMI rather than her percentage of fat. Significance level was set at an α level of P ≤ .01 to account for multiple comparisons.
RESULTS
Athlete Characteristics
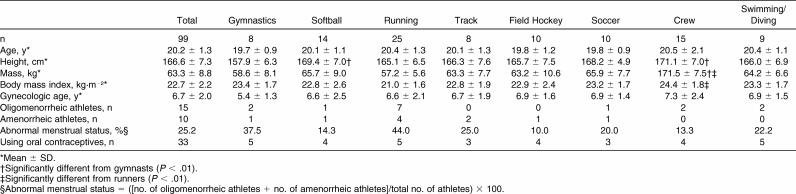
Complete data were available on 99 female athletes (approximately 50% of all female athletes) representing 8 varsity teams, including gymnastics (n = 8), softball (n = 14), running (cross-country and track runners with events of 800 m or longer; n = 25), track (field events and sprinters with events of less than 800 m; n = 8), field hockey (n = 10), soccer (n = 10), crew (n = 15), and swimming/diving (n = 9). Athletes in 3 other sports (basketball, golf, and tennis) chose not to participate due to scheduling conflicts. Participant characteristics are displayed in Table 1. Despite differences in height and mass measurements ( P < .01), BMI was markedly similar among groups, with the only significant difference being between runners (21.0 kg·m −2) and rowers (24.4 kg·m −2).
Table 1. Participant Characteristics.
Although age at menarche ranged from 11 to 18 years, no differences were noted among sport means for gynecologic age ( Table 1). Based on our definitions of menstrual dysfunction, 15 of 99 athletes were oligomenorrheic and 10 had amenorrhea. The number of oligomenorrheic and amenorrheic athletes was summed and divided by the total number of athletes per sport to determine the percentage of women with menstrual dysfunction. In our total sample of 99 athletes, 25.2% experienced some degree of menstrual dysfunction. Runners and gymnasts had the highest percentage of menstrual dysfunction (44.0% and 37.5%, respectively), whereas field hockey (10.0%) and crew athletes (13.3%) had the lowest. About one third of study participants were currently using oral contraceptives and of these, 6 reported menstrual disturbances in the past year.
Bone Mineral Density
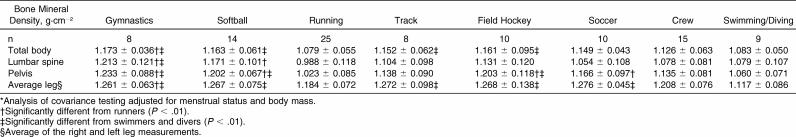
Comparisons of BMD among sports are shown in Table 2. Runners had significantly lower BMD than gymnasts had at every site ( P < .01). Mean pelvis BMD of runners was also lower than athletes in every sport except track, crew, and swimming/diving ( P < .01). Swimmers/divers showed significantly lower average leg BMD scores than all other athletes except for runners and rowers ( P < .01).
Table 2. Total and Site-Specific Bone Mineral Density by Sport (Mean ± SD)*.
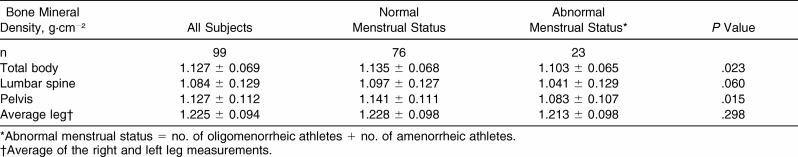
Total-body and site-specific BMD values compared by menstrual status are presented in Table 3. Although eumenorrheic athletes tended to have higher BMD scores, no significant differences were found. Statistics were not calculated on BMD differences by menstrual status within each sport due to the low number of athletes per group with menstrual dysfunction.
Table 3. Total-Body and Site-Specific Bone Mineral Density by Menstrual Status (Mean ± SD).
Correlations and Regression Analysis
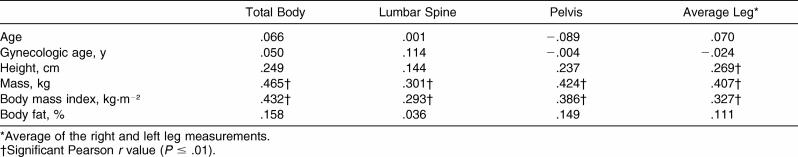
Total-body and site-specific BMD correlations are displayed in Table 4. Mass and BMI were correlated significantly with every BMD measurement recorded ( P < .01). Height was related significantly to average leg BMD score ( P < .01). Total percentage of body fat as determined by DXA was not correlated with any BMD measurement and, thus, was not included in the regression analysis.
Table 4. Total-Body and Site-Specific Bone Mineral Density Correlations.
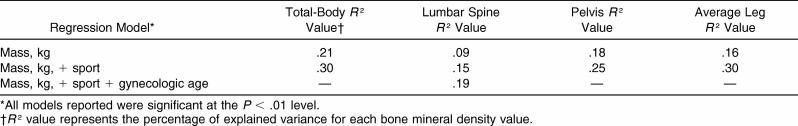
Among all possible explanatory variables (age, gynecologic age, height, mass, BMI, sport, and oral contraceptive use), only mass and sport consistently predicted BMD ( Table 5). Gynecologic age also entered the model as a significant predictor of lumbar spine BMD ( Table 5). The variance inflation factors ranged from 1.052 to 1.130 for each regression; hence, these models were not violated. When menstrual status as a dichotomous variable was forced into the model, the predictive value did not change significantly.
Table 5. Explained Variance for Total-Body and Site-Specific Bone Mineral Density Values, g·cm −2 .
DISCUSSION
Our purpose was to describe and compare BMD at a variety of sites among Division I female athletes and to determine predictor variables for each BMD measurement. Significant differences were noted in total-body and site-specific BMD values among athletic groups. Mass and sport were significant predictors of total-body, pelvic, and average leg BMD, whereas mass, sport, and gynecologic age predicted lumbar spine BMD in female collegiate athletes. In general, the BMD values reported here are similar to those reported for other athletes, despite differences in the subject population and the use of different DXA scan protocols and machines. 2, 3, 5, 8, 13
Swimmers/divers had lower average leg BMD values than other athletes, a finding that is consistent with past literature. 1, 3 Athletes who compete in non–weight-bearing activities, such as swimming, typically show lower BMD in the lumbar spine and lower limb sites than athletes who perform weight-bearing activities. 1, 4 Due to the small number of study participants, swimming (n = 6) and diving (n = 3) were combined for our analysis; thus, our results cannot differentiate between the sports. Although swimmers and divers displayed lower BMD values, the nature of their sport also protects them from impact-related bone injuries during competition. However, research suggests an increased risk of lower extremity injuries during cross-training sessions, which may be due to BMD deficiencies. 21
Runners also demonstrated lower BMD at several sites when compared with athletes in the other sports and had the lowest total-body, lumbar spine, and pelvis BMD values. This finding was surprising because running is a high-impact activity that would be expected to increase lower body BMD. However, other authors 22 have shown similar trends toward lower total-body and lumbar spine BMD values for runners versus gymnasts. The previous investigators hypothesized that gymnastics training invokes greater bone loading; thus, the athletes may benefit from an enhanced osteogenic stimulus. Athletic trainers should be aware of the high incidence of low BMD among female runners and should keep them under close observation for stress fractures and other bone injuries.
Our runners had total-body and site-specific BMD values similar to those reported previously for amenorrheic runners using a similar protocol for measuring whole-body, lumbar spine, and pelvis BMD. 23 Possible reasons for this include decreased calcium intake, disordered eating, or insufficient energy intake relative to energy expenditure (or a combination of these factors). Investigators 24 have shown that disordered eating and energy deficits are related to low BMD even without menstrual disturbances. In addition, a high incidence of eating disorders may exist among long-distance runners. 25 Although we did not collect nutritional information on athletes participating in our study and excluded 1 athlete for a suspected eating disorder, it is still plausible that the running group as a whole may have experienced a greater energy deficit as a result of high training volume and disordered eating than the athletes in other sports. 14
Our data showed no differences in BMD values by menstrual status when sports were collapsed and all athletes were considered together; it is possible, however, that our study was underpowered to detect significant differences, because strong trends existed for higher total-body, lumbar spine, and pelvis BMD values among the normally menstruating athletes. Past researchers have been divided as to the importance of menstrual dysfunction with regard to BMD in athletes. Although Meyer et al 2 found that menstrual history was not associated with BMD in Olympic-level winter sport athletes, most other authors 6, 26 have reported that menstrual dysfunction is strongly linked to decreased BMD. Regarding lumbar spine BMD specifically, some investigators 27, 28 have found no differences between groups of normally and abnormally menstruating athletes. In contrast, a strong body of literature suggests that duration of menstrual dysfunction is related to decreased BMD at the lumbar spine. 8–10, 13, 15, 16 It appears that decreased estrogen levels affect the more metabolically active trabecular bone before the cortical bone. 8, 29 Our findings partially support this hypothesis, because gynecologic age significantly added to the prediction of lumbar spine BMD, which, being composed mostly of trabecular bone, appears to be sensitive to less estrogen exposure. Thus, one measure of menstrual status (gynecologic age) was related to lumbar spine BMD, even though the mean value was not lower when comparing athletes with abnormal versus normal menstrual status. Different scan protocols (ie, site specific versus total body) and menstrual status measurement procedures may account partially for contradictory results in past studies. Additionally, some women in our sample with abnormal menstrual status reported taking oral contraceptives (n = 6 of 23). This factor could lead to a misclassification error and could dilute the effect of menstrual disturbance, because these women were still exposed to normal hormone levels, which may have helped to maintain BMD.
Mass was the best predictor of BMD for female athletes in our study. Although sport also was significantly related to each BMD measurement and added some predictive ability, mass alone explained most of the variance in BMD at each site measured. Mechanistically, a higher mass would be expected to lead to greater gains in BMD among athletes as a result of greater loading with every movement, thereby providing a greater osteogenic stimulus. 1, 3 Adding menstrual status to the regression models did not increase our ability to predict variance in BMD. The majority of athletes with menstrual disturbances also had lower body masses; thus, it is possible that menstrual status did not add to the predictive value because the 2 measurements were interrelated.
Regression analysis results were in partial agreement with previous literature. Although they did not report menstrual status, Quintas et al 5 found that low body weight and insufficient energy intakes were associated with lower BMD values. Other groups have shown body weight and menstrual status to be the best predictors of BMD at the lumbar spine. 8, 13, 23 Weight-bearing exercise or high-impact activities (or both) appear to be somewhat protective for the BMD at weight-bearing sites in amenorrheic athletes. 10, 22 Similar to our results, Robinson et al 22 found that gymnasts had higher total-body and leg BMD values than runners had, despite having a greater incidence of menstrual disturbances. However, other researchers have found that extended periods of menstrual irregularity resulted in low bone density even at weight-bearing sites among athletes involved in high-impact activities. 13 Past investigators used different menstrual status categories, wide age ranges, and various physical activity levels, which make comparisons difficult. Results from our study are more specific to National Collegiate Athletic Association Division I college athletes and, hence, may be of more practical value to athletic trainers and sports physicians than the past literature is. These findings should be replicated in a larger, more diverse sample, however, before it will be possible to validate a BMD prediction equation using clinically available information.
Some limitations of the current study include the use of region-of-interest BMD values from a total-body scan and a lack of dietary information, training and injury histories, and detailed menstrual histories. The use of region-of-interest BMD values limits our ability to compare our results with those of authors using site-specific bone scans, yet our results were generally in agreement with those using more exact measures. 2, 3, 5, 8, 13 Although we collected information on menstruation during the past year and the age at menarche, athletes were not questioned on prior irregularities. Complete menstrual history may be more important than recent menstrual status when considering BMD. 8, 13, 15 In addition, it is possible that the number of years spent training or the number of skeletal injuries sustained (or both) may affect BMD. 2, 30 However, it is reasonable to assume that female athletes on Division I varsity teams have similarly intense training histories. Dietary history may have added to our bone density predictive ability, but valid data are difficult to collect in normal clinical settings.
The results of this study add significantly to our understanding of bone health in Division I female athletes. Although it included only 99 women, this is one of the largest cohorts of collegiate female athletes with information regarding BMD published to date. In addition, no other groups have specifically considered the bone health of athletes in several sports included here (eg, softball, field hockey, crew). Our data showed that despite different training modes, total-body BMD was markedly similar among athletes in the various sports except for running and swimming/diving. Greater differences among sports were seen when comparing lumbar spine, pelvis, and average leg BMD; however, only the runners and swimmers and divers showed significantly lower values, which may have clinical implications. Athletic trainers working with female runners, swimmers, and divers should keep in mind their higher incidence of low BMD and be wary of overtraining. Future investigators examining the BMD of female athletes should consider using more detailed training, injury, menstrual, and dietary histories on a larger sample of athletes. In addition, longitudinal studies involving the BMD of varsity female athletes may yield more information on how bone health changes in response to intense training over time.
REFERENCES
- Creighton DL, Morgan AL, Boardley D, Brolinson PG. Weight-bearing exercise and markers of bone turnover in female athletes. J Appl Physiol. 2001;90:565–570. doi: 10.1152/jappl.2001.90.2.565. [DOI] [PubMed] [Google Scholar]
- Meyer NL, Shaw JM, Manore MM. Bone mineral density of Olympic-level female winter sport athletes. Med Sci Sports Exerc. 2004;36:1594–1601. doi: 10.1249/01.mss.0000139799.20380.da. et al. [DOI] [PubMed] [Google Scholar]
- Fehling PC, Alekel L, Clasey J, Rector A, Stillman RJ. A comparison of bone mineral densities among female athletes in impact loading and active loading sports. Bone. 1995;17:205–210. doi: 10.1016/8756-3282(95)00171-9. [DOI] [PubMed] [Google Scholar]
- Taaffe DR, Snow-Harter C, Connolly DA, Robinson TL, Brown MD, Marcus R. Differential effects of swimming versus weight-bearing activity on bone mineral status of eumenorrheic athletes. J Bone Miner Res. 1995;10:586–593. doi: 10.1002/jbmr.5650100411. [DOI] [PubMed] [Google Scholar]
- Quintas ME, Ortega RM, Lopez-Sobaler AM, Garrido G, Requejo AM. Influence of dietetic and anthropometric factors and of the type of sport practised on bone density in different groups of women. Eur J Clin Nutr. 2003;57:S-58–S-62. doi: 10.1038/sj.ejcn.1601817. (suppl 1) [DOI] [PubMed] [Google Scholar]
- Nattiv A, Agostini R, Drinkwater B, Yeager KK. The female athlete triad: the inter-relatedness of disordered eating, amenorrhea, and osteoporosis. Clin Sports Med. 1994;13:405–418. [PubMed] [Google Scholar]
- Myburgh KH, Hutchins J, Fataar AB, Hough SF, Noakes TD. Low bone density is an etiologic factor for stress fractures in athletes. Ann Intern Med. 1990;113:754–759. doi: 10.7326/0003-4819-113-10-754. [DOI] [PubMed] [Google Scholar]
- Drinkwater BL, Bruemner B, Chesnut CH., III. Menstrual history as a determinant of current bone density in young athletes. JAMA. 1990;263:545–548. [PubMed] [Google Scholar]
- Cann CE, Martin MC, Genant HK, Jaffe RB. Decreased spinal mineral content in amenorrheic women. JAMA. 1984;251:626–629. [PubMed] [Google Scholar]
- Young N, Formica C, Szmukler G, Seeman E. Bone density at weight-bearing and nonweight-bearing sites in ballet dancers: the effects of exercise, hypogonadism, and body weight. J Clin Endocrinol Metab. 1994;78:449–454. doi: 10.1210/jcem.78.2.8106634. [DOI] [PubMed] [Google Scholar]
- Zeni AI, Street CC, Dempsey RL, Staton M. Stress injury to the bone among women athletes. Phys Med Rehabil Clin N Am. 2000;11:929–947. [PubMed] [Google Scholar]
- Fast facts. National Osteoperosis Foundation. Available at: http://www.nof.org/osteoporosis/diseasefacts.htm. Accessed June 12, 2007 .
- Rencken ML, Chesnut CH, III, Drinkwater BL. Bone density at multiple skeletal sites in amenorrheic athletes. JAMA. 1996;276:238–240. [PubMed] [Google Scholar]
- Zanker CL, Swaine IL. Bone turnover in amenorrhoeic and eumenorrhoeic women distance runners. Scand J Med Sci Sports. 1998;8:20–26. doi: 10.1111/j.1600-0838.1998.tb00224.x. [DOI] [PubMed] [Google Scholar]
- Micklesfield LK, Lambert EV, Fataar AB, Noakes TD, Myburgh KH. Bone mineral density in mature, premenopausal ultramarathon runners. Med Sci Sports Exerc. 1995;27:688–696. [PubMed] [Google Scholar]
- Tomten SE, Falch JA, Birkeland KI, Hemmersbach P, Hostmark AT. Bone mineral density and menstrual irregularities: a comparative study on cortical and trabecular bone structures in runners with alleged normal eating behavior. Int J Sports Med. 1998;19:92–97. doi: 10.1055/s-2007-971888. [DOI] [PubMed] [Google Scholar]
- Duncan CS, Blimkie CJ, Cowell CT, Burke ST, Briody JN, Howman-Giles R. Bone mineral density in adolescent female athletes: relationship to exercise type and muscle strength. Med Sci Sports Exerc. 2002;34:286–294. doi: 10.1097/00005768-200202000-00017. [DOI] [PubMed] [Google Scholar]
- Egan E, Reilly T, Giacomoni M, Redmond L, Turner C. Bone mineral density among female sports participants. Bone. 2006;38:227–233. doi: 10.1016/j.bone.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Warner ER, Fornetti WC, Jallo JJ, Pivarnik JM. A skinfold model to predict fat-free mass in female athletes. J Athl Train. 2004;39:259–262. [PMC free article] [PubMed] [Google Scholar]
- Fornetti WC, Pivarnik JM, Foley JM, Fiechtner JJ. Reliability and validity of body composition measures in female athletes. J Appl Physiol. 1999;87:1114–1122. doi: 10.1152/jappl.1999.87.3.1114. [DOI] [PubMed] [Google Scholar]
- McFarland EG, Wasik M. Injuries in female collegiate swimmers due to swimming and cross training. Clin J Sport Med. 1996;6:178–182. doi: 10.1097/00042752-199607000-00007. [DOI] [PubMed] [Google Scholar]
- Robinson TL, Snow-Harter C, Taaffe DR, Gillis D, Shaw J, Marcus R. Gymnasts exhibit higher bone mass than runners despite similar prevalence of amenorrhea and oligomenorrhea. J Bone Miner Res. 1995;10:26–35. doi: 10.1002/jbmr.5650100107. [DOI] [PubMed] [Google Scholar]
- Pettersson U, Stalnacke B, Ahlenius G, Henriksson-Larsen K, Lorentzon R. Low bone mass density at multiple skeletal sites, including the appendicular skeleton in amenorrheic runners. Calcif Tissue Int. 1999;64:117–125. doi: 10.1007/s002239900589. [DOI] [PubMed] [Google Scholar]
- Zanker CL, Cooke CB. Energy balance, bone turnover, and skeletal health in physically active individuals. Med Sci Sports Exerc. 2004;36:1372–1381. doi: 10.1249/01.mss.0000135978.80362.aa. [DOI] [PubMed] [Google Scholar]
- Rosen LW, McKeag DB, Hough DO, Curley V. Pathogenic weight-control behavior in female athletes. Physician Sportsmed. 1986;14(1):79–86. doi: 10.1080/00913847.1986.11708966. [DOI] [PubMed] [Google Scholar]
- Zanker CL. Bone metabolism in exercise associated amenorrhoea: the importance of nutrition. Br J Sports Med. 1999;33:228–229. [PubMed] [Google Scholar]
- Moen SM, Sanborn CF, DiMarco NM. Lumbar bone mineral density in adolescent female runners. J Sports Med Phys Fitness. 1998;38:234–239. et al. [PubMed] [Google Scholar]
- Punpilai S, Sujitra T, Ouyporn T, Teraporn V, Sombut B. Menstrual status and bone mineral density among female athletes. Nurs Health Sci. 2005;7:259–265. doi: 10.1111/j.1442-2018.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- Wolman RL, Clark P, McNally E, Harries M, Reeve J. Menstrual state and exercise as determinants of spinal trabecular bone density in female athletes. BMJ. 1990;301:516–518. doi: 10.1136/bmj.301.6751.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U, Nordstrom P, Alfredson H, Henriksson-Larsen K, Lorentzon R. Effect of high impact activity on bone mass and size in adolescent females: a comparative study between two different types of sports. Calcif Tissue Int. 2000;67:207–214. doi: 10.1007/s002230001131. [DOI] [PubMed] [Google Scholar]