Abstract
Context: Rectal temperature is recommended by the National Athletic Trainers' Association as the criterion standard for recognizing exertional heat stroke, but other body sites commonly are used to measure temperature. Few authors have assessed the validity of the thermometers that measure body temperature at these sites in athletic settings.
Objective: To assess the validity of commonly used temperature devices at various body sites during outdoor exercise in the heat.
Design: Observational field study.
Setting: Outdoor athletic facilities.
Patients or Other Participants: Fifteen men and 10 women (age = 26.5 ± 5.3 years, height = 174.3 ± 11.1 cm, mass = 72.73 ± 15.95 kg, body fat = 16.2 ± 5.5%).
Intervention(s): We simultaneously tested inexpensive and expensive devices orally and in the axillary region, along with measures of aural, gastrointestinal, forehead, temporal, and rectal temperatures. Temporal temperature was measured according to the instruction manual and a modified method observed in medical tents at local road races. We also measured forehead temperatures directly on the athletic field (other measures occurred in a covered pavilion) where solar radiation was greater. Rectal temperature was the criterion standard used to assess the validity of all other devices. Subjects' temperatures were measured before exercise, every 60 minutes during 180 minutes of exercise, and every 20 minutes for 60 minutes of postexercise recovery. Temperature devices were considered invalid if the mean bias (average difference between rectal temperature and device temperature) was greater than ±0.27°C (±0.5°F).
Main Outcome Measure(s): Temperature from each device at each site and time point.
Results: Mean bias for the following temperatures was greater than the allowed limit of ±0.27°C (±0.5°F): temperature obtained via expensive oral device (−1.20°C [−2.17°F]), inexpensive oral device (−1.67°C [−3.00°F]), expensive axillary device (−2.58°C [−4.65°F]), inexpensive axillary device (−2.07°C [−3.73°F]), aural method (−1.00°C [−1.80°F]), temporal method according to instruction manual (−1.46°C [−2.64°F]), modified temporal method (−1.36°C [−2.44°F]), and forehead temperature on the athletic field (0.60°C [1.08°F]). Mean bias for gastrointestinal temperature (−0.19°C [−0.34°F]) and forehead temperature in the pavillion (−0.14°C [−0.25°F]) was less than the allowed limit of ±0.27°C (±0.5°F). Forehead temperature depended on the setting in which it was measured and showed greater variation than other temperatures.
Conclusions: Compared with rectal temperature (the criterion standard), gastrointestinal temperature was the only measurement that accurately assessed core body temperature. Oral, axillary, aural, temporal, and field forehead temperatures were significantly different from rectal temperature and, therefore, are considered invalid for assessing hyperthermia in individuals exercising outdoors in the heat.
Keywords: core body temperature, hyperthermia, tympanic membrane temperature
Key Points
Oral, axillary, tympanic (aural), temporal, and forehead measurements did not accurately assess core body temperature in athletes exercising intensely in the heat.
The sports medicine staff must be prepared and willing to obtain a rectal temperature when necessary to assess the degree of hyperthermia for an athlete who has been exercising in the heat.
Core body temperature measured with an ingestible thermistor provides a valid indication of the body temperature rise and fall associated with the onset and cessation of exercise in the heat.
When athletes perform intense exercise in the heat, exertional heat stroke is a risk. One of the key diagnostic determinants between nonlethal heat exhaustion and exertional heat stroke (a medical emergency) is an accurate and immediate assessment of core body temperature. Athletic trainers, team physicians, and other emergency personnel often depend on measurement devices that have not been validated. 1 Any delay in or absence of treatment due to spuriously low body temperature measurements could prove fatal. 2 Indeed, the delay in obtaining an accurate temperature measurement has cost the lives of many athletes with exertional heat stroke. Their deaths were unnecessary because a rapid and accurate temperature assessment followed by rapid cooling (ie, ice or cold-water immersion) would have ensured survival. 3 This scenario of lack of proper temperature assessment and rapid cooling continues to occur in the United States every summer, when many athletes begin preseason practices. The sports medicine staff should take appropriate action to prevent these unnecessary tragedies.
In a recent pilot study, 1 athletic trainers were found to most often rely on tympanic (aural) and oral assessment of body temperature when evaluating suspected hyperthermia. This finding is especially surprising because the 2002 National Athletic Trainers' Association position statement regarding exertional heat illnesses 4 clearly directed that temperature assessment, for a suspected hyperthermic athlete who has been exercising in the heat, should be obtained rectally. This document further stated that “… the ATC should not rely on the oral, tympanic [aural], or axillary temperature for athletes because these are inaccurate and ineffective measures of body-core temperatures during and after exercise.” 4 Authors of a recent review 2 also seriously questioned the use of these devices (as well as temporal temperature) and pointed out that many of them have not been tested on athletes performing intense exercise in the heat. Speculation about accuracy arises because these devices may be influenced by skin temperature, evaporating sweat, ingestion of fluids, and wind. 2, 5, 6
Our purpose was to test the most common field measurement devices for core temperature assessment during intense exercise in hot outdoor environments. We assessed the validity of devices that measure oral, axillary, aural, gastrointestinal, forehead, and temporal temperature before, during, and after outdoor exercise in the heat. We tested the following commonly used temperature devices: inexpensive and expensive digital thermistors for oral measurement, inexpensive and expensive digital thermistors for axillary measurement, tympanic thermistor for aural measurement, intestinal thermistor for gastrointestinal measurement, liquid crystal sticker (2 methods) for forehead measurement, and scanner (2 methods) for temporal measurement. To our knowledge, no other authors have simultaneously evaluated these devices on athletes exercising outdoors in the heat.
Rectal temperature (RCT), as recommended by the National Athletic Trainers' position statement, 4 was selected as the criterion standard because of its validity 7 and practicality of use in this setting. Rectal temperature is both valid and reliable for temperature measurement in individuals at rest and while exercising 2, 7–10 and is considered the criterion standard for temperature measurement in hyperthermic athletes. 5, 6, 11 Despite a reported lag in response time (ie, versus esophageal or pulmonary artery temperature), RCT provides a valid core temperature measurement in the field for diagnosis and treatment of exertional heat stroke. 11–17
Given the consistent findings that gastrointestinal temperature closely matches RCT in hyperthermic individuals, 18–20 we hypothesized that measurement at the former site via an ingestible thermistor would match RCT with the least degree of bias when measurements were taken on subjects during outdoor exercise in the heat. For obvious reasons, temperatures were not elevated to the point of exertional heat stroke, but the 40°C (104°F) limit provided a window into the potential ability of these devices to track hyperthermia in a consistent and meaningful manner.
METHODS
After signing an informed consent form approved by the institutional review board (which also approved the study), recruited subjects completed a self-administered medical history questionnaire and were excluded if contraindications for exercise in the heat were present (eg, heat intolerance or heat stroke within the last 3 years). All subjects were physically active (≥2 workouts per week and/or ≥4 hours of exercise per week). Before testing, sex, age, height, mass (scale model BWB-800A; Tanita Corp, Tokyo, Japan), and skinfold thickness at 3 sites to estimate percentage of body fat 21–23 were measured.
To help achieve a euhydrated state, subjects were instructed to drink 473 mL (16 ounces) of fluid both the night before testing and in the morning before arrival at the playing fields. Three hours before their scheduled arrival, subjects swallowed an intestinal thermistor (CorTemp; HQ Inc, Palmetto, FL) that has been approved by the US Food and Drug Administration. Subjects were contacted the night before testing to ensure compliance with instructions.
Measurement Sites and Devices
Temperature measurements were taken by a team of 4 trained researchers as follows: gastrointestinal, RCT, and forehead values (researcher A); oral values (researcher B); aural (ie, ear canal) and temporal values (researcher C); and axillary values (researcher D). To maintain consistency and accuracy of placement of the axillary, aural, and temporal measures, the same researchers measured these sites for each subject.
Oral temperature was measured using an inexpensive (model VT-801BWT; Walgreen Co, Deerfield, IL) and expensive (SureTemp model 679; Welch Allyn Medical Products, Skaneateles Falls, NY) digital thermometer (ORL IE and ORL E, respectively); both were used according to the instruction manuals (tip placed below the tongue, toward the back of the mouth). Axillary temperature was measured by inexpensive (model VT-801BWT; Walgreen Co) and expensive (DataTherm model 00703; RG Medical Diagnostics, Southfield, MI) temperature devices (AXL IE and AXL E, respectively) placed high in the central axillary region, with the subjects' arms adducted after being wiped free of sweat. Before data analysis, we adjusted AXL E measures according to the instruction manual in order to “estimate rectal temperature” (add 1°C [1.8°F]). Aural temperature (AUR) was measured using a “tympanic” ear thermometer (Braun Thermoscan ExacTemp model IRT 4520; Braun, South Boston, MA) according to the instruction manual. Forehead skin temperature (FST) was measured using a forehead sticker (SportsTemp; Greenwood Village, CO) affixed vertically in the middle of the forehead above the left eyebrow. Temporal artery temperature was assessed via a temporal artery scanner (model 2000C; Exergen Corp, Watertown, MA) using the method described in the instruction manual (with no visible sweat, swipe from forehead to hairline; with sweat, hold behind the ear just anterior to the mastoid process; TEM INST) and a modified method observed at local road races (swipe from forehead to hairline and then around the back edge of the ear, ending just anterior to the mastoid process; TEM MOD). Rectal temperature was measured using a rectal thermistor (model 401; Yellow Springs Instruments, Inc, Yellow Springs, OH) inserted at least 10 cm beyond the anal sphincter. Thermal sensation was evaluated at each time point using a visual 0 to 8 scale ( unbearably cold to unbearably hot) adapted from Toner et al. 24 Subjects observing the scale were asked, “How hot or cold do you feel right now?”
Measurements at each site were started at the same time. The order of measurements for each researcher was as follows:
Researcher A: RCT, INT, FST
Researcher B: ORL E, ORL IE
Researcher C: AUR (twice), TEM INST (twice), TEM MOD (twice)
Researcher D: AXL E in left axilla and AXL IE in right axilla
Measurements took less than 2 minutes except for those made with the inexpensive thermometers (ORL IE, AXL IE). These devices took up to 5 minutes to stabilize and to provide a reading. At the end of each series of measurements, researcher A repeated his or her measures, and the continuous reading of AXL E was recorded.
Protocol
Upon arrival and after the subject's forehead was cleaned with rubbing alcohol and dried, an FST was affixed as described above. Other measurement devices (RCT, ORL E, ORL IE, AXL E, AXL IE, AUR, TEM INST, TEM MOD) were put in place, were used, and were removed as described above during each temperature measurement period.
The initial temperature measurement (minute 0) occurred just before subjects began to play various team sports (ie, soccer, ultimate Frisbee). Subjects remained active throughout data collection except for short breaks to allow for temperature measurements (5 to 10 minutes) every hour. Subjects' temperatures were assessed after 60, 120, and 180 minutes of exercise and 20, 40, and 60 minutes postexercise (minutes 200, 220, and 240, respectively). Approximately 5 minutes before temperature assessment, a measurement of FST was taken on the field (FST FLD) and subjects proceeded to a nearby bathroom (30 m), where they inserted a rectal thermistor. Temperature measurements were then obtained from the subjects in a covered pavilion adjacent to the field no longer than 5 minutes after they exited the playing field. The FST FLD was measured while the subjects remained in the sun because we hypothesized that FST measures would be influenced by solar radiation.
During exercise, subjects consumed fluid and food ad libitum except within 5 minutes of the beginning of temperature measurement. We measured the wet bulb, dry bulb, and globe temperatures to provide the wet bulb globe temperature on the field every 60 minutes.
Statistical Analyses
The RCT value was an average of the RCTs at the beginning and end of the 5-minute temperature-measuring period. Measurements from other devices that were taken twice in a given time period (AUR, INT, FST, TEM INST, TEM MOD) also were averaged for comparison with RCT.
We conducted a 2-way (temperature device × time) repeated-measures analysis of variance to test the significance of mean differences in devices over time. To evaluate differences with a given device versus RCT, follow-up repeated-measures t tests with the Bonferroni alpha correction were used. Greenhouse-Geisser corrections were performed when the assumption of sphericity was violated.
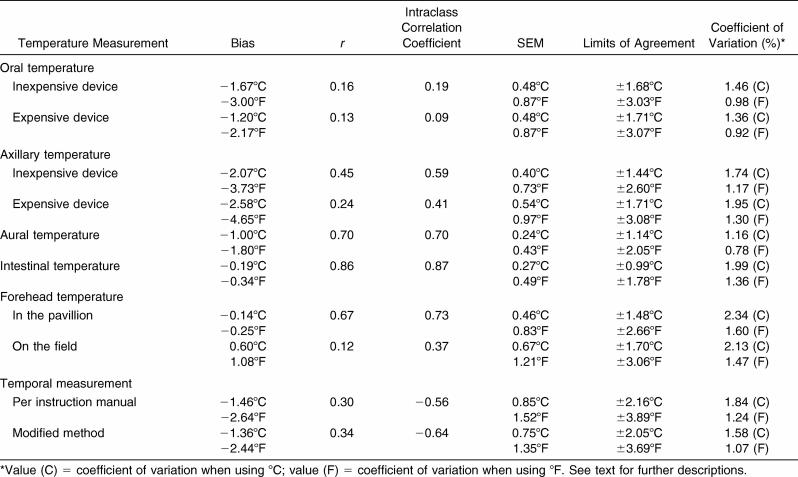
Validity for each device, using RCT as the criterion measure, was evaluated with a range of measurement error statistics. Mean bias and limits of agreement were calculated as described by Bland-Altman. 25 Bias is defined as the mean difference between RCT and device (device temperature minus RCT); limits of agreement were calculated by multiplying the SD of the mean difference between the temperature device measurement and RCT by 1.96 (2 SDs). 25 The difference between the temperature device reading and RCT, with a 95% probability, is expected to lie within the limits of agreement. 26 Intraclass correlation coefficients (2-way mixed-effects model), SEM, and coefficient of variation were calculated between each device measurement and RCT as outlined by Atkinson and Nevill. 26 Pearson product moment coefficients of correlation ( r), corrected for repeated measures, 27 were calculated only to evaluate relative agreement of device measurements. Although r, intraclass correlation coefficients, SEM, limits of agreement, and coefficient of variation all provide insight into the validity of a device, we determined that for simplicity and practicality for the medical staff, a mean bias of a given device greater than ±0.27°C (0.5°F) from RCT would bring a device's validity into question. This level of bias is similar to that used by other authors examining the validity of body temperature measuring devices. 18 All statistical tests were performed with SPSS (version 10 for Windows; SPSS, Inc, Chicago, IL) with α set at .05.
RESULTS
Rectal probe calibration was verified by comparing each probe with a certified glass thermometer in water baths of various water temperatures (24.5°C to 41°C [76.1°F to 105.8°F]) and measuring a mean difference of −0.12°C ± 0.12°C (−0.22°F ± 0.21°F). Fifteen men and 10 women (mean age = 26.5 ± 5.3 years, height = 174.3 ± 11.1 cm, mass = 72.73 ± 15.95 kg, body fat = 16.2 ± 5.5%) participated in this study. On the 2 days of data collection, the dry bulb, wet bulb, and wet bulb globe temperatures on the athletic fields were 32.6°C ± 2.0°C (90.8°F ± 3.6°F), 23.9°C ± 2.0°C (75.0°F ± 3.6°F), and 29.4°C ± 1.4°C (84.9°F ± 2.5°F), respectively. In the covered pavilion, the dry bulb, wet bulb, and wet bulb globe temperatures were 29.7°C ± 2.1°C (85.5°F ± 3.7°F), 22.6°C ± 1.2°C (72.7°F ± 2.2°F), and 24.7°C ± 1.1°C (76.4°F ± 2.1°F), respectively.
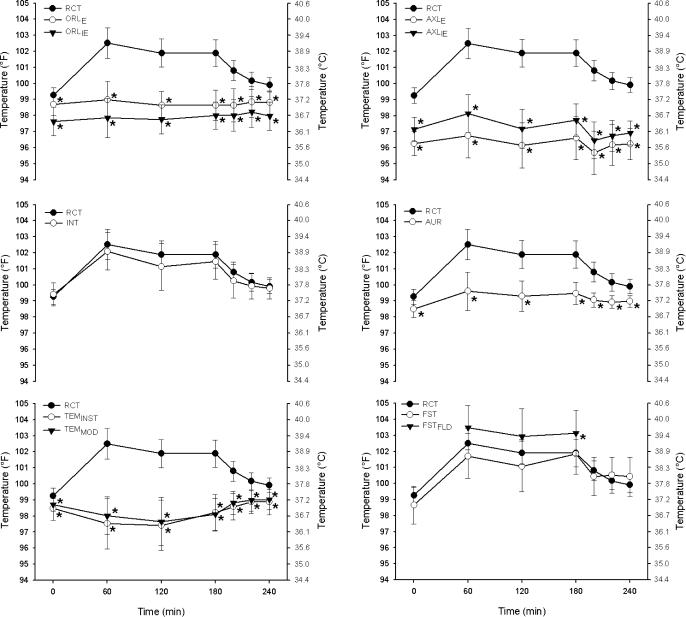
Subjects' core body temperature at each time point, as measured by RCT, decreased slightly from beginning to end of each 5-minute temperature measurement period (38.30°C ± 0.74°C [100.94°F ± 1.33°F] and 38.28°C ± 0.73°C [100.90°F ± 1.32°F]; F 1,170 = 8.93, P = 0.007). The interaction of time and temperature device was significant (F 6,54 = 21.89, P < .001; Figure 1).
Figure 1. Mean ± SD of each temperature device over time compared with RCT. RCT = rectal temperature, ORL IE = oral temperature with inexpensive thermometer, ORL E = oral temperature with expensive thermometer, AXL IE = axillary temperature with inexpensive thermometer, AXL E = axillary temperature with expensive thermometer, INT = intestinal temperature, AUR = aural temperature, TEM INST = temporal temperature measured with the method described by the instructional manual, TEM MOD = temporal temperature measured in a modified method, FST = forehead sticker temperature, and FST FLD = forehead temperature measured on the field. (See text for further description.) *Indicates significant difference from RCT at the same time point ( P < .05) .
Temperature Before Exercise
Before exercise began, the device measurements were significantly different (F 9,216 = 55.38, P < .001). Compared with RCT, the ORL IE ( P < .001), ORL E ( P = .031), AXL IE ( P < .001), AXL E ( P < .001), AUR ( P < .001), TEM INST ( P = .001), and TEM MOD ( P < .001) were all significantly lower ( Figure 1). Only the INT and FST measurements were not different from pre-exercise RCT ( P = 1.000 and .078, respectively).
Temperature During Exercise
At each exercise time point (minutes 60, 120, and 180), ORL E, ORL IE, AXL E, AXL IE, AUR, TEM INST, and TEM MOD were all significantly lower than RCT (all P < .001; Figure 1). The INT and RCT were not significantly different at 60, 120, and 180 minutes ( P = 1.00, .291, and .239, respectively). The FST was also not different from RCT during exercise ( P = .503, .284, and 1.00 for 60, 120, and 180 minutes, respectively). The FST FLD was not statistically different from RCT at 60 and 120 minutes ( P = .131, and .294, respectively) but was higher than RCT at 180 minutes ( P = .044). On approximately 2 occasions during exercise, the FST became dislodged from the subject's forehead and due to the presence of sweat could not be reattached. Therefore, FST data for these subjects were no longer collected. Three measurements of FST FLD during exercise were at the upper limit of the device's measurement scale (∼41°C [∼106°F]).
Temperature Postexercise
At each postexercise time point (minutes 200, 220, and 240) ORL E, ORL IE, AXL E, AXL IE, AUR, TEM INST, and TEM MOD were all significantly lower than RCT (all P ≤ .001; Figure 1). The INT and RCT were not significantly different at 200, 220, and 240 minutes ( P = .254, 1.00, and 1.00, respectively). The FST was also not different from RCT postexercise ( P = 1.00).
Temperature Device Validity
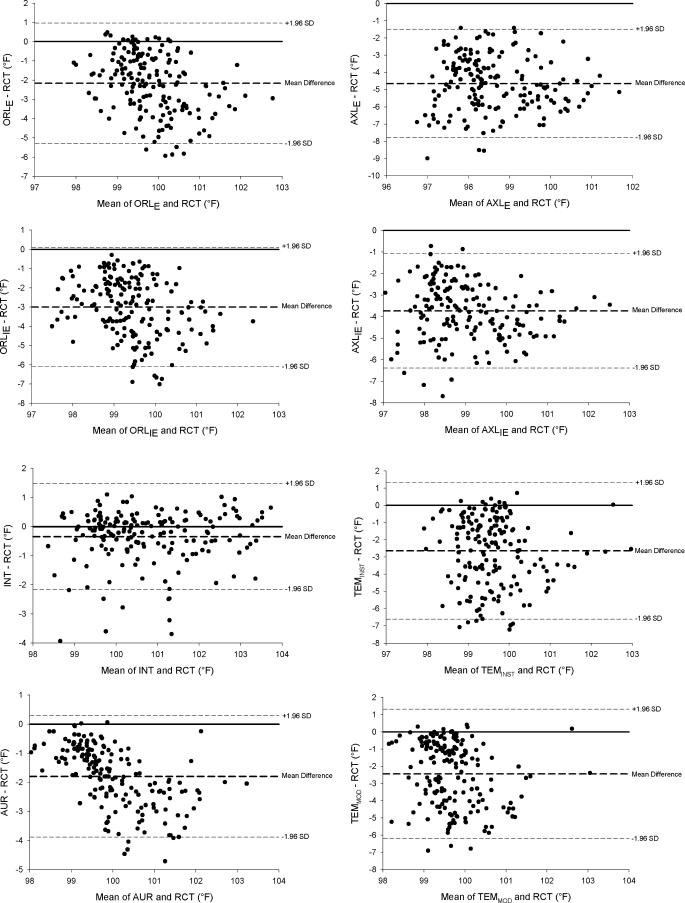
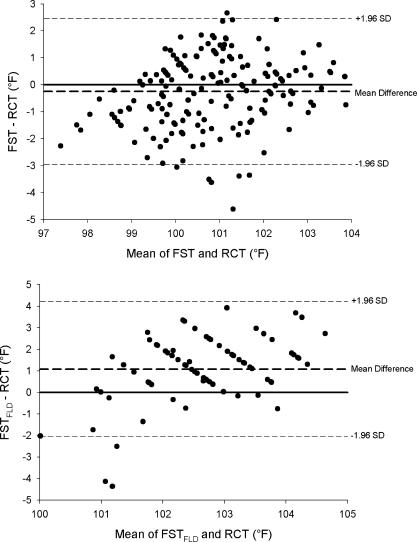
Mean bias, r, intraclass correlation coefficients, SEM, limits of agreement, and coefficients of variation are presented in the Table. Mean bias and limits of agreement are represented graphically with Bland-Altman plots in Figure 2. According to the criteria set, only the mean bias of INT and FST (but not FST FLD) fell within the accepted mean bias range (±0.27°C [±0.5°F]).
Measures of Validity Using Rectal Temperature as the Reference.
Figure 2. Bland-Altman plots indicating the mean bias (bold dashed line) and limits of agreement (dashed lines) for each temperature device compared with RCT. RCT = rectal temperature, ORL IE = oral temperature with inexpensive thermometer, ORL E = oral temperature with expensive thermometer, AXL IE = axillary temperature with inexpensive thermometer, AXL E = axillary temperature with expensive thermometer, INT = intestinal temperature, AUR = aural temperature, TEM INST = temporal temperature measured with the method described by the instructional manual, TEM MOD = temporal temperature measured in a modified method, FST = forehead sticker temperature, and FST FLD = forehead temperature measured on the field. (See text for further description) .
Thermal Sensation
Because thermal sensation ranged from 0 to 8, the only direct comparison to RCT we made was a Pearson correlation. Thermal sensation was significantly correlated with RCT ( r = .72, P < .001).
DISCUSSION
Temperature readings from many devices (at various body sites) that are commonly used by sports medicine professionals are not valid for assessing hyperthermia in individuals who are exercising outdoors in the heat. We defined a valid device as one that provided a temperature reading within ±0.27°C (±0.5°F) of our criterion standard (RCT). The devices that measured body temperature at the following sites were shown to be invalid and should not be used during outdoor exercise in the heat: oral, axillary, aural (tympanic), and temporal. Given the common use of these devices at these sites by sports medicine staff and the extremely serious nature of temperature assessment (ie, evaluating the likelihood of exertional heat stroke), we believe that these findings have life-saving implications. The forehead sticker, which is used less often than the other devices, is not valid for assessing exertional heat stroke due to its limited temperature range, different temperature readings in different settings, and its potential to fall off. Ultimately, we believe that only the RCT and INT methods provide a valid assessment of hyperthermia during intense outdoor exercise in the heat and during recovery. Sports medicine staff professionals should always be able to measure RCT when athletes exercise in a hot environment. Although valid, an INT will not be available or possible in every situation in which a temperature reading is necessary.
Oral Temperature
An oral thermometer often is used to obtain body temperature in resting individuals, 5, 28 but its clinical validity is uncertain. 2, 8, 29 Conflicting findings could be the result of oral temperature being affected by eating, drinking, breathing, swallowing, facial fanning, saliva temperature, and air temperature. 5, 6, 11, 14, 15 Although it appears that oral temperature is consistently lower than deep body temperature, 30 corrected values (ie, via predetermined algorithms) do not accurately represent RCTs. 10 At every time point we measured, both ORL E and ORL IE were significantly lower than RCT ( P < .05; Figure 1). At peak temperature (minute 60), RCT averaged 39.17°C ± 0.53°C (102.51°F ± 0.95°F), whereas ORL E (37.21°C ± 0.64°C [98.98°F ± 1.14°F]) and ORL IE (36.58°C ± 0.68°C [97.85°F ±1.22°F]) were lower (both P < .001). The large mean difference from RCT at peak temperature in ORL IE (−2.59°C ± 0.78°C [−4.66°F ± 1.40°F]) and ORL E (−1.97°C ± 0.73°C [−3.55°F ± 1.31°F]) in this study further confirms that using ORL IE and ORL E is not valid for assessing hyperthermia in exercising individuals. Mean bias increased for ORL IE (−3.00°C ± 0.74°C [−5.40°F ± 1.33°F]) and ORL E (−2.35°C ± 0.69°C [−4.23°F ± 1.24°F]) when we examined the 8 highest responders to outdoor exercise (those individuals with the highest RCT at minute 60). The ORL IE and ORL E detected the least degree of temperature change of all the measurements taken ( r = .13 and .16, respectively). The intraclass correlation coefficients of both ORL IE and ORL E were closer to zero than were the measurements from any other devices tested. Additionally, other measures of validity (SEM, limits of agreement, and coefficients of variation) indicate that these devices were not valid. Although it does appear that ORL E was consistently higher than ORL IE (average difference = 0.46°C [0.83°F]), both measurements were still substantially lower than RCT. From this, we conclude that the devices used to measure oral temperature are invalid for measuring body temperature under these conditions.
Aural (Tympanic) Temperature
Body temperature measured by inserting a commercially available device into the ear canal often is referred to as a tympanic temperature. These devices are not valid measures of tympanic membrane temperature because they do not touch the tympanic membrane and, thus, represent an average of various sites (ie, tympanic membrane, air within the ear canal, heat radiated from the inner canal wall). 2, 8, 29, 31 Because aural temperature is often low, conversions are made by manufacturers (some devices do this automatically) to improve accuracy. 14, 15, 28, 30, 32–34 Pre-exercise AUR temperature (36.95°C ± 0.30°C [98.50°F ± 0.54°F]) in our study was significantly lower than RCT (37.36°C ± 0.27°C [99.26°F ± 0.48°F]); this finding is similar to the results of other groups examining aural temperature in resting individuals.
As RCT increased with exercise, AUR remained significantly lower at every time point. Postexercise AUR was also lower than RCT at every time point. Other authors 15, 29, 35 examining the aural temperature of athletes exercising in the heat have drawn similar conclusions. The low sensitivity of AUR to internal temperature changes (especially with cooling) likely result from changes in blood flow to the skin, air or sweat evaporative cooling of the heat, and moisture and sweat in the ear. *
Peak AUR temperature (37.56°C ± 0.65°C [99.60°F ± 1.18°F]) occurred at the same time as RCT (minute 60) but was 1.62°C ± 0.59°C (2.91°F ± 1.07°F) lower. Interestingly, AUR tracked RCT (ie, when RCT increased, AUR increased) but not with the same magnitude (eg, in the first 60 minutes of exercise, RCT increased 1.81°C [3.26°F], whereas AUR increased 0.61°C [1.10°F]). This tracking resulted in a deceivingly high r value (.70) and supports our use of a Bland-Altman plot. The mean bias of −1.00°C (−1.80°F) confirms that AUR temperature was not appropriate for measuring body temperature. It is noteworthy that the mean RCT at minute 60 for the 8 highest responders was 39.77°C ± 0.17°C (103.59°F ± 0.30°F), whereas mean AUR for these same subjects was only 37.91°C ± 0.65°C (100.23°F ± 1.16°F), representing a mean difference of 1.86°C (3.36°F).
Axillary Temperature
Axillary temperature is an easy, safe and convenient measure but may not be valid as a clinical measure 2, 8–10, 39 because it involves a sheltered skin temperature, not a core temperature measurement. 8–10, 40 Axillary measures, like those from many other external sites, typically are lower than core body temperature. 5, 29, 30, 33, 40 In our study, both axillary thermometers measured significantly lower than RCT pre-exercise (average = 1.4°C [2.6°F]).
Even though axillary temperature is not commonly measured in clinical settings with individuals suspected of exertional heat stroke, we know of no previous researchers who tested its validity with individuals exercising outdoors in the heat. Although peak temperature with AXL IE (36.74°C ± 0.66°C [98.13°F ± 1.19°F]) and AXL E (35.98°C ± 0.77°C [96.76°F ± 1.39°F]) occurred at the same time point as RCT (minute 60), the temperature differences were large for AXL IE (−2.44°C ± 0.43°C [−4.39°F ± 0.77°F]) and AXL E (−3.19°C ± 0.61°C [−5.74°F ± 1.10°F]). Temperatures measured with both axillary devices were significantly lower than RCT at every time point during exercise and postexercise. The mean biases of AXL IE and AXL E [−2.07°C [−3.73°F] and −2.58°C [−4.64°F], respectively) for all time points were larger than for any other device measured. Additionally, the limits of agreement between the 2 devices at the same body site differed greatly (±1.44°C [±2.60°F] for AXL IE and ±1.71°C [±3.08°F] for AXL E). Thus, we conclude that devices measuring axillary temperature are invalid for measuring body temperature in individuals exercising and recovering from outdoor exercise in the heat. Axillary temperature is likely invalid because of the influence of air temperature, skin temperature, and skin blood flow and the continued sweating in the axillary region. 11
Ingestible Thermistors
Ingestible thermistors were originally developed in the 1980s by the US National Aeronautic and Space Administration and the John Hopkins University Applied Physics Laboratory to monitor core body temperature during exercise or walking. 20, 41–44 The existing scientific literature indicates that this is a valid technique, accurate within ± 0.1°C (± 0.18°F) of the criterion standard. 2, 7, 20, 41, 43, 45
The INT was the only device in our study that was not statistically different from RCT at any time point. The mean bias of INT for all subjects at all time points (−0.19°C [−0.34°F]) was the smallest of any device tested; thus, INT was the only device that met our criterion for validity. Gant et al 18 recently measured a similar mean bias (−0.15°C [−0.27°F]) when intestinal thermistor measurements were compared with RCT in exercising individuals. Additionally, INT had the largest r, largest intraclass correlation coefficient, and lowest limits of agreement of all devices tested ( Table). Other research in different settings and populations (astronauts, divers, soldiers, swimmers, and firefighters) confirms that intestinal thermistors are appropriate for monitoring core body temperature. 7, 18, 20, 41–43, 45, 46 In the present study, the difference between RCT and INT increased when we examined the 8 highest responders (−0.35°C [−0.63°F]), so future authors should validate this device in individuals experiencing exertional heat stroke.
Ingestible thermistors provide an excellent alternative to more invasive temperature measures (eg, rectal, esophageal) with 2 caveats. The intestinal thermistor pill must be ingested well before temperature measurement and must remain in the gastrointestinal tract (ie, not be expelled through bowel movements or vomit). The pill also must be at a point in the gastrointestinal tract at which food or fluid ingestion will not compromise accuracy, which may take 1 hour or more after ingestion. Therefore, intestinal thermistors cannot be used in a collapsed athlete suspected of exertional heat stroke who has not already ingested and retained a thermistor.
Temporal Temperature
Researchers using temporal measures have found TEM INST to be valid at rest 47, 48 but not during indoor exercise in the heat. 29 We found no published studies on the validity of this device for individuals exercising outdoors in the heat. Our data show that TEM INST and TEM MOD were significantly lower than RCT before, during, and after exercise in the heat ( P < .05). Mean bias for all time points was −1.46°C (−2.64°F) for TEM INST and −1.36°C (−2.44°F) for TEM MOD. A correction factor would not validate this device because changes over time in TEM INST and TEM MOD were opposite those in RCT. At each time point when RCT increased, TEM INST and TEM MOD decreased, and vice versa ( Figure 1). These findings are similar to those of others measuring temporal temperature during indoor exercise. 29 Additionally, both TEM INST and TEM MOD had the largest SEM and limits of agreement of all devices tested ( Table). At peak temperature, TEM INST (2.77°C [4.98°F]) and TEM MOD (2.50°C [4.51°F]) were lower than RCT. These large differences remained when we examined the 8 highest responders (−2.98°C [−5.37°F] and −2.80°C [−5.05°F], respectively). Such large discrepancies may have resulted from sweat production or ambient or skin temperature changes. 29 Despite our using different techniques to improve the accuracy of the device (TEM INST and TEM MOD), we found the TEM device invalid for measuring hyperthermia in exercising individuals.
Liquid Crystal Forehead Strips
Another method of purportedly measuring the temperature of the blood in the temporal artery is with a liquid crystal forehead strip. Investigators have shown forehead stickers to be both valid 39, 49 and invalid 31 for subjects at rest. In our study, FST before exercise was not significantly different from RCT, but future authors need to confirm this in a variety of field and laboratory settings.
Because forehead stickers are placed superficially over the temporal artery, we hypothesized that FST measures would be influenced adversely by skin temperature and solar radiation. 31 In order to test this hypothesis, measurements were taken while the subjects remained in the sun (FST FLD) and while standing inside a shaded pavilion (FST). The FST was not statistically different from RCT (mean bias of −0.14°C [−0.25°F]), but FST FLD was consistently greater than FST (mean = 0.46°C [0.82°F]) and overestimated body temperature by 0.60°C (1.08°F). Because measures in the sun greatly influenced temperature readings, the validity of this device is questionable in the sun. Further, the reliability and validity of FST and FST FLD are questionable because they had the largest coefficients of variation of all devices tested ( Table). Because most collapsed athletes are found in outdoor settings, the maximal limit of the sticker's scale is 41.11°C (106°F), and there was a tendency for it to peel away from sweat-covered skin, FST is not appropriate for the detection of exertional heat stroke in individuals exercising outdoors in the heat. Future authors should examine the effect of covering the sticker during exercise with a helmet, pads, hat, or sweat band (ie, where a microclimate is present). Given the large differences between FST and FST FLD, future groups also should examine this device in a variety of environmental settings.
Thermal Sensation Scale
Perceived heat stress can be evaluated with a thermal sensation or physiologic strain index (ie, wet bulb globe temperature). With the lack of a universally accepted heat stress scale, 50 we chose a commonly used scaling adapted from Toner et al. 24 Direct comparisons to RCT could not be made due to different scaling, but the correlation between thermal sensation and RCT was moderate ( r = .72). Heat indexes may be appropriate for a specific environmental condition, but they are not transferable to other settings. 50, 51 Due to the subjective nature of this measure and the absence of a direct transfer of thermal sensation to RCT, this scale should not be used to diagnose exertional heat stroke. Interestingly, subjects in our study with an RCT higher than 39.7°C (103.5°F) had a thermal sensation rating of 5.5 or higher.
CONCLUSIONS
The impetus for the present study emanated from personal experiences of working in various medical tents over many years. At one race, for example, the medical staff used temporal measurements as the initial diagnostic device to determine body core temperature, which influenced triage and treatment decisions. The race took place on a hot day (air temperature reached 30.6°C [87°F]) and more than 1000 runners sought care in the medical facility. Some of us wondered if temporal measurements, along with those from other commonly used temperature devices, had been validated for use in athletes exercising outdoors in the heat. The answer at that point was no.
Our data demonstrate that several commonly used devices should, in fact, not be used to assess or even to estimate the deep body temperature of an athlete. When measuring the degree of hyperthermia, all sports medicine professionals should assess temperature rectally. Although intestinal thermistors provide accurate readings, these ordinarily will not be in place during athletes' participation in training or competition. The intestinal thermistor should be considered strongly as a preventive tool, though, by those who work with athletes exercising in the heat and have the means to purchase the temperature pills.
These results support the need for all athletic trainers, team physicians, and other emergency medical personnel to be fully trained, equipped, ready, and willing to assess temperature rectally. Additionally, a flexible thermistor (versus a standard, nonflexible rectal thermometer) provides the added benefit of continuous RCT monitoring during cold-water immersion cooling of patients with exertional heat stroke. This eliminates the need to periodically remove the athlete from the immersion tub for temperature assessment.
A temperature assessment method that can be influenced by such factors as the environment, skin temperature, sweat, and fluids ingested should not be used when measuring the temperature of an athlete who has been performing outdoor exercise in the heat. Because RCT may not be possible or available in some situations, instead of an invalid device, the clinician should use other skills (eg, evaluate central nervous system function) when assessing an individual suspected of exertional heat stroke.
In preparation for proper assessment of exertional heat stroke, any impediments to measuring RCT should be fully investigated and overcome. The situation, without hyperbole, is life or death, and until another accurate field measure is available that provides viable, rapid, and accurate temperature measures, RCT must be used.
Acknowledgments
We thank all the researchers who provided the assistance to make this study such a success. They included Lindsay Boots, Paul Boyd, Chris Casa, Tutita Casa, Mike D'Alfonso, Christy Eason, Brian Gallagher, Ashleigh Gauvain, Camille James, Nick Kalra, Jennifer Klau, Elaine Lee, Stephanie Mazerolle, Ian Scruggs, Barry Spiering, Kristin Stroly, Jacob Vingren, Greig Watson, Linda Yamamoto, and Brad Yeargin. We also thank Curt Vincente and the town of Mansfield for the use of the fields and facilities during this study.
Footnotes
*References 5,11,13,15,17,28,30,35–38.
REFERENCES
- Dombek PM, Casa DJ, Yeargin SW. Athletic trainers' knowledge and behavior regarding the prevention, recognition, and treatment of exertional heat stroke at the high school level [abstract] J Athl Train. 2006;41:S–47. et al. (suppl) [Google Scholar]
- Casa DJ, Armstrong LE, Ganio MS, Yeargin SW. Exertional heat stroke in competitive athletes. Curr Sports Med Rep. 2005;4:309–317. doi: 10.1097/01.csmr.0000306292.64954.da. [DOI] [PubMed] [Google Scholar]
- Costrini A. Emergency treatment of exertional heatstroke and comparison of whole body cooling techniques. Med Sci Sports Exerc. 1990;22:15–18. [PubMed] [Google Scholar]
- Binkley HM, Beckett J, Casa DJ, Kleiner DM, Plummer PE. National Athletic Trainers' Association position statement: exertional heat illnesses. J Athl Train. 2002;37:329–343. [PMC free article] [PubMed] [Google Scholar]
- Moran DS, Mendal L. Core temperature measurement: methods and current insights. Sports Med. 2002;32:879–885. doi: 10.2165/00007256-200232140-00001. [DOI] [PubMed] [Google Scholar]
- Casa DJ, Roberts WO. Considerations for the medical staff: preventing, identifying, and treating exertional heat illnesses. In: Exertional Heat Illnesses. Armstrong LE, ed. Champaign, IL: Human Kinetics; 2003:169– 196 .
- Lee SM, Williams WJ, Fortney Schneider SM. Core temperature measurement during supine exercise: esophageal, rectal, and intestinal temperatures. Aviat Space Environ Med. 2000;71:939–945. [PubMed] [Google Scholar]
- Jensen BN, Jensen FS, Madsen SN, Lossl K. Accuracy of digital tympanic, oral, axillary, and rectal thermometers compared with standard rectal mercury thermometers. Eur J Surg. 2000;166:848–851. doi: 10.1080/110241500447218. [DOI] [PubMed] [Google Scholar]
- Lefrant JY, Muller L, de La Coussaye JE. Temperature measurement in intensive care patients: comparison of urinary bladder, oesophageal, rectal, axillary, and inguinal methods versus pulmonary artery core method. Intens Care Med. 2003;29:414–418. doi: 10.1007/s00134-002-1619-5. et al. [DOI] [PubMed] [Google Scholar]
- Chaturvedi D, Vilhekar KY, Chaturvedi P, Bharambe MS. Comparison of axillary temperature with rectal or oral temperature and determination of optimum placement time in children. Indian Pediatr. 2004;41:600–603. [PubMed] [Google Scholar]
- Roberts WO. Assessing core temperature in collapsed athletes: what's the best method? Physician Sportsmed. 1994;22(8):49–55. doi: 10.1080/00913847.1994.11947680. [DOI] [PubMed] [Google Scholar]
- Brown GA, Williams GM. The effect of head cooling on deep body temperature and thermal comfort in man. Aviat Space Environ Med. 1982;53:583–586. [PubMed] [Google Scholar]
- Livingstone SD, Grayson J, Frim J, Allen CL, Limmer RE. Effect of cold exposure on various sites of core temperature measurements. J Appl Physiol. 1983;54:1025–1031. doi: 10.1152/jappl.1983.54.4.1025. [DOI] [PubMed] [Google Scholar]
- Zehner WJ, Terndrup TE. The impact of moderate ambient temperature variance on the relationship between oral, rectal, and tympanic membrane temperatures. Clin Pediatr (Phila) 1991;30:61–72. doi: 10.1177/0009922891030004S19. [DOI] [PubMed] [Google Scholar]
- Deschamps A, Levy RD, Cosio MG, Marliss EB, Magder S. Tympanic temperature should not be used to assess exercise induced hyperthermia. Clin J Sport Med. 1992;2:27–32. [Google Scholar]
- Cabanac M, Caputa M. Natural selective cooling of the human brain: evidence of its occurrence and magnitude. J Physiol. 1979;286:255–264. doi: 10.1113/jphysiol.1979.sp012617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraki K, Sagawa S, Tajima F, Yokota A, Hashimoto M, Brengelmann GL. Independence of brain and tympanic temperatures in an unanesthetized human. J Appl Physiol. 1988;65:482–486. doi: 10.1152/jappl.1988.65.1.482. [DOI] [PubMed] [Google Scholar]
- Gant N, Atkinson G, Williams C. The validity and reliability of intestinal temperature during intermittent running. Med Sci Sports Exerc. 2006;38:1926–1931. doi: 10.1249/01.mss.0000233800.69776.ef. [DOI] [PubMed] [Google Scholar]
- Kolka MA, Levine L, Stephenson LA. Use of an ingestible telemetry system to measure core temperature under chemical protective clothing. J Therm Biol. 1997;22:343–349. [Google Scholar]
- O'Brien C, Hoyt RW, Buller MJ, Castellani JW, Young AJ. Telemetry pill measurement of core temperature in humans during active heating and cooling. Med Sci Sports Exerc. 1998;30:468–472. doi: 10.1097/00005768-199803000-00020. [DOI] [PubMed] [Google Scholar]
- Jackson AS, Pollock ML. Practical assessment of body composition. Physician Sportsmed. 1985;13(5):76–90. doi: 10.1080/00913847.1985.11708790. [DOI] [PubMed] [Google Scholar]
- Pollock ML, Schmidt DH, Jackson AS. Measurement of cardiorespiratory fitness and body composition in the clinical setting. Comp Ther. 1980;6:12–17. [PubMed] [Google Scholar]
- Heyward VH, Stolarczyk LM. Applied Body Composition Assessment. Champaign, IL: Human Kinetics; 1996.
- Toner MM, Drolet LL, Pandolf KB. Perceptual and physiological responses during exercise in cool and cold water. Percept Mot Skills. 1986;62:211–220. doi: 10.2466/pms.1986.62.1.211. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998;26:217–238. doi: 10.2165/00007256-199826040-00002. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: part 1—correlation within subjects. Br Med J. 1995;310:446. doi: 10.1136/bmj.310.6977.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker EA, Houston H. Screening for fever in an adult emergency department: oral vs tympanic thermometry. South Med J. 1996;89:230–234. doi: 10.1097/00007611-199602000-00016. [DOI] [PubMed] [Google Scholar]
- Kistemaker JA, Den Hartog EA, Daanen HA. Reliability of an infrared forehead skin thermometer for core temperature measurements. J Med Eng Technol. 2006;30:252–261. doi: 10.1080/03091900600711381. [DOI] [PubMed] [Google Scholar]
- Togawa T. Body temperature measurement. Clin Phys Physiol Meas. 1985;6:83–108. doi: 10.1088/0143-0815/6/2/001. [DOI] [PubMed] [Google Scholar]
- Patel N, Smith CE, Pinchak AC, Hagen JF. Comparison of esophageal, tympanic, and forehead skin temperatures in adult patients. J Clin Anesth. 1996;8:462–468. doi: 10.1016/0952-8180(96)00103-1. [DOI] [PubMed] [Google Scholar]
- Stewart JV, Webster D. Re-evaluation of the tympanic thermometer in the emergency department. Ann Emerg Med. 1992;21:158–161. doi: 10.1016/s0196-0644(05)80151-9. [DOI] [PubMed] [Google Scholar]
- Cattaneo CG, Frank SM, Hesel TW, El-Rahmany HK, Kim LJ, Tran KM. The accuracy and precision of body temperature monitoring methods during regional and general anesthesia. Anesth Analg. 2000;90:938–945. doi: 10.1097/00000539-200004000-00030. [DOI] [PubMed] [Google Scholar]
- Amoateng-Adjepong Y, Del Mundo J, Manthous CA. Accuracy of an infrared tympanic thermometer. Chest. 1999;115:1002–1005. doi: 10.1378/chest.115.4.1002. [DOI] [PubMed] [Google Scholar]
- Armstrong LE, Maresh CM, Crago AE, Adams R, Roberts WO. Interpretation of aural temperatures during exercise, hyperthermia, and cooling therapy. Med Exerc Nutr Health. 1994;3:9–16. [Google Scholar]
- McCaffrey TV, McCook RD, Wurster RD. Effect of head skin temperature on tympanic and oral temperature in man. J Appl Physiol. 1975;39:114–118. doi: 10.1152/jappl.1975.39.1.114. [DOI] [PubMed] [Google Scholar]
- Morgans LF, Nunneley SA, Stribley RF. Influence of ambient and core temperatures on auditory canal temperature. Aviat Space Environ Med. 1981;52:291–293. [PubMed] [Google Scholar]
- Fraden J, Lackey RP. Estimation of body sites temperatures from tympanic measurements. Clin Pediatr (Phila) 1991;30:65–72. doi: 10.1177/0009922891030004S20. (4 suppl) [DOI] [PubMed] [Google Scholar]
- Allen GC, Horrow JC, Rosenberg H. Does forehead liquid crystal temperature accurately reflect “core” temperature? Can J Anaesth. 1990;37:659–662. doi: 10.1007/BF03006486. [DOI] [PubMed] [Google Scholar]
- Shann F, Mackenzie A. Comparison of rectal, axillary, and forehead temperatures. Arch Pediatr Adolesc Med. 1996;150:74–78. doi: 10.1001/archpedi.1996.02170260078013. [DOI] [PubMed] [Google Scholar]
- Leclerc S, Lacroix VJ, Montgomery DL. Body temperature homeostasis during a 40 km open water swim. J Swim Res. 2000;14:26–32. [Google Scholar]
- Fortney SM, Mikhaylov V, Lee SM, Kobzev Y, Gonzalez RR, Greenleaf JE. Body temperature and thermoregulation during submaximal exercise after 115-day spaceflight. Aviat Space Environ Med. 1998;69:137–141. [PubMed] [Google Scholar]
- Menze R, McMullen MJ, White LJ, Dougherty JM. During hazardous materials training sessions. Prehosp Disaster Med. 1996;11:42–45. doi: 10.1017/s1049023x00042746. [DOI] [PubMed] [Google Scholar]
- White LJ, Jackson F, McMullen MJ, Lystad J, Jones JS, Hubers RH. Continuous core temperature monitoring of search and rescue divers during extreme conditions. Prehosp Emerg Care. 1998;2:280–284. doi: 10.1080/10903129808958880. [DOI] [PubMed] [Google Scholar]
- Cutchis PN, Hogrefe AF, Lesho JC. The ingestible thermal monitoring system. Johns Hopkins APL Tech Digest. 1988;9:16–21. [Google Scholar]
- Byrne C, Lim CL. The ingestible telemetric body core temperature sensor: a review of validity and exercise applications. Br J Sports Med. 2007;41:126–133. doi: 10.1136/bjsm.2006.026344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenes DS, Fleisher GR. When body temperature changes, does rectal temperature lag? J Pediatr. 2004;144:824–826. doi: 10.1016/j.jpeds.2004.02.037. [DOI] [PubMed] [Google Scholar]
- Greenes DS, Fleisher GR. Accuracy of a noninvasive temporal artery thermometer for use in infants. Arch Pediatr Adolesc Med. 2001;155:376–381. doi: 10.1001/archpedi.155.3.376. [DOI] [PubMed] [Google Scholar]
- Brull SJ, Cunningham AJ, Connelly NR, O'Connor TZ, Silverman DG. Liquid crystal skin thermometry: an accurate reflection of core temperature? Can J Anaesth. 1993;40:375–381. doi: 10.1007/BF03009638. [DOI] [PubMed] [Google Scholar]
- Moran DS, Shitzer A, Pandolf KB. A physiological strain index to evaluate heat stress. Am J Physiol. 1998;275:R-129–R-134. doi: 10.1152/ajpregu.1998.275.1.R129. [DOI] [PubMed] [Google Scholar]
- Maresh CM, Herrera-Sota JA, Armstrong LE. Perceptual responses in the heat after brief intravenous versus oral rehydration. Med Sci Sports Exerc. 2001;33:1039–1045. doi: 10.1097/00005768-200106000-00025. et al. [DOI] [PubMed] [Google Scholar]