Abstract
Context: Phonophoresis is purported to represent a method to apply topical medications through the skin to treat soft tissue injuries and inflammatory conditions. Few data are available to demonstrate the clinical effectiveness of the treatment.
Objective: To determine the effect of ultrasound on the transcutaneous absorption of dexamethasone when occluded with a dressing.
Design: Crossover design.
Setting: University general clinical research center.
Patients or Other Participants: Ten healthy subjects (age = 29.2 ± 8.8 years; height = 170.0 ± 3.9 cm; mass = 67.5 ± 18.4 kg).
Intervention(s): Two grams of 0.33% dexamethasone cream were applied to a 10-cm 2 area on the anterior forearm. The drug was applied to the skin and occluded with a dressing for 30 minutes before the ultrasound and sham ultrasound treatments. The treatments were applied over the drug and occlusive dressing. Ultrasound treatments were delivered at an intensity of 1.0 W/cm 2 (50% pulsed) at an output frequency of 3 MHz for 5 minutes and compared with sham ultrasound treatments that were delivered at an intensity of 0.0 W/cm 2 (50% pulsed) at an output frequency of 3 MHz for 5 minutes. All subjects received both the ultrasound and sham treatments, and the order in which subjects received the treatments was counterbalanced.
Main Outcome Measure(s): Serum samples were drawn before treatment and immediately posttreatment and at 2, 4, 6, 8, and 10 hours posttreatment. Using high-performance liquid chromatography, we analyzed serum to determine dexamethasone concentrations.
Results: A 2-way repeated-measures analysis of variance (condition × time) revealed a significant main effect for ultrasound treatment ( P = .047). The rate of appearance and the total concentration of dexamethasone in the serum were greater in subjects after phonophoresis than after sham ultrasound. The sham group had only trace amounts of dexamethasone in the serum, indicating that drug absorption was negligible without the ultrasound energy. The effect size of the phonophoresis condition fell within a 95% confidence interval after the baseline measurement.
Conclusions: We found that a phonophoretic effect occurred with dexamethasone when its application saturated the skin.
Keywords: ultrasound, therapeutic ultrasound, skin saturation
Key Points
Ultrasound enhanced the absorption of dexamethasone when the drug was applied to the skin and covered with an occlusive dressing.
Drug absorption increased with longer exposure time and use of an occlusive dressing.
Phonophoresis is a technique by which therapeutic ultrasound is used to introduce pharmacologic agents, usually anti-inflammatory or analgesic drugs, through intact skin into the subcutaneous tissues. Theoretically, phonophoresis can provide a safe and painless alternative to injections for treatment of common inflammatory conditions such as bursitis, sprains, strains, and tendinitis. Phonophoresis has been studied in vivo with several anti-inflammatory drugs, including hydrocortisone, 1–6 benzydamine, 7 dexamethasone, 4, 8–10 and salicylates, 11–14 and with anesthetics, such as lidocaine, 15–17 with variable results. Authors 18, 19 of in vitro studies of the phonophoretic effect of ultrasound reported that ultrasound enabled a greater transport of whole molecules across synthetic or organic semipermeable membranes than was afforded by sham ultrasound.
Researchers have noted varying results with regard to the therapeutic benefits of phonophoresis (such as pain relief and improved range of motion) when it was used to treat lateral epicondylitis, 3, 20, 21 temporomandibular joint pain, 22 and osteoarthritic conditions. 1, 23, 24 Most authors 1, 20–22 have shown that, when compared with placebo treatments or ultrasound alone, phonophoresis provides clinical improvement by decreasing pain and increasing function. In contrast, Halle et al 20 and Stratford et al 3 reported no statistical difference between phonophoresis and ultrasound alone when treating pain and dysfunction in patients with lateral epicondylitis. However, in many of these studies, 1, 4–6, 21–23 the inclusion criteria, ultrasound factors, drug dosages, and transmission of ultrasound through the drugs were controlled poorly.
One potential problem with phonophoresis may be related to the clinical procedure used. Most clinicians apply the drug directly to clean skin, apply ultrasound conductive gel, and deliver the treatment for a period of 4 to 6 minutes, based on the size of the treatment area. 25 The drug and gel generally are removed immediately after the ultrasound treatment. Researchers 6, 10 have not shown an increased penetration of a topically administered drug in humans when ultrasound is applied with this technique. This technique may not allow adequate saturation of the stratum corneum, which is the rate-limiting barrier to transcutaneous drug absorption. 26, 27 Investigators 28–31 have reported that a longer contact time and occlusion of the drug with an impermeable film enhance transcutaneous drug penetration.
The absence of a laboratory test to accurately measure the amount and depth of penetration of anti-inflammatory drugs has limited phonophoresis research. An effective technique that accurately can determine drug accumulation in the subcutaneous area of humans has not been established. 5–7, 11, 12, 14, 16 Furthermore, investigators 1, 32 have used various ultrasound frequencies, intensities, and treatment durations that may not be indicated for use on an inflamed area. The commonly recommended factors 33, 34 for the treatment of acute or subacute inflammatory conditions are low intensity, high frequency, and pulsed mode to minimize thermal effects. Therefore, the ultrasound intensity for phonophoresis should be consistent with an intensity used in the treatment of a subacute inflammatory condition. Mitragotri et al 35 examined the minimal ultrasound energy for a phonophoretic effect, but the frequency that they suggested (20 kHz) is not consistent with the ultrasound used in physical rehabilitation.
Our purpose was to determine the effect of ultrasound on the transcutaneous absorption of dexamethasone when occluded with a dressing.
METHODS
Pilot Testing
An assay to determine the amount of dexamethasone in the blood is not commercially available. Clinically, endocrinologists use a measure of serum cortisol to determine the cumulative effect of therapy with glucocorticoids. In pilot testing, we attempted to use an enzyme-linked immunosorbent assay (ELISA) that detects steroid dosing in horses before races. When using radiomarked dexamethasone to examine the usefulness and accuracy of the ELISA, we could not control the sensitivity of the assay, especially in the lower ranges that we were anticipating. Although we were unable to power the study a priori, we were able to show significant differences between groups; therefore, retrospectively powering the analysis was not necessary. We did not use the ELISA in our study, but our finding is relevant for developing methods for future studies.
Subjects
Ten healthy subjects (3 men, 7 women; age = 29.2 ± 8.8 years; height = 170.0 ± 3.9 cm; mass = 67.5 ± 18.4 kg) volunteered to participate in our study. A physician examined all subjects before they entered the study. All subjects met the inclusion criteria: they had no skin lesions in the treated area; they had not used oral steroids or topical steroid creams within 1 month before the study; they had not received an intramuscular or intra-articular injection within the year before the study; they had no contraindications to the use of therapeutic ultrasound; and they had no history of vascular or heart disease. The institutional review board of the university and the General Clinical Research Center (GCRC) approved the study. All subjects gave informed consent.
Instruments
To administer ultrasound treatments, we used an Ultrasound 2000 (Accelerated Care Plus, Reno, NV) that had a beam uniformity ratio of 4:1 and an effective radiating area of 4.2 cm 2. The manufacturer calibrated the ultrasonic output of the generator 1 week before testing, and the use of the machine was reserved for this investigation. Blood samples were analyzed via high-performance liquid chromatography (HPLC) using a lambda-max ultraviolet spectrophotometer (model 486; Waters Associates Inc, Milford, MA), an autosampler (model 717 plus; Waters Associates Inc), and a Millenium chromatography manager (Waters Associates Inc). We set the wavelength to 245 N·m and followed the manufacturer's guidelines for use. All solvents and reagents were liquid chromatography grade (Waters Associates Inc). We obtained standards for the dexamethasone and hydrocortisone (product numbers D1756 and H5885, respectively; Sigma Chemical Co, St Louis, MO). The stationary phase was a μBondapak C18 column, and the liquid chromatograph was a pump with an automated gradient controller (model 501; Waters Associates Inc).
Procedures
The subjects were admitted to the GCRC for 1 night on 2 separate occasions. During 1 admission, sham ultrasound was applied to the volar surface of the forearm, and during the other admission, a therapeutic dosage of ultrasound was applied to the same area. All other aspects of the experimental procedure remained identical. The order in which these tests were performed was counterbalanced, and a minimum of 2 weeks elapsed between treatments.
An intravenous line was placed in a vein of the forearm that was not receiving the treatment, and it remained in position for the duration of the subject's stay. The untreated arm was chosen to ensure that a systemic, rather than a local, effect would be measured. If needed, excess hair was clipped carefully from a 10-cm 2 area on the volar surface of the forearm. A 10-cm 2 template was traced onto the forearm to ensure proper localization and treatment consistency. An individually prepared and equivalent 2-g dose of 0.33% dexamethasone cream (Vann Healthcare Services Inc, Glasgow, KY) from the same lot was applied to this area at 8:00 pm to control for a possible diurnal effect of the steroid. We used a topical dexamethasone mixture in an inert carbocol gel because Byl et al 4 and J. C. Castel (unpublished data, 1998) found that ultrasound penetrates well through this drug. An occlusive dressing (Tegaderm; 3M, St Paul, MN) was applied over the drug to enhance the saturation of the stratum corneum with the drug.
The same clinician administered all treatments to ensure consistent ultrasound delivery. The subjects were blinded to their treatment groups. However, the clinician was not blinded because, unlike when the sham treatment was used, she could not disengage the ultrasound treatment timer when the ultrasound energy was used. The laboratory technicians were blinded to the condition of each serum sample so that they could not determine group identity.
After a 30-minute exposure to the drug, each subject had the ultrasound or sham ultrasound treatment. Each treatment was performed by applying 4 g of Aquasonic gel (Parker Labs, Fairfield, NJ), which was premeasured, over the dressing that was occluding the drug. The experimental dosage was pulsed ultrasound (50%) applied with an intensity of 1.0 W/cm 2 at a 3-MHz frequency for 5 minutes. The sham group received the same treatment, but the output intensity was 0.0 W/cm 2. Both the drug and the ultrasound or sham ultrasound treatment remained within the designated area for the entire treatment. The clinician moved the transducer approximately from 2 cm/s to 4 cm/s; the distance was measured using a metronome during pilot testing. The transducer was moved in a circular pattern throughout the treatment area.
The ultrasound gel, occlusive dressing, and remaining dexamethasone cream were removed after each treatment. Nurses extracted a 10-mL sample of blood before treatment, immediately posttreatment, and at 2, 4, 6, 8, and 10 hours posttreatment. The blood was centrifuged within 30 minutes and frozen so that we could analyze the serum with HPLC. The samples were labeled so that the laboratory would not be able to determine group identity.
Subjects were allowed to eat, sleep, and participate in daily activities during their stay. They were discharged after breakfast.
High-Performance Liquid Chromatography
The HPLC was performed according to the procedure outlined by Caldarella et al. 36 We performed the assay with known standards in serum to check the accuracy of the quantification method. Several dilution techniques were tested to ensure that the standard curve was consistent and reliable at very low concentrations (less than 20 ng/dL). The assay was accurate at all concentrations that were more than 5 ng/dL. All standard curves had a correlation of 0.99 to known standards.
For each sample, we prepared the mobile phase of the HPLC in the following manner. We shook 4 mL of serum with methylene chloride to extract steroids. The aqueous phase was removed by aspiration, and the organic phase was washed with sodium hydroxide and water. Five-milliliter aliquots were evaporated to dryness in a water bath with a temperature of 37°C. The dried samples were reconstituted into a mobile phase and injected into the HPLC. Subject samples and controls were compared with a standard curve to determine the actual dexamethasone concentration.
The standard curve was prepared by spiking aliquots of known concentrations of hydrocortisone and dexamethasone. The curve specimens were extracted in the same way as the subject samples. Corrections for sample volumes and total serum volume were incorporated into the data reduction formula.
Statistical Analysis
We used 2-way repeated-measures analysis of variance (ANOVA) (condition × time) to analyze the data. Post hoc means comparisons were performed if we found significant main effects or interactions. An α level of P < .05 was set for analysis. We used SPSS (version 11.0 for MacIntosh; SPSS Inc, Chicago, IL) to analyze the data.
RESULTS
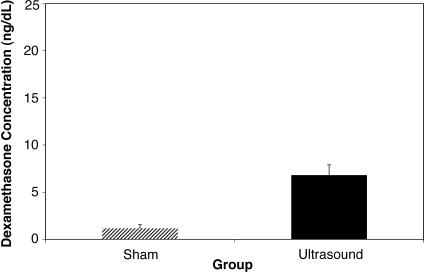
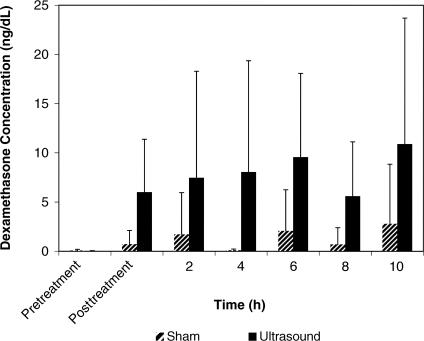
The 2-way repeated-measures ANOVA indicated that the treatment had a main effect (F 1,9 = 5.28, P = .047), with a significantly greater concentration of dexamethasone in the serum when ultrasound was used than when sham ultrasound was used ( Table; Figure 1). We found a significant main effect for time ( P = .003) with all posttreatment levels, more so than at the baseline, but at each time, they were not different from each other ( Figure 2). No significant treatment × time interaction was observed.
Results of Treatment With Ultrasound and Sham Ultrasound.
Figure 1. Effect of group on dexamethasone concentration. The mean concentration of dexamethasone in overall measurements of the sham group was well below the 5-ng/dL level that was considered to be an accurately measurable amount. The error bars represent SDs.
Figure 2. The mean concentrations of dexamethasone as a function of time (ng/dL). Although the drug concentration increased over time, the interaction of the condition and time was not significant. The error bars represent SDs.
The main effect for time indicated a difference in the concentrations at various times. Examination by post hoc means comparisons demonstrated that this difference existed at a significant level only from the baseline measurement. The effect size for comparing the phonophoresis treatment condition with the sham condition (−0.5) was within a 95% confidence interval at all times after the baseline measurement (see the Table).
DISCUSSION
Our primary finding was a significantly greater accumulation of dexamethasone concentration in the serum when ultrasound was used than when sham ultrasound was used. The drug concentration increased over time, and it was still elevated 10 hours posttreatment. We found no significant condition × time interaction. Although our results are inconsistent with the results found by authors 1, 3, 6, 7, 10 of other phonophoresis studies on human subjects, we expected the experimental protocol of the extended exposure time and the use of an occlusive dressing to saturate the stratum corneum and enhance the effect of phonophoresis. We also thought that the choice of drug and the ultrasound factors would optimize the chance of increasing drug penetration. Using the occlusive dressing with sham ultrasound caused only a trace change in concentration of dexamethasone in the serum. Therefore, the massaging effect of repetitively moving the transducer over the occluded drug did not cause an appreciable amount of drug absorption.
We chose treatment factors at the high end of the dosage range to maximize the potential phonophoretic effect. The factors were expected to cause a mild thermal effect, which may not be ideal for an inflammatory condition but which would increase the nonthermal effects of ultrasound. 37–39 Michlovitz 40 reported that no investigators have shown evidence for the role of phonophoresis in treating tendinitis. However, we chose the mild thermal dosage based on the premise that the ultrasound energy would cause a phonophoretic effect without treating a potentially inflamed condition with an aggressive thermal dosage.
The serum samples had a high degree of variance. Some subjects had relatively little change in the drug concentrations even when ultrasound was applied, but other subjects had a remarkable increase in absorption. We did not have enough data to determine the variable absorption among subjects. Chien and Liu 41 reported that skin type, age, and hydration can affect topical drug absorption; however, each subject in our study had both treatments, so the variance within subjects was minimized. Sex of the subjects is not a factor in skin permeability. 42 We did not evaluate factors that may have contributed to variance in topical drug absorption. In addition, although dexamethasone is a steroid, it is not produced endogenously.
We examined the effect of a single exposure to ultrasound. Clinical treatments involving both ultrasound and phonophoresis in athletic training and physical therapy are often delivered several times per week for up to 2 or 3 weeks. 24 Franklin et al 9 examined the effect of phonophoresis with dexamethasone on adrenal function when 8 treatments were delivered during a 2-week period. They found no immunosuppressant effect even with the repetitive treatments; however, they used the typical clinical protocol of a 5-minute exposure. We found an increased absorption of dexamethasone. Investigators must explore the ramifications of repeated treatments because of the risk of an immunosuppressant effect.
With the exception of Franklin et al, 9 researchers conducting controlled experiments have used only a single application of phonophoresis. The biologic half-life of dexamethasone is from 26 to 54 hours, and we found that greater dexamethasone levels in the serum were present up to 10 hours after a single exposure of ultrasound than were present after sham ultrasound. Therefore, a cumulative effect could occur if treatments were administered daily. The immunosuppressive effects of steroids have serious ramifications on numerous physiologic systems and must be examined. Clearly, the serum levels of the drug must be monitored to prevent adverse effects, and a physician must prescribe the administration of phonophoresis in this manner.
No clinical test is available to determine the amount of dexamethasone in the serum; the effects of the drug are interpreted from blood cortisol levels. We wanted to determine the amount of absorption of dexamethasone rather than the clinical or therapeutic dosage. The serum values that we measured were low (mean concentration = 6.7 ng/dL), but because a clinical laboratory test for this drug was not available, we could not determine whether this value represented a therapeutic range. More research is needed to evaluate the treatment effect. The effect sizes were calculated to compare the treatment condition with the sham condition and ranged from 1.34 (10 hours) to 61.23 (4 hours). These values exemplify the magnitude of the treatment effect.
Whether taken orally or applied topically, anti-inflammatory drugs have a local target that coincides with the injured part. Topically applied drugs diffuse through the epidermis to the dermis to reach the capillary networks, which causes systemic uptake of the drug. 41 The systemic effects of the drug can be monitored by analyzing the blood serum content or the excretion of the drug in the urine. Researchers 13, 43 have found evidence that some topically administered drugs are absorbed at the treatment site in a greater concentration than in other areas of the body. We measured a systemic effect, but a drug concentration may localize in the area immediately below the administration site. This topical application over the muscle, synovium, ligaments, and tendons also could benefit the patient by providing a therapeutic dosage at the injured area.
CONCLUSIONS
Our results indicate that changes should be implemented with regard to the clinical procedure of phonophoresis. The clinician must choose a drug that enables the transmission of ultrasound. Dexamethasone is a strong anti-inflammatory drug that can be used without attenuating the ultrasound energy as it is delivered to the tissues. Other types of drugs and vehicles should be investigated for transmission of ultrasound before their use in phonophoresis. One of our most significant findings is the use of longer exposure times with an occlusive dressing. The commonly used method of applying the drug to the skin only for the duration of the ultrasound treatment has not produced favorable results in human or animal studies, even when dexamethasone has been used. 10 Therefore, clinical applications that use treatment procedures similar to the procedures we used should produce more effective clinical results.
Acknowledgments
This research was funded by the University of Virginia General Clinical Research Center and the National Athletic Trainers' Association Research & Education Foundation (Dallas, TX). We thank Frank McCue III, Ethan Saliba, Don Ball, William Webright, Chris Castel, and Chad Starkey for their research assistance and support of this endeavor.
REFERENCES
- Kleinkort JA, Wood F. Phonophoresis with 1 percent versus 10 percent hydrocortisone. Phys Ther. 1975;55:1320–1324. doi: 10.1093/ptj/55.12.1320. [DOI] [PubMed] [Google Scholar]
- Davick JP, Martin RK, Albright JP. Distribution and deposition of tritiated cortisol using phonophoresis. Phys Ther. 1988;68:1672–1675. doi: 10.1093/ptj/68.11.1672. [DOI] [PubMed] [Google Scholar]
- Stratford PW, Levy DR, Gauldie S, Miseferi D, Levy K. The evaluation of phonophoresis and friction massage as treatments for extensor carpi radialis tendinitis: a randomized controlled trial. Physiother Can. 1989;41:93–99. [Google Scholar]
- Byl NN, McKenzie AL, Halliday B, Wong T, O'Connell J. The effects of phonophoresis with corticosteroids: a controlled pilot study. J Orthop Sports Phys Ther. 1993;18:590–600. doi: 10.2519/jospt.1993.18.5.590. [DOI] [PubMed] [Google Scholar]
- Bare AC, McAnaw MB, Pritchard AE. Phonophoretic delivery of 10% hydrocortisone through the epidermis of humans as determined by serum cortisol concentrations. Phys Ther. 1996;76:738–749. doi: 10.1093/ptj/76.7.738. et al. [DOI] [PubMed] [Google Scholar]
- Kuntz AR, Griffiths CM, Rankin JM, Armstrong CW, McLoughlin TL. Cortisol concentrations in human skeletal muscle tissue after phonophoresis with 10% hydrocortisone gel. J Athl Train. 2006;41:321–324. [PMC free article] [PubMed] [Google Scholar]
- Benson HA, McElnay JC, Harland R. Use of ultrasound to enhance percutaneous absorption of benzydamine. Phys Ther. 1989;69:113–118. doi: 10.1093/ptj/69.2.113. [DOI] [PubMed] [Google Scholar]
- Moll M. A new approach to pain: lidocaine and decadron with ultrasound. USAF Med Serv Dig. 1979;30:8–10. [Google Scholar]
- Franklin ME, Smith ST, Chenier TC, Franklin RC. Effect of phonophoresis with dexamethasone on adrenal function. J Orthop Sports Phys Ther. 1995;22:103–107. doi: 10.2519/jospt.1995.22.3.103. [DOI] [PubMed] [Google Scholar]
- Darrow H, Schulthies S, Draper D, Ricard M, Measom GJ. Serum dexamethasone levels after Decadron phonophoresis. J Athl Train. 1999;34:338–341. [PMC free article] [PubMed] [Google Scholar]
- Ciccone CD, Leggin BG, Callamaro JJ. Effects of ultrasound and trolamine salicylate phonophoresis on delayed-onset muscle soreness. Phys Ther. 1991;71:666–678. doi: 10.1093/ptj/71.9.666a. [DOI] [PubMed] [Google Scholar]
- Oziomek RS, Perrin DH, Herold DA, Denegar CR. Effect of phonophoresis on serum salicylate levels. Med Sci Sports Exerc. 1991;23:397–401. [PubMed] [Google Scholar]
- Baldwin JR, Carrano RA, Imondi AR. Penetration of trolamine salicylate into the skeletal muscle of the pig. J Pharm Sci. 1984;73:1002–1004. doi: 10.1002/jps.2600730739. [DOI] [PubMed] [Google Scholar]
- Hill DW, Richardson JD. Effectiveness of 10% trolamine salicylate cream on muscular soreness induced by a reproducible program of weight training. J Orthop Sports Phys Ther. 1989;11:19–23. doi: 10.2519/jospt.1989.11.1.19. [DOI] [PubMed] [Google Scholar]
- Novak EJ. Experimental transmission of lidocaine through intact skin by ultrasound. Arch Phys Med Rehabil. 1964;45:231–232. [PubMed] [Google Scholar]
- Benson HAE, McElnay JC, Harland R. Phonophoresis of lignocaine and prilocaine from EMLA cream. Int J Pharm. 1988;44:65–69. [Google Scholar]
- McElnay JC, Matthews MP, Harland R, McCafferty DF. The effect of ultrasound on the percutaneous absorption of lignocaine. Br J Clin Pharmacol. 1985;20:421–424. doi: 10.1111/j.1365-2125.1985.tb05089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez J, Franklin B, Kollias J. Effect of ultrasound on the permeability of D2O through cellulose membranes. Biomedicine. 1976;25:121–122. [PubMed] [Google Scholar]
- Lenart I, Ausländer D. The effect of ultrasound on diffusion through membranes. Ultrasonics. 1980;18:216–218. doi: 10.1016/0041-624x(80)90123-7. [DOI] [PubMed] [Google Scholar]
- Halle JS, Franklin RJ, Kralfa BL. Comparison of four treatment approaches for lateral epicondylitis of the elbow. J Orthop Sports Phys Ther. 1986;8:62–68. doi: 10.2519/jospt.1986.8.2.62. [DOI] [PubMed] [Google Scholar]
- Holdsworth LK, Anderson DM. Effectiveness of ultrasound used with a hydrocortisone coupling medium or epicondylitis clasp to treat lateral epicondylitis: pilot study. Physiotherapy. 1993;79:19–25. [Google Scholar]
- Wing M. Phonophoresis with hydrocortisone in the treatment of temporomandibular joint dysfunction. Phys Ther. 1982;62:32–33. doi: 10.1093/ptj/62.1.32. [DOI] [PubMed] [Google Scholar]
- Griffin JE, Echternach JL, Price RE, Touchstone JC. Patients treated with ultrasonic driven hydrocortisone and with ultrasound alone. Phys Ther. 1967;47:594–601. doi: 10.1093/ptj/47.7.594. [DOI] [PubMed] [Google Scholar]
- Pottenger FJ, Karalfa BL. Utilization of hydrocortisone phonophoresis in United States Army physical therapy clinics. Mil Med. 1989;154:355–358. [PubMed] [Google Scholar]
- Quillen W. Phonophoresis: a review of the literature and technique. Athl Train. 1980;15:109–110. [Google Scholar]
- Bommannan D, Okuyama H, Stauffer P, Guy RH. Sonophoresis, I: the use of high-frequency ultrasound to enhance transdermal drug delivery. Pharm Res. 1992;9:559–564. doi: 10.1023/a:1015808917491. [DOI] [PubMed] [Google Scholar]
- Bommannan D, Menon GK, Okuyama H, Elias PM, Guy RH. Sonophoresis, II: examination of the mechanism(s) of ultrasound-enhanced transdermal drug delivery. Pharm Res. 1992;9:1043–1047. doi: 10.1023/a:1015806528336. [DOI] [PubMed] [Google Scholar]
- Feldman RJ, Maibach HI. Penetration of 14C-hydrocortisone through normal skin. Arch Dermatol. 1965;91:661–666. doi: 10.1001/archderm.1965.01600120093023. [DOI] [PubMed] [Google Scholar]
- Evers H, von Dardel O, Juhlin L, Ohlsen L, Vinnars E. Dermal effects of compositions based on the eutectic mixture of lignocaine and prilocaine (EMLA): studies in volunteers. Br J Anaesth. 1985;57:997–1005. doi: 10.1093/bja/57.10.997. [DOI] [PubMed] [Google Scholar]
- Brown L, Langer R. Transdermal delivery of drugs. Annu Rev Med. 1988;39:221–229. doi: 10.1146/annurev.me.39.020188.001253. [DOI] [PubMed] [Google Scholar]
- Schwandt RE, Hayes JM. Clinical overview of topical corticosteroids. Available at: http://www.helix.com/helix/resc/trends/pharmacy/aug96_ce.htm. Accessed June 13, 2002 .
- Griffin JE, Touchstone JC. Ultrasonic movement of cortisol into pig tissues, I: movement into skeletal muscle. Am J Phys Med. 1963;42:77–85. [PubMed] [Google Scholar]
- Dyson M. Mechanisms involved in therapeutic ultrasound. Physiotherapy. 1987;73:116–120. [Google Scholar]
- ter Haar G, Dyson M, Oakley EM. The use of ultrasound by physiotherapists in Britain, 1985. Ultrasound Med Biol. 1987;13:659–663. doi: 10.1016/0301-5629(87)90064-0. [DOI] [PubMed] [Google Scholar]
- Mitragotri S, Farrell J, Tang H, Terahara T, Kost J, Langer R. Determination of threshold energy dose for ultrasound-induced transdermal drug transport. J Control Release. 2000;63:41–52. doi: 10.1016/s0168-3659(99)00178-9. [DOI] [PubMed] [Google Scholar]
- Caldarella AM, Reardon GE, Canalis E. Analysis for cortisol in serum by liquid chromatography. Clin Chem. 1982;28:538–543. [PubMed] [Google Scholar]
- Draper DO, Castel JC, Castel D. Rate of temperature increase in human muscle during 1 MHz and 3 MHz continuous ultrasound. J Orthop Sports Phys Ther. 1995;22:142–150. doi: 10.2519/jospt.1995.22.4.142. [DOI] [PubMed] [Google Scholar]
- Draper DO, Ricard MD. Rate of temperature decay in human muscle following 3 MHz ultrasound: the stretching window revealed. J Athl Train. 1995;30:304–307. [PMC free article] [PubMed] [Google Scholar]
- Gallo JA, Draper DO, Brody LT, Fellingham GW. A comparison of human muscle temperature increases during 3-MHz continuous and pulsed ultrasound with equivalent temporal average intensities. J Orthop Sports Phys Ther. 2004;34:395–401. doi: 10.2519/jospt.2004.34.7.395. [DOI] [PubMed] [Google Scholar]
- Michlovitz SL. Is there a role for ultrasound and electrical stimulation following injury to tendon and nerve? J Hand Ther. 2005;18:292–296. doi: 10.1197/j.jht.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Chien YW, Liu JC. Transdermal drug delivery systems. J Biomater Appl. 1986;1:183–206. doi: 10.1177/088532828600100202. [DOI] [PubMed] [Google Scholar]
- Reed JT, Ghadially R, Elias PM. Skin type, but neither race nor gender, influence epidermal permeability barrier function. Arch Dermatol. 1995;131:1134–1138. [PubMed] [Google Scholar]
- McNeill SC, Potts RO, Francoeur ML. Local enhanced topical delivery (LETD) of drugs: does it truly exist? Pharm Res. 1992;9:1422–1427. doi: 10.1023/a:1015854728278. [DOI] [PubMed] [Google Scholar]