Abstract
Context: An arthrogenic muscle response (AMR) of the soleus and peroneal muscles has been previously demonstrated in individuals with chronic ankle instability (CAI), but the presence of AMR in muscles acting on joints proximal to unstable ankles has not been previously explored.
Objective: To determine if AMR is present in the quadriceps and hamstrings muscles of those with and without unilateral CAI.
Design: Case control.
Setting: University research laboratory.
Patients or Other Participants: Twenty subjects with unilateral CAI (12 males, 8 females: age = 19.9 ± 3.7 years; height = 170.3 ± 15.6 cm; mass = 78.0 ± 23.1 kg) and 21 controls (16 males, 5 females: age = 23.2 ± 5.4 years; height = 173.9 ± 12.7 cm; mass = 87.2 ± 24.6 kg) with no previous ankle injuries.
Main Outcome Measure(s): The central activation ratio (CAR), a measure of motoneuron pool excitability during maximal voluntary isometric contraction, for the hamstrings and quadriceps muscles was measured in both limbs using the superimposed burst technique.
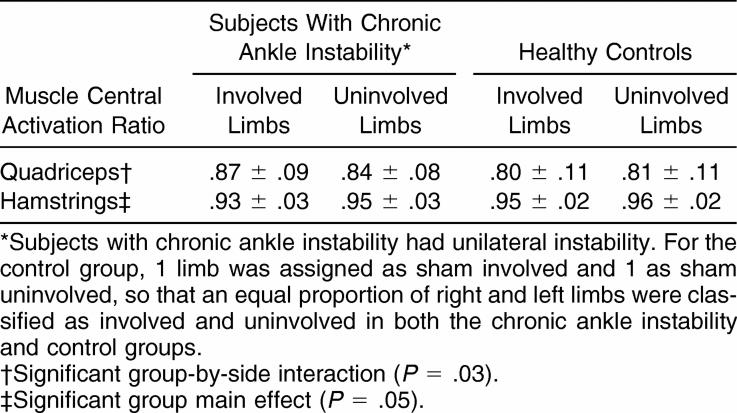
Results: The CAI group demonstrated quadriceps CARs that were significantly larger in their involved limbs (.87 ± .09), as compared with their uninvolved limbs (.84 ± .08), whereas no significant side-to-side difference was seen in the control group (sham involved = .80 ± .11, sham uninvolved = .81 ± .11). When values from both the involved and uninvolved limbs were averaged, the hamstrings CAR was significantly lower for the CAI group (.94 ± .03) than for the control group (.96 ± .03).
Conclusions: Arthrogenic inhibition of the hamstrings muscles bilaterally and facilitation of the quadriceps muscle ipsilateral to the involved limb were noted in subjects with unilateral CAI. Motoneuron pool excitability appears to be altered in muscles that act on joints proximal to the ankle in those with unilateral CAI.
Keywords: ankle sprain, functional ankle instability, muscle inhibition
Key Points
Differences in motoneuron pool excitability of the quadriceps and hamstrings muscles were found between those subjects with chronic ankle instability and control subjects.
Proximal deficits in motor control should be considered when assessing and treating patients with lateral ankle sprains and chronic ankle instability.
Ankle injuries are one of the most common injuries in both the athletic and general populations. 1, 2 Once an individual has suffered an ankle sprain, he or she is more susceptible to recurrent sprains. 3, 4 Furthermore, 40% of individuals suffering lateral ankle sprains will develop chronic ankle instability (CAI), or frequent “giving way” of the ankle, during functional activities. 5 Chronic ankle instability may be caused by mechanical instability, functional instability, or a combination of the two. 3 The focus of our study is neuromuscular deficits, which are often referred to as functional instability. Acute sprains may result in permanent disruption of the mechanoreceptors in the injured ligaments, 5 and these changes may lead to neuromuscular alterations in the lower limb. Chronic ankle instability is associated with neuromuscular changes at the involved ankle, as well as proximally in the ipsilateral limb 6, 7 and in the contralateral limb. 8
Arthrogenic muscle response (AMR), an important and often-unrecognized consequence of joint injury, 9 is defined as an ongoing reflex reaction of the musculature surrounding a joint after distension or damage to structures of that joint. 10, 11 Potentially involving inhibition or facilitation of a muscle's motoneuron pool excitability, AMR can be independent of pain and swelling and may be present long after the acute injury has resolved. 11–13 Arthrogenic muscle response may be associated with altered neuromuscular activation patterns around injured joints and is thought to occur in response to distorted articular sensory receptors after joint injury. 9 Although AMR has primarily been studied in the knee, 10–21 it has also been studied in the shoulder, 22, 23 elbow, 22, 23 and ankle. 15, 24–26
A common method of measuring AMR is through the central activation ratio (CAR). 27–30 The CAR is the ratio of the force exerted in a maximal voluntary isometric contraction (MVIC) divided by the force exerted when an MVIC is performed concurrently with a superimposed burst of electric stimulation. When performing this maneuver, ideally motor units that are not recruited during voluntary contraction should be contracted with supramaximal stimulation of the muscle fibers. 31 A smaller CAR reflects a greater number of motor units having been recruited involuntarily with the supramaximal stimulation, thus indicating that the muscle is more inhibited during voluntary contraction. In theory, a CAR of 1.0 represents no muscle inhibition, whereas lower ratios represent escalating levels of inhibition.
Arthrogenic muscle inhibition is present in the ipsilateral peroneal and soleus muscles of subjects with CAI. 32 Although peroneal muscle dysfunction has frequently been associated with CAI, the role of soleus dysfunction is less understood. Multiple authors have demonstrated concurrent changes in the motoneuron pool excitability of the soleus and quadriceps muscles. 33–36 Three groups assessing the effects of artificial knee effusion have shown soleus muscle facilitation and quadriceps muscle inhibition with knee effusion. 36–38 Building on these findings, soleus inhibition associated with CAI may be related to accompanying quadriceps facilitation. Furthermore, using the principle of reciprocal inhibition, 39, 40 it is plausible that facilitation of the quadriceps motoneuron pool may be related to inhibition of the hamstrings motoneuron pool.
Our purpose was to examine subjects with and without unilateral CAI for the presence of AMR in their quadriceps and hamstrings muscle groups. Our hypothesis was that, in comparison with healthy subjects, subjects with CAI would exhibit quadriceps facilitation and hamstring inhibition.
METHODS
A case-control design was used to evaluate the group and side differences in the quadriceps and hamstrings muscles of subjects with and without unilateral CAI. The dependent variables were the CAR of the quadriceps and hamstrings muscle groups, as measured using the superimposed burst technique. The 2 independent variables each had 2 levels: (1) group (CAI, control) and (2) side (involved, uninvolved). Each control subject was side matched to a subject in the CAI group, so that one limb was assigned as sham involved and one as sham uninvolved. An equal proportion of right and left limbs were classified as involved and uninvolved in the CAI group and as sham involved and sham uninvolved in the control group. Power for this investigation was determined from a similar study that evaluated the AMR of the lower leg musculature in subjects with CAI. 32
Subjects
Twenty volunteers (12 males, 8 females; age = 19.9 ± 3.7 years; height = 170.3 ± 15.6 cm; mass = 78.0 ± 23.1 kg) with a history of unilateral CAI were tested in the experimental group. Twenty-one volunteers (16 males, 5 females; age = 23.2 ± 5.4 years; height = 173.9 ± 12.7 cm; mass = 87.2 kg ± 24.6 kg) with no significant history of lower extremity conditions, knee injury, or low back pain within the last year; no history of surgery in the lower extremity; and no neurologic deficits comprised the control group. All subjects read and signed informed consent forms before initiation of testing. The informed consent form and protocol were approved by the Human Investigations Committee at our university.
Subjects completed 2 ankle questionnaires, the Ankle Instability Instrument (AII) and the Foot and Ankle Instability Disability index (FADI), including the FADI Sport Subscale, to determine inclusion criteria. The AII is reliable and valid in the assessment of patients with CAI. 41 To be classified into the CAI group, subjects had to have a history of ankle sprain and must have answered yes to at least 4 other ankle symptom questions. Additionally, volunteers reporting bilateral ankle sprains or any fracture to the lower extremity or significant knee injuries were excluded. The FADI is also reliable and valid in detecting self-reported disability in those with CAI. 42 Subjects had to self-report disability on at least 2 items on the FADI to be included in the study.
Instruments
Isometric torque data collection was performed using the Biodex System 3 Pro isokinetic dynamometer (Biodex Medical Systems Inc, Shirley, NY). The data were exported, in real time, from the Biodex System 3 using a custom-made cable to the BIOPAC 150 system (DA100B; BIOPAC Systems Inc, Santa Barbara, CA). The AcqKnowledge software (version 3.7.1; Harvard Apparatus Institute, Holliston, MA) was used to collect and analyze data on a Lenovo ThinkPad T42 laptop computer (Lenovo Corp, Morrisville, NC). The S88 dual-channel Grass Stimulator (Grass Telefactor, West Warwick, RI) with the SIU8T transformer stimulus isolation unit was used to deliver the superimposed burst to the quadriceps and hamstrings muscle groups.
Subject Preparation
The skin over the quadriceps and hamstrings stimulation sites was cleaned with alcohol before electrode placement. To stimulate the quadriceps muscle, 2 carbon electrodes (12 × 8 cm) were applied with a thin layer of conducting gel. The first pad was placed on the proximal quadriceps muscle about 5 cm from the inguinal crease. The second pad was placed over the vastus medialis.
For the hamstrings, a 5 × 5-cm adhesive electrode pad was placed in line with the ischial tuberosity just inferior to the gluteal fold. The second electrode was placed slightly medial and distal to the first electrode on the bulk of the hamstring musculature, as identified during submaximal isometric contraction against manual resistance.
Testing Procedures
A coin toss randomized the order of side and muscle group testing for each subject. For quadriceps muscle testing, the subject was secured in an upright sitting position in the dynamometer with waist and shoulder straps and the test knee at 90° of flexion. For hamstrings testing, the subject was tested in the sitting position reclined to 45° with the test knee at 45° of flexion. The subject then performed submaximal isometric contractions and was stimulated with submaximal stimulatory twitches for familiarization with the procedures. All subjects were given 3 minutes of rest between trials to avoid fatigue. Verbal encouragement and visual feedback from the software's real-time display of force output were provided during contraction to motivate the subject.
After becoming familiar with the procedures, the subject was asked to perform an MVIC for either knee extension or knee flexion for at least 3 seconds. Once the force plateau was visually identified on the computer screen by the investigator, a superimposed burst (SIB) of electric stimulation was administered. The stimulator delivered one 100-millisecond train of 10 square-wave pulses at 125 V, with an interpulse duration of 600 μs and a carrier frequency of 100 pulses/s, to elicit the SIB in the quadriceps. 30 To elicit the SIB in the hamstrings, one 100-millisecond train of 10 square-wave pulses at 100 V, with an interpulse duration of 600 μs and a carrier frequency of 60 pulses/s, was applied. These settings were chosen after extensive pilot testing revealed that the same stimulation settings could not be used for both muscle groups.
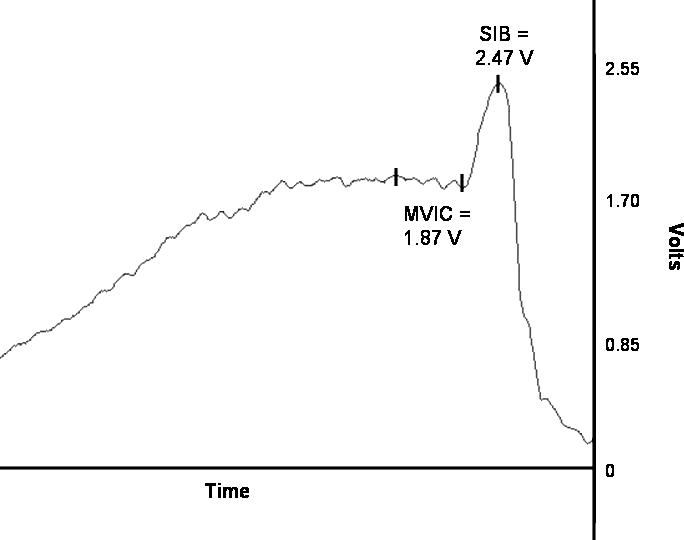
The electric stimulation caused a transient increase in torque, called the SIB torque, over the MVIC torque ( Figure). The CAR was calculated as the ratio of the MVIC torque signal over the combined MVIC+SIB torque signal; the MVIC and SIB values were then identified using the AcqKnowledge software. Subjects performed 3 trials each for the hamstrings and quadriceps muscle groups in each limb. The subject was able to discard and repeat any trial if he or she did not perceive that maximal effort was exerted when the SIB was applied.
The central activation ration (CAR) incorporates torque signal measurements from (1) a maximal voluntary isometric contraction (MVIC) and (2) MVIC and a superimposed burst (SIB) of electric stimulation. The CAR is calculated as the ratio of the mean of the plateau (x̄) during the MVIC over the peak in the combined MVIC+SIB force. In this example for the quadriceps, the CAR is calculated as 1.87/2.47 and is equal to 0.76.
Statistical Analysis
Independent t tests were used to compare the results of the FADI, FADI Sport, and AII scores among the groups. Intraclass correlation coefficients (3,1) and standard error of measurement (SEM) were calculated across all subjects to establish intrasession reliability and precision in the CAR measures for the quadriceps and hamstrings muscle groups. Lastly, 2 separate, mixed-model, 2 × 2 analyses of variance were calculated, one for the quadriceps data and one for the hamstrings data. The between-subjects factor was group (CAI, control), and the within-subjects factor was side (involved, uninvolved). The a priori α level was set at P ≤ .05.
RESULTS
The CAI and control groups were significantly different in terms of ankle disability status as assessed by the FADI, FADI Sport, and AII ( P < .05). The CAI group scored 90.5% ± 10.2% on the FADI, 75.6% ± 11.8% on the FADI Sport, and 5.2% ± 1.3% on the AII; the control group scored 99.8% ± 0.5% on the FADI, 99.7% ± 1.4% on the FADI Sport, and 0.05% ± 0.2% on the AII.
The intrasession reliability estimate for the quadriceps CAR measures was .77, and the SEM was 0.05. For the hamstrings, the intrasession reliability estimate was .61, and the SEM was 0.02.
The CAR results, as detailed in the Table, were notable for the quadriceps; a significant group-by-side interaction ( P = .03) was evident. Tukey post hoc comparisons revealed that, for the CAI group, the quadriceps CAR was significantly greater in the involved limbs (.87 ± .09) than in the uninvolved limbs (.84 ± .08), whereas no significant side-to-side difference was seen in the control group (sham involved = .80 ± .11, sham uninvolved = .81 ± .11, P = .22).
Central Activation Ratios in Subjects With and Without Chronic Ankle Instability (Mean ± SD).
A significant group main effect was found for the hamstrings CAR measures ( P = .05). When values from both the involved and uninvolved limbs were averaged, the hamstrings CAR was significantly lower for the CAI group (.94 ± .03) as compared with the control group (.96 ± .03). However, we were only able to obtain valid hamstrings CAR measures in 29 (15 with CAI, 14 controls) of our 41 subjects.
DISCUSSION
Subjects with and without unilateral CAI were evaluated for AMR in the quadriceps and hamstrings muscle groups. As hypothesized, the CAI group exhibited quadriceps facilitation and hamstrings inhibition. The CAI group demonstrated facilitation of the quadriceps muscle in the involved limb in comparison with the uninvolved limb and both limbs of the control group. Interestingly, the CAI group also had bilateral inhibition of the hamstrings compared with the control group. These motoneuron pool responses are likely due to the residual effects of CAI that stem from a loss of, or change in, sensory information emanating from the injured ankle joint. It is unclear if these changes represent a coping mechanism—an effort to protect the injured ankle while still retaining some semblance of lower extremity function.
Quadriceps Facilitation
Previous authors 32 have shown soleus and peroneal muscle inhibition in subjects with CAI. The soleus muscle and quadriceps muscle motoneuron pools are reciprocally linked. 35–37 For example, with an artificial knee effusion, the soleus is facilitated and the quadriceps is inhibited. 36–38 An earlier finding of soleus muscle inhibition, combined with our current finding of quadriceps facilitation in those with CAI, provides further evidence of the reciprocal relationship between soleus and quadriceps motoneuron pool excitability. The functional relationship between the quadriceps and soleus may exist because both muscles are knee extensors in the closed chain. 43–45 As one muscle is inhibited, its agonist may be facilitated in an effort to maintain normal joint mechanics.
Soleus muscle inhibition in those with CAI was previously demonstrated using Hoffmann-reflex measures, 31 as opposed to the SIB maneuver used to assess quadriceps muscle activation in our study. The relationship between the SIB and the Hoffmann-reflex has yet to be established. However, they are both techniques used to estimate motoneuron pool excitability. One limitation of our present study is that soleus inhibition could not be verified. Future researchers should evaluate soleus and quadriceps motoneuron pool excitability in subjects reporting CAI.
Hamstrings Inhibition
Our finding of hamstrings inhibition in the CAI group may have 2 potential explanations. The first is reciprocal inhibition, 27 which may result from the inhibition of the soleus muscle, leading to a facilitation of the quadriceps muscle. Artificial effusions have provided evidence that the soleus muscle becomes facilitated when the quadriceps muscle is inhibited. 36–38 Currently, we have no reason to believe that the opposite may occur. Our findings support facilitation in the quadriceps muscle. These findings, coupled with earlier research showing soleus muscle inhibition, strengthen the argument. If the quadriceps is facilitated to compensate for the potential decrease in closed chain soleus function, the hamstrings must, according to reciprocal inhibition, be inhibited to allow for smoother coordinated muscle contraction of the quadriceps. The presence of hamstrings inhibition in our CAI group begins to address that theory.
A second plausible explanation for hamstrings inhibition relates to their secondary role as hip extensors. The gluteus maximus, the primary hip extensor, has been shown to have altered activation patterns in both limbs in subjects recovering from severe unilateral ankle sprains. 46 Similarly, we identified bilateral decreases in motoneuron pool excitability of the hamstrings in subjects with unilateral CAI. Although we tested hamstrings activation during knee flexion, it may be that hamstrings inhibition also manifests itself in hip extension.
The limitations of our assessment technique for the hamstrings must be acknowledged. To our knowledge, ours is the first study to use the SIB maneuver on the hamstrings. We were only able to obtain valid measures of the CAR in 29 of our 41 subjects, and this may have reduced the statistical power needed to identify a significant group-by-side interaction, as was seen in the quadriceps. We could not identify a plausible neurophysiologic explanation for why an agonist muscle group (eg, quadriceps) would have a unilateral motoneuron pool activation response and an antagonist muscle group (eg, hamstrings) would have a bilateral response. Additionally, the intrasession reliability of the hamstrings measure was only moderate (intraclass correlation coefficient = .61). We recognized the subjects were sitting on the electrode pads in the hamstrings protocol and that pad pressures may have varied across subjects. Another issue we must consider is that only 100 V was used to stimulate the hamstrings. This value is important because not all subjects may have had fully stimulated muscle fibers, which may account for some subjects having an undetectable SIB. Also, inhibition may have been underestimated, because it is uncertain if there was actually a supramaximal stimulus; however, we did detect torque values with SIB that were clearly greater than the MVIC. The study was limited to 100 V because, in the pilot testing, stimulation values greater than 100 V caused “cross-talk” stimulation into other muscles, particularly the thigh adductors. In some subjects, the SIB may have been unable to fully stimulate the hamstrings in order to produce an appreciable burst; in others, it may have elicited an appreciable burst but not a supramaximal stimulus.
A final potentially confounding factor in acquiring usable tracings for the hamstrings is the possibility of synergistic muscle contribution of the gastrocnemius, a secondary knee flexor. The contribution of the gastrocnemius to knee flexion may have been more than adequate to diminish any effect of the SIB on the hamstrings. Some subjects may have been more capable than other subjects of “shutting off” the gastrocnemius during contraction. We did not monitor the gastrocnemius during our testing sessions. Further refinements of the methods used to assess the CAR in the hamstrings using the SIB maneuver, perhaps with subjects lying prone, are warranted.
Clinical Implications
Mounting evidence demonstrates proximal neuromuscular changes in those with ankle instability. Such changes have been identified in both the ipsilateral and contralateral limbs of individuals with unilateral ankle injuries. Proximal changes ipsilaterally after acute ankle sprain have been shown in altered electromyographic and strength measures of the hip extensors. 46 Contralateral changes associated with acute ankle sprain have been illustrated via altered activation patterns of the hip extensors 46 and impaired postural control in the limb contralateral to the acutely sprained ankle. 47 In those with CAI, ipsilateral proximal changes have previously been demonstrated via reduced strength of the hip and knee extensors 48 and altered knee and hip kinematics during jump landings 7 and dynamic balance tasks. 6 Contralateral changes in postural control in subjects with CAI have also previously been shown. 8 The current findings add to this existing body of literature, showing changes in proximal and contralateral neuromuscular control related to CAI. Clinicians should be cognizant of these issues when designing rehabilitation programs for patients with acute ankle sprains or CAI. Rehabilitating the entire lower extremity on the side of impairment should be emphasized, and treatments that have been shown to reverse the effects of arthrogenic muscle inhibition, such as cryotherapy, transcutaneous electric nerve stimulation, 10 and joint manipulation, 49 should be used immediately before therapeutic exercise is performed in an effort to activate motor units that may have been previously inhibited. Consideration must also be given to assessing and restoring function to the limb contralateral to the side of injury.
Although the differences in quadriceps CAR were statistically significant between the involved and uninvolved limbs of CAI subjects, the magnitude of differences was only 3%. Similarly, the significant difference between CAI and control groups for hamstrings CAR was only of a 2% magnitude. Some may question the physiologic importance of a difference this small. Further research is needed to assess the functional consequences of such deficits. Lastly, the potential limitation of the assessment of motoneuron pool excitability in an open kinetic chain task must be acknowledged, as it is unclear what the consequences of the identified differences in CAR are in closed kinetic chain functional activities.
In conclusion, we demonstrated bilateral arthrogenic inhibition of the hamstrings and ipsilateral facilitation of the quadriceps in subjects with unilateral CAI. In those with unilateral CAI, motoneuron pool excitability appears to be altered in muscles that act on joints proximal to the ankle.
REFERENCES
- Garrick JG, Requa RK. The epidemiology of foot and ankle injuries in sports. Clin Sports Med. 1988;7:29–36. [PubMed] [Google Scholar]
- Garrick JG. The frequency of injury, mechanism of injury, and epidemiology of ankle sprains. Am J Sports Med. 1977;5:241–242. doi: 10.1177/036354657700500606. [DOI] [PubMed] [Google Scholar]
- Hertel J. Functional anatomy, pathomechanics, and pathophysiology of lateral ankle instability. J Athl Train. 2002;37:364–375. [PMC free article] [PubMed] [Google Scholar]
- McKay GD, Goldie PA, Payne WR, Oakes BW. Ankle injuries in basketball: injury rate and risk factors. Br J Sports Med. 2001;35:103–108. doi: 10.1136/bjsm.35.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MA, Dean MR, Hanham IW. The etiology of functional instability of the foot. J Bone Joint Surg Br. 1965;47:678–685. [PubMed] [Google Scholar]
- Gribble PA, Hertel J, Denegar CR, Buckley WE. The effects of fatigue and chronic ankle instability on dynamic postural control. J Athl Train. 2004;39:321–329. [PMC free article] [PubMed] [Google Scholar]
- Caulfield BM, Garrett M. Functional instability of the ankle: differences in patterns of ankle and knee movement prior to and post landing in a single leg jump. Int J Sports Med. 2002;23:64–68. doi: 10.1055/s-2002-19272. [DOI] [PubMed] [Google Scholar]
- Hertel J, Olmsted-Kramer LC. Deficits in time-to-boundary measures of postural control with chronic ankle instability. Gait Posture. 2007;25:33–39. doi: 10.1016/j.gaitpost.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Hopkins JT, Ingersoll CD. Arthrogenic muscle inhibition: a limiting factor in joint rehabilitation. J Sport Rehabil. 2000;9:135–159. [Google Scholar]
- Hopkins J, Ingersoll CD, Edwards J, Klootwyk TE. Cryotherapy and transcutaneous electric neuromuscular stimulation decrease arthrogenic muscle inhibition of the vastus medialis after knee joint effusion. J Athl Train. 2002;37:25–31. [PMC free article] [PubMed] [Google Scholar]
- Young A. Current issues in arthrogenous inhibition. Ann Rheum Dis. 1993;52:829–834. doi: 10.1136/ard.52.11.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A, Stokes M, Iles JF. Effects of joint pathology on muscle. Clin Orthop Relat Res. 1987;219:21–27. [PubMed] [Google Scholar]
- Stokes M. The contribution of reflex inhibition to arthrogenous muscle weakness. Clin Sci (Lond) 1984;67:7–14. doi: 10.1042/cs0670007. [DOI] [PubMed] [Google Scholar]
- Fahrer H, Rentsch HU, Gerber NJ, Beyeler C, Hess CW, Grunig B. Knee effusion and reflex inhibition of the quadriceps. J Bone Joint Surg Br. 1988;70:635–638. doi: 10.1302/0301-620X.70B4.3403614. [DOI] [PubMed] [Google Scholar]
- Hopkins JT, Ingersoll CD, Edwards J, Cordova ML. Changes in soleus motoneuron pool excitability after artificial knee joint effusion. Arch Phys Med Rehabil. 2000;81:1199–1203. doi: 10.1053/apmr.2000.6298. [DOI] [PubMed] [Google Scholar]
- Hopkins JT, Wagie N. Intrasession and intersession reliability of the quadriceps Hoffmann reflex. Electromyogr Clin Neurophysiol. 2003;43:85–89. [PubMed] [Google Scholar]
- Huber A, Suter E, Herzog W. Inhibition of the quadriceps muscle in elite male volleyball players. J Sports Sci. 1998;16:281–289. doi: 10.1080/026404198366812. [DOI] [PubMed] [Google Scholar]
- Iles JF, Stokes M, Young A. Reflex actions of knee joint afferents during contraction of the human quadriceps. Clin Physiol. 1990;10:489–500. doi: 10.1111/j.1475-097x.1990.tb00828.x. [DOI] [PubMed] [Google Scholar]
- Stokes M, Young A. Investigations of quadriceps inhibition: implications for clinical practice. Physiotherapy. 1984;70:425–428. [Google Scholar]
- Suter E, Herzog W. Extent of muscle inhibition as a function of knee angle. J Electromyogr Kinesiol. 1996;7:123–130. doi: 10.1016/s1050-6411(96)00028-4. [DOI] [PubMed] [Google Scholar]
- Suter E, Herzog W, Bray RC. Quadriceps inhibition following arthroscopy in patients with anterior knee pain. Clin Biomech (Bristol, Avon) 1998;13:314–319. doi: 10.1016/s0268-0033(98)00098-9. [DOI] [PubMed] [Google Scholar]
- MacDougall JD, Elder GC, Sale DG, Moroz JR, Sutton JR. Effects of strength training and immobilization on human muscle fibers. Eur J Appl Physiol Occup Physiol. 1980;43:25–34. doi: 10.1007/BF00421352. [DOI] [PubMed] [Google Scholar]
- MacDougall JD, Ward GR, Sale DG, Sutton JR. Biomechanical adaptation of human skeletal muscle to heavy resistance training and immobilization. J Appl Physiol. 1977;43:700–703. doi: 10.1152/jappl.1977.43.4.700. [DOI] [PubMed] [Google Scholar]
- Hopkins JT, Palmieri RM. Effects of ankle joint effusion on lower leg function. Clin J Sport Med. 2004;14:1–7. doi: 10.1097/00042752-200401000-00001. [DOI] [PubMed] [Google Scholar]
- Palmieri RM, Ingersoll CD, Hoffman MA. Arthrogenic muscle response to a stimulated ankle effusion. Br J Sports Med. 2004;38:26–30. doi: 10.1136/bjsm.2002.001677. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri RM, Hoffman MA, Ingersoll CD. Intersession reliability for H-reflex measurements arising from the soleus, peroneal, and tibialis anterior musculature. Int J Neurosci. 2002;112:841–850. doi: 10.1080/00207450290025851. [DOI] [PubMed] [Google Scholar]
- Machner A, Pap G, Awiszus F. Evaluation of quadriceps strength and voluntary activation after unicompartmental arthroplasty for medial osteoarthritis of the knee. J Orthop Res. 2002;20:108–111. doi: 10.1016/S0736-0266(01)00068-7. [DOI] [PubMed] [Google Scholar]
- Berth A, Urbach D, Awiszus F. Improvement of voluntary quadriceps muscle activation after total knee arthroplasty. Arch Phys Med Rehabil. 2002;83:1432–1436. doi: 10.1053/apmr.2002.34829. [DOI] [PubMed] [Google Scholar]
- Mizner RL, Stevens JE, Snyder-Mackler L. Voluntary activation and decreased force production of the quadriceps femoris muscle after total knee arthroplasty. Phys Ther. 2003;83:359–365. [PubMed] [Google Scholar]
- Hart JM, Kerrigan DC, Fritz JM, Saliba EN, Gansneder B, Ingersoll CD. Contribution of hamstring fatigue to quadriceps inhibition following lumbar extension exercise. J Sports Sci Med. 2006;5:70–79. [PMC free article] [PubMed] [Google Scholar]
- Hales JP, Gandevia SC. Assessment of maximal voluntary contraction with twitch interpolation: an instrument to measure twitch responses. J Neurosci Methods. 1988;25:97–102. doi: 10.1016/0165-0270(88)90145-8. [DOI] [PubMed] [Google Scholar]
- McVey ED, Palmieri RM, Docherty CL, Zinder SM, Ingersoll CD. Arthrogenic muscle inhibition in the leg muscles of subjects exhibiting functional ankle instability. Foot Ankle Int. 2005;26:1055–1061. doi: 10.1177/107110070502601210. [DOI] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E, Simonetta M. Pattern of monosynaptic heteronymous Ia connections in the human lower limb. Exp Brain Res. 1993;96:534–544. doi: 10.1007/BF00234121. [DOI] [PubMed] [Google Scholar]
- Koceja DM, Kamen G. Interactions in human quadriceps-triceps surae motoneuron pathways. Exp Brain Res. 1991;86:433–439. doi: 10.1007/BF00228969. [DOI] [PubMed] [Google Scholar]
- Koceja DM. Quadriceps mediated changes in soleus motoneuron excitability. Electromyogr Clin Neurophysiol. 1995;35:25–30. [PubMed] [Google Scholar]
- Hopkins JT, Ingersoll CD, Krause BA, Edwards JE, Cordova ML. Effect of knee joint effusion on quadriceps and soleus motoneuron pool excitability. Med Sci Sports Exerc. 2001;33:123–126. doi: 10.1097/00005768-200101000-00019. [DOI] [PubMed] [Google Scholar]
- Hopkins JT, Ingersoll CD, Edwards JE, Cordova ML. Changes in soleus motoneuron pool excitability after artificial knee joint effusion. Arch Phys Med Rehabil. 2000;81:1199–1203. doi: 10.1053/apmr.2000.6298. [DOI] [PubMed] [Google Scholar]
- Palmieri RM, Tom JA, Edwards JE. Arthrogenic muscle response induced by an experimental knee joint effusion is mediated by pre- and post-synaptic spinal mechanisms. J Electromyogr Kinesiol. 2004;14:631–640. doi: 10.1016/j.jelekin.2004.06.002. et al. [DOI] [PubMed] [Google Scholar]
- DeLee J, Drez D, Miller MD. DeLee and Drez's Orthopaedic Sports Medicine. St Louis, MO: WB Saunders; 2003.
- Leonard CT, Sandholdt DY, McMillan JA. Long-latency contributions to reciprocal inhibition during various levels of muscle contraction. Brain Res. 1999;817:1–12. doi: 10.1016/s0006-8993(98)01096-8. [DOI] [PubMed] [Google Scholar]
- Docherty CL, Gansneder BM, Arnold BL, Hurwitz SR. Development and reliability of the Ankle Instability Instrument. J Athl Train. 2006;41:154–158. [PMC free article] [PubMed] [Google Scholar]
- Hale SA, Hertel J. Reliability and sensitivity of the Foot and Ankle Disability Index in subjects with chronic ankle instability. J Athl Train. 2005;40:35–40. [PMC free article] [PubMed] [Google Scholar]
- Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantarflexors to support, forward progression and swing initiation during walking. J Biomech. 2001;34:1387–1398. doi: 10.1016/s0021-9290(01)00105-1. [DOI] [PubMed] [Google Scholar]
- Perry J. Gait Analysis: Normal and Pathological Function. Thorofare, NJ: Slack Inc; 1992.
- Nordin M, Frankel VH. Basic Biomechanics of the Musculoskeletal System. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2001.
- Bullock-Saxton JE, Janda V, Bullock MI. The influence of ankle sprain injury on muscle activation during hip extension. Int J Sports Med. 1994;15:330–334. doi: 10.1055/s-2007-1021069. [DOI] [PubMed] [Google Scholar]
- Evans T, Hertel J, Sebastianelli W. Bilateral deficits in postural control following lateral ankle sprain. Foot Ankle Int. 2004;25:833–839. doi: 10.1177/107110070402501114. [DOI] [PubMed] [Google Scholar]
- Gribble PA, Hertel J, Denegar C. Chronic ankle instability is associated with strength deficits in proximal lower extremity muscle groups [abstract] J Athl Train. 2005;40:S-28. (suppl) [Google Scholar]
- Hillermann B, Gomes AN, Korporaal C, Jackson D. A pilot study comparing the effects of spinal manipulative therapy with those of extra-spinal manipulative therapy on quadriceps muscle strength. J Manipulative Physiol Ther. 2006;29:145–149. doi: 10.1016/j.jmpt.2005.12.003. [DOI] [PubMed] [Google Scholar]