Abstract
Context: Deficits in static and dynamic stability during single-leg stance have been noted in individuals with chronic ankle instability (CAI), but few investigators have tested subjects for subtle deficits in dynamic balance. Subtle deficits in dynamic balance during a double-leg stance may reveal changes in the sensorimotor system because of CAI.
Objective: To use a standardized tibial nerve stimulation as a perturbation to test for dynamic balance deficits between a group of recreational athletes with CAI and a group of recreational athletes with stable ankles.
Design: Case-control study.
Setting: Laboratory.
Patients or Other Participants: Twenty recreational athletes with CAI and 20 recreational athletes with stable ankles.
Intervention(s): Balance deficits were assessed for each subject during static and dynamic trials.
Main Outcome Measure(s): Time to stabilization and center-of-pressure excursion path length, velocity, and area from ground reaction forces during double-leg stance were collected through a forceplate. We used an accelerometer to measure tibial acceleration. Data were collected during static stance and during a bilateral perturbation using maximal motor neuron recruitment elicited by electric stimulation of the tibial nerve.
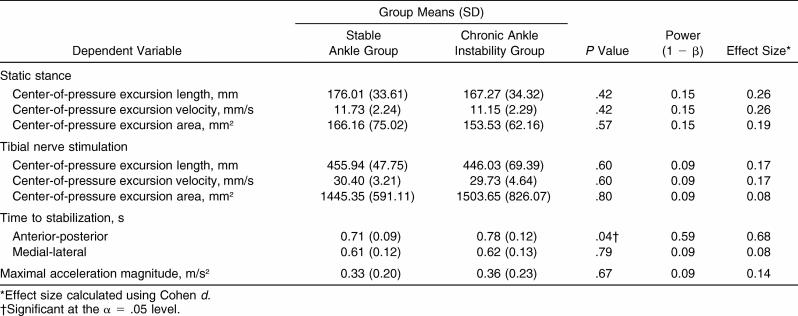
Results: Only time to stabilization in the anterior-posterior direction was significantly different between groups ( P = .04), with the CAI group taking longer to return to a stable range of ground reaction forces. We found no other differences in stability measures between the groups.
Conclusions: Dynamic balance in double-leg stance as measured by time to stabilization appears to be affected in individuals with CAI. Deficits in the response to external perturbation may indicate subtle central sensorimotor changes.
Keywords: ankle sprains, proprioception, stability, postural control
Key Points
When measured by time to stabilization, dynamic balance in double-leg stance appeared to be affected in individuals with chronic ankle instability.
Compared with individuals with stable ankles, individuals with chronic ankle instability had longer time-to-stabilization values in the anterior-posterior direction after tibial nerve stimulation in a double-leg stance.
Longer time-to-stabilization values in subjects with chronic ankle instability may indicate increased relaxation time and longer time until a stable pattern is reached after external perturbation.
Ankle sprains are one of the most common lower extremity injuries. According to the National Collegiate Athletic Association, 1 ankle sprains are the most common injury in men and women who participate in soccer, basketball, and volleyball. Most ankle sprains are inversion injuries that damage the lateral ligaments of the ankle. 2 Up to 73% of individuals who sprain their ankles have residual symptoms including pain, repeated sprains, and episodes of “giving way.” 3 Individuals with chronic ankle instability (CAI) experience frequent sprains, pain, and instability and have unknown long-term consequences to joint health. As the joint becomes unstable over time and continues to “roll” past its physiologic limits, the risk of damaging the articular surfaces within the joint and developing osteoarthritis increases. 4
In previous research, investigators 5–10 have identified static and dynamic balance deficits during single-leg stance in the affected limb of individuals with CAI; however, other authors 11–13 have reported no differences. Static balance means maintaining the center of mass over a stationary base of support, 14 such as maintaining balance during quiet stance. Dynamic balance means maintaining the center of mass over the base of support when the base of support is moving or when an external perturbation is applied to the body. In these studies, the investigators used different measures of postural stability, which may have contributed to the differences in results. 5–14 Additionally, in their study of an active population, Hoffman and Koceja 15 asserted that static measures may not relate well to dynamic measures, which are more indicative of functional ability and may be more applicable to a physically active population. The way that unilateral or bilateral CAI affects the contralateral limb and the spontaneous self-organization of the sensorimotor system is unclear. Rozzi et al 8 reported minor balance deficits in the uninvolved limb in individuals with unilateral CAI, and Caulfield and Garrett 16 attributed changes in landing pattern to “alterations in central programming at the spinal level.” Using a double-leg stance may reveal possible central programming deficits and emphasize the interplay between the involved limb and overall control of body motion. Balance deficits in double-leg stance in subjects with CAI have not been assessed. Deficits identified in this stable position may provide evidence for the change in basic motor control strategies in subjects with CAI.
Time to stabilization (TTS) is a measure of dynamic stability that analyzes the anterior-posterior (A/P), medial-lateral (M/L), and vertical ground reaction forces (GRFs) during the period the subject is recovering from a perturbation and returning to a static stance. 7, 9, 11, 17, 18 It assesses how long the individual takes to return the GRF to a stable range. The TTS has been used to evaluate dynamic balance in subjects with CAI after landing from a single-leg jump. 7, 9, 11, 18 Investigators 7, 9, 11 have demonstrated that individuals with CAI take longer than individuals with stable ankles to return their A/P GRF to a stable range after jump landing. However, jump landings are difficult to standardize and introduce considerable variability into the testing protocol in terms of jump height, trunk and arm positioning, and landing strategy. 9 Differences in these factors may have accounted for differences in the reported TTS values. We elected to change the perturbation to a movement more centered on the ankle to test if less complex and lower amplitude movements elicited the same TTS deficits. Hoffman and Koceja 15 established the reliability of a perturbation protocol and measured subjects' ability to regain stability. Electric stimulation applied to the tibial nerve resulted in an involuntary contraction of the triceps surae muscles, and the subjects had to shift weight to maintain balance. Some leg, trunk, and upper extremity movement occurred, but the subjects did not move their feet. Because movement occurred, it was a measure of dynamic balance, but it enabled the individual to remain standing upright. Consistent levels of perturbation were observed between trials because the strength of the perturbation (electric stimulation) was always the same. They 15 reported the intrasession reliability as 0.71 to 0.98. Although this external perturbation is not necessarily applicable outside the laboratory, it does minimize some of the limitations encountered in previous jump-landing studies. 7, 9, 11
The dynamic systems theory states that sensorimotor system organization involves the interaction of various constraints, including the task, environment, and organism, to produce movement. Interaction of these constraints produces spontaneous self-organization of the sensorimotor system. 19 When an external perturbation is applied and the model of self-organizing constraints is used, the components of the network interact under the influence of higher brain-center inputs and peripheral inputs. 19, 20 As the dynamics of the network change, patterns develop, and multiple patterns may be created under different conditions. 19, 20 One may consider CAI as a constraint in the sensorimotor system or as a feature that interacts with others to limit the attempt of a biological system to organize optimally; the effect is evident as organismic deficits in balance. 19 As an added constraint, individuals with CAI may not be able to develop adequate patterns to address external perturbations. Longer TTS values in subjects with CAI may reflect that lack of flexibility to adjust to different perturbations. Additionally, longer TTS values in subjects with CAI may reflect an increase in relaxation time, as explained in the Haken phase transition theory. 21, 22 Relaxation time is the amount of time needed for the relative phase relationship to return to its original state after a perturbation and indicates the stability of the movement pattern. 21, 22 If a longer relaxation time is noted, the organization of the neuromuscular system is considered less stable. 21, 22 Thus, longer TTS values in subjects with CAI may reflect the longer time required for the sensorimotor system to reorganize to a stable pattern after external perturbation. Even at low levels of perturbation, dynamic balance deficits in double-leg stance that are detected with TTS values provide evidence that basic motor-control strategies are altered adversely in individuals with CAI. The TTS eventually may be developed into a screening tool to place individuals with CAI into a continuum of severity, establish more homogenous research groups, and potentially test the efficacy of rehabilitation programs.
By using both traditional static balance measures and novel dynamic measures, researchers and clinicians may be able to detect subtle differences in sensorimotor function in individuals with CAI. A standardized perturbation is lacking in CAI research and may be useful. If compromises in dynamic balance exist in individuals with CAI, rehabilitation programs that emphasize rapid stabilizations after perturbation may be designed. 23 Time to stabilization is a relatively new measure and may identify and quantify CAI in individuals; however, it needs more investigation. The purpose of our study was to use tibial nerve stimulation as a standardized perturbation to test for deficits in static and dynamic balance between a group of recreational athletes with CAI and a group of recreational athletes with stable ankles.
METHODS
Subjects
Forty healthy, recreationally active subjects aged 18 to 36 years volunteered to participate in our study. No subject was receiving physical therapy treatment for any lower extremity injuries. We defined recreationally active as participating in at least 20 minutes of physical activity 3 times per week. We divided subjects into 2 groups: stable ankles and CAI. We matched 20 subjects with stable ankles (10 men, 10 women) to 20 subjects with CAI (10 men, 10 women) by sex, age (±2 years), height (±10%), mass (±10%), test-limb dominance, and activity type and level. Subjects with stable ankles had a mean age, height, and mass of 22.10 ± 4.15 years, 173.69 ± 11.68 cm, and 72.10 ± 13.24 kg, respectively. Subjects with CAI had a mean age, height, and mass of 21.45 ± 3.41 years, 173.16 ± 10.48 cm, and 69.34 ± 12.68 kg, respectively. The dominant limb was defined as the limb used in at least 2 of the 3 following tests: recovering balance after a posterior push, stepping up onto a box, and kicking a ball with maximal accuracy through a goal. 24 The involved unstable ankle of subjects in the CAI group was assessed for dominance or nondominance and was matched to the dominant or nondominant limb of the subjects in the stable ankle group, accordingly. In each group, the dominant leg of 12 subjects and the nondominant leg of 8 subjects were tested. Subjects reported participating in at least 1 of the following activities on a weekly basis: basketball, running, weight training, volleyball, tennis, swimming, biking, softball, football, soccer, or racquetball. The average physical activity level of each participant was 6.8 hours (range, 1–18 hours) per week. Subjects in the CAI group reported an average of 10 sprains to the right and 7 sprains to the left ankle (range, 2–70 sprains).
Inclusion criteria for subjects with stable ankle were no history of the ankle giving way with activity and no ankle sprains within the 8 weeks before the study. Inclusion criteria for subjects with CAI were a history of the ankle giving way with activity and a minimum of 2 ankle sprains or “episodes of giving way” in the year before the study. 9, 16 Exclusion criteria for both groups were a history of lower extremity surgery, current signs or symptoms of an ankle sprain (ie, swelling, discoloration, heat, pain, or acute loss of function), vestibular or balance disorders, hereditary nerve disorders, or current use of drugs that affect the central nervous system. All subjects in the CAI group had at least 2 ankle sprains in the 12 months before the study, and most of them had sprained their ankles in the 6 months before the study. The damage occurred at the distal ligament. Subjects provided informed consent. Our study was approved by the university's institutional review board.
Electromyography
We used the 8-channel Bagnoli Desktop EMG System (Delsys Inc, Boston, MA) with differential amplification, common mode rejection ratio >80 dB; input impedance >10 15//0.2 ohm//pF, signal-to-noise ratio >40 dB; and an amplifier to measure electromyographic (EMG) activity of the tibialis anterior, peroneal, lateral gastrocnemius, and soleus muscles in the test leg and of the soleus muscle in the nontest leg. A 16-bit analog-to-digital converter (model PCI-MIO-16E-1; National Instruments, Austin, TX) sampled the EMG data and passed the signals from the receiver to the computer. We used surgical tape and adhesive stickers to fix bar-shaped Ag/AgCl single differential surface electrodes (model DE-2.1; Delsys Inc) on the midpoint of each muscle belly. After shaving, abrading, and cleansing the skin with alcohol, we placed electrodes according to previously published guidelines 25, 26 over the muscle belly for the tibialis anterior, peroneals, and lateral gastrocnemius. The soleus electrode placement was inferior to the gastrocnemius and proximal to the insertion of the Achilles tendon on the calcaneus. The electrodes were 19 mm wide and 35 mm long with 10 mm between contacts. We placed a carbon reference electrode over the lateral malleolus. Manual muscle tests were performed on each muscle, and the output was viewed on an oscilloscope to ensure proper electrode placement and to minimize cross-talk. We sampled at 1000 Hz over a bandwidth of 20 to 500 Hz. Total gain on the system was 1000, and the EMG signal was corrected for direct current (DC) bias. Electromyography was used solely to visualize the tibial nerve stimulation during dynamic balance trials.
Accelerometer
A triaxial accelerometer (model 356A22; PCB Piezoelectronics, Depew, NY) was attached to the anterior tibial crest using a custom orthoplast device. We secured the accelerometer to the orthoplast and the leg with underwrap and surgical tape. The x-axis was aligned in the A/P direction; positive acceleration occurred in the same direction as the subject was facing. We connected the accelerometer (voltage sensitivity = 100.0 mV/g, frequency range = 0.5–4000 Hz, output bias level = 8–12 VDC) to a BNC breakout box (model 482A17; PCB Piezoelectronics) that amplified the signal by a factor of 10 and was connected to the same A/D board as the EMG system.
Tibial Nerve Stimulation
A telefactor stimulator with 2 constant current units (CCUs) and 2 stimulus isolation units (SIUs) (models S88, CCU1, and SIU5-D, respectively; Grass Instruments Co, Warwick, RI) transmitted a bilateral 150-V electrical stimulation with a square-wave DC pulse to 1-cm Ag/AgCl stimulating electrodes. On the stimulus unit, the S1 and S2 stimulus switches were set to “on,” with the S1 function set to single and the S2 function set to S1 and S2. The train rate was set to 1 pulse per second with the meter multiplier set to ×1.0, and the S1 sync-out channel was connected to the analog-to-digital board. The S1 voltage was set to 15, and the meter multiplier was set to ×10 SIUs. The S1 duration was set to 1 ms at ×1, with S1 delay set to zero, with the S1 rate set to less than 1 and ×.01. On both CCUs, the output adjustment I was set to normal, with the current adjustment set to maximum and the meter multiplier set to ×10.
The cathodes were applied to the popliteal fossa over the tibial nerve where it passed close to the surface of the skin in the back of the knee. A 4-cm carbon electrode (anode) was placed over the anterior portion of the thigh just proximal to the knee to complete the circuit. 15 Each electrode was separated from the skin by conductive gel and attached to the skin with surgical tape. A 1-millisecond stimulation was applied, causing a brief, strong contraction of the triceps surae muscles. Consequently, each subject's center of gravity was displaced posteriorly over the base of support, causing an anterior weight shift in response. 15
Forceplate
A piezoelectric forceplate (model 4060A; Bertec Co, Columbus, OH) with a frequency response of 400 Hz in the vertical direction and 300 Hz in both of the horizontal directions collected 3-dimensional forces and moments. Forceplate data were collected through Digital Acquire (version 1.4.5; Bertec Co), which was the standard software that the manufacturer provided with the forceplate. The sampling frequency for that program was set to 1000 Hz with a 500-Hz filter.
Testing
We performed 3 practice tibial nerve stimulation trials to check for stimulating and EMG electrode placement, electric signal wave-form, patient comfort, and motor response. The stimulus can be uncomfortable, but subjects tolerated it well for the short test. Once the check was complete, the subjects performed 3 static standing trials without tibial nerve stimulation and 7 dynamic test trials with tibial nerve stimulation. The single test session lasted 1 hour. During the static trials, subjects stood in a comfortable stance with both feet on the force plate, approximately 1 shoulderwidth apart with their hands on their hips and looking straight ahead. 15 They were instructed to remain standing as still as possible. During the tibial nerve stimulation trials, subjects were in the same position, and the stimulation was applied after a period of variable length (from 5 to 10 seconds). The length of the period was varied to attempt to decrease anticipatory effects of muscle contraction. We collected data from the beginning of the period to 5 to 10 seconds after the stimulation, depending on the length of the period, so that the total trial time was 15 seconds. 15 Subjects were instructed to regain balance as quickly as possible. 15 Hoffman and Koceja 15 reported that stability was recovered well in their subjects within 3 seconds after the ankle perturbation. At least 30 seconds of rest was provided between trials. We did not map a motor response, but, by setting the stimulus unit to maximum, we ensured that the subjects experienced a maximal M wave.
Data Processing
The forceplate collected GRFs via the Acquire software, sampling at 180 Hz, with amplifier gains set at 2. We used a custom LabVIEW software program (version 6i; National Instruments) to process the data and calculate balance measures (center-of-pressure [COP] excursion path length, velocity, and area) from the GRF data. These traditional COP balance measures were calculated during both the static and dynamic trials. The COP excursion path length was calculated as the sum of the linear distance between consecutive data points collected during the trial. The COP velocity was calculated as the path length of that trial divided by 15 seconds, and it represented the average velocity during the trial. The COP excursion area was calculated by taking the mean location of all the points and connecting that mean to the first point recorded. A triangle was drawn from point 1 to point 2 and from point 2 back to the mean. The program calculated the area of that triangle and repeated the procedure, sweeping out the area in triangles from each successive point and the mean and calculating and summing all those areas. 15
We used the oscillating GRF in each direction to manually identify A/P TTS and M/L TTS. The researcher was blinded to the subjects' group assignment and used the following rules for identifying TTS. First, the researcher identified a stable range before the perturbation in which minimal COP excursion occurred. Second, the time of stimulus onset was identified as the first deviation in the x direction. This time was cross-checked with the EMG data to ensure accuracy of stimulus onset identification. Third, the COP location was tracked as it oscillated after the perturbation, with all subjects following a regular damped oscillation. When that oscillation decreased in magnitude and appeared stable about the previous baseline level, TTS was established. An intraclass correlation coefficient (ICC 2,1) 27 was used to establish observer reliability in determining TTS. Using 6 subjects (3 from each group), the researcher identified A/P and M/L TTS for 1 trial of each subject 5 separate times. The researcher was blinded to group membership and to the TTS values identified in previous trials. The ICC value for A/P TTS was 0.99, with a standard error of measurement (SEM) value of 51.62 ms. For M/L TTS, the ICC value was 0.98, with an SEM value of 42.56 ms.
We used custom LabVIEW programs to collect and process the EMG and accelerometer data. Raw EMG signal was bandpass filtered using a fourth-order Butterworth filter from 10 to 350 Hz, was notch filtered at 59.5 to 60.5 Hz, and was rectified. No smoothing procedures were performed. No additional postprocessing was necessary on the accelerometer signal. We also identified the magnitude of the maximal acceleration in the A/P direction in each trial.
Data Analysis
All data analyses were performed using SPSS statistical software (version 12.0; SPSS Inc, Chicago, IL). We discarded the highest and lowest values of each variable in the 7 tibial nerve stimulation trials for each subject, leaving 5 test trials for data analysis. The variables removed were not necessarily from the same trial. Each subject's 5 trials were averaged for that subject on each variable. The 3 static stance trials were averaged and included in the analyses.
We performed independent-samples t tests with an α level of .05 to ensure groups were appropriately matched for age, height, and mass and that no statistical differences between groups existed for those variables. Independent-samples t tests also were used to compare accelerometer maximal acceleration in the anterior direction.
We calculated independent-samples t tests for each dependent variable, with an a priori α level of .05. The independent variable or factor variable was group (stable or CAI), and the dependent variables were A/P and M/L TTS and COP excursion length, velocity, and area in both static and dynamic trials. An ICC (2,1) also was calculated for each dependent variable and maximal acceleration with SEM to assess reliability and precision in each group. 27 Because of equipment malfunction, EMG data were available for only 37 of the 40 subjects. The 3 subjects for whom we did not have EMG data were in the CAI group.
RESULTS
Age, height, and mass were not significantly different between the stable ankle and CAI groups ( P > .05). Maximal acceleration magnitude also was not significantly different between the groups ( P = .67; Table 1). Only the A/P TTS was significantly different between groups, with the CAI group demonstrating longer TTS values than the stable ankle group ( P = .04). No differences in M/L TTS or traditional balance measures were observed between the groups ( Table 1). The observed power and effect sizes also are reported in the Table.
Table 1. Independent-Samples t Test Results .
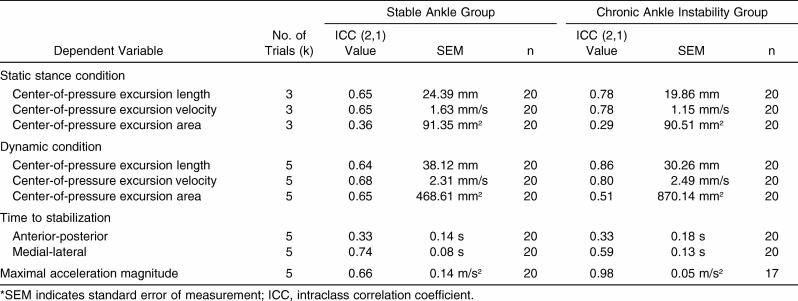
The ICC (2,1) results for each dependent variable by group are reported in Table 2. The maximal acceleration magnitude demonstrated good within-subjects reliability for the CAI group (ICC = 0.98, SEM = 0.05 m/s 2) but moderate reliability for the stable ankle group (ICC = 0.66, SEM = 0.14 m/s 2). Reliability of the COP excursion area and A/P TTS was low. The CAI group demonstrated good reliability for the COP excursion length and velocity in the static and dynamic conditions; this group's reliability was better than that of the stable ankle group.
Table 2. Intraclass Correlation Coefficients and Standard Errors of Measurement*.
DISCUSSION
Our most important finding was that individuals with CAI had longer A/P TTS after a standardized perturbation in a double-limb stance. This was the only deficit in static or dynamic balance that we observed. Balance in individuals with CAI, for the most part, was not significantly affected in a stable double-leg stance. However, after a perturbation, subjects with CAI did not return to a stable stance in the A/P direction as quickly as individuals with stable ankles. This delay provides evidence for the central effects of CAI on the sensorimotor system. Chronic ankle instability may be viewed as a constraint on the sensorimotor system, limiting its ability to quickly generate new patterns of movement to control posture and return to a steady stance after an external perturbation is applied. Decreases in the ability of the self-organizing sensorimotor system to generate and implement movement patterns quickly after a perturbation may indicate an increased relaxation time and, thus, a less stable organization of the neuromuscular system. 21 Although damage occurred only at a distal ligament, this organism-level change may indicate that an individual with CAI may have overall sensorimotor response to perturbation that is altered and that may be unable to adapt to an external perturbation as easily as an individual without CAI. The longer TTS values in subjects with CAI may indicate increased relaxation time and, thus, longer time until a stable pattern is reached after external perturbation.
Time to stabilization is a unique measure of stability because it is dynamic and potentially more functional than traditional balance measures. It also reflects a global measure of the sensorimotor system, potentially indicating a relationship between CAI and central patterning. 16 The magnitude of the TTS differences between groups that we observed was smaller than differences reported by other investigators. 7, 9, 28 This observation is reasonable because standing on 2 feet is much less demanding than a single-leg jump landing, and Wikstrom et al 28 found shorter TTS values with less demanding tasks. However, even a difference of a few milliseconds may increase the risk of injury and potential joint damage if GRF cannot be controlled and minimized quickly. 29
The ICCs for each group revealed low to moderate reliability for most dependent variables. The CAI group was more reliable than the stable ankle group in the static and dynamic COP excursion length and velocity and the maximal acceleration magnitude. However, the CAI group was less reliable in static and dynamic COP excursion area and M/L TTS. The stable ankle and CAI groups had similar low ICC values for the A/P TTS. Interpreting these results is difficult because ICCs typically measure reliability or repeatability, and the nature of healthy human movement may be variable, especially with low-demand tasks. 30, 31 Reliability, variability, and the task difficulty may interact. 32 The CAI subjects appear to produce more consistent COP excursion length and velocity measures during static and dynamic conditions than stable subjects produce, but the COP area, which is a broader measure of balance performance, is more variable. If CAI is a constraint to the sensorimotor system, the greater consistency in COP excursion length and velocity may actually produce fewer successful movement patterns to decrease the COP excursion area. 31 We instructed subjects to stand as still as possible, and the decrease in reliability of subjects with CAI in the COP excursion area may mean that the sensorimotor system was constrained by the CAI condition and had a more difficult time spontaneously self-organizing to successfully complete the task. The large SEM value for the CAI group in the dynamic COP excursion area reinforces this interpretation ( Table 2). However, the ICC values for A/P TTS and the SEM values were very similar between groups. This finding may indicate TTS as a novel measure can capture a different component of stability than the more traditional measures can capture.
Using a standardized tibial nerve stimulation with TTS in our study evoked consistent speed and amount of displacement as anticipated; we observed no differences in the maximal acceleration of the tibia. The accelerometer was used to ensure that the ankle perturbation was standardized between groups in amplitude and rate of acceleration. Additionally, movement during the perturbation was concentrated at the ankle, but completely isolating the joint during a postural task is impossible. Movement in the rest of the kinetic chain, including at the knee, hip, and trunk, occurred. The sensorimotor system may be able to compensate for deficiencies at the ankle by relying on sensory input from and movement strategies at the hip and knee. This compensation may explain why we observed no differences in the traditional stability measures. The differences we did observe in TTS were small, which may be due to the robustness of the sensorimotor system despite CAI and its ability to compensate at proximal joints during movement.
No other variables (ie, COP excursion path length, velocity, or area) in the static or dynamic conditions demonstrated differences between groups during a double-leg stance. Whether deficits in static balance exist in subjects with CAI remains controversial. Nakagawa and Hoffman 5 noted deficits in COP total excursion during single-leg stance, and other authors 8, 10 have noted deficits in single-leg balance in individuals with CAI. However, Ross and Guskiewicz 11 reported no differences in COP mean excursion, and Bernier et al 12 reported no differences in stability index measures during single-leg stance. In individuals with CAI in our study, the dynamic balance condition challenged the sensorimotor system more than the static balance condition challenged it because TTS was the only dependent variable that detected group differences after the change in the sensorimotor system from the perturbation. The TTS may be a better measure of sensorimotor function in the presence of the constraint of CAI.
The limitations of our study include the low intertrial reliability of some of the variables, including COP excursion area and A/P TTS; the latter was the only variable with group differences. Variability should be considered a separate entity to measurement reliability. If CAI is a constraint on the sensorimotor system, the network components may not be able to interact to develop adequate patterns to address external perturbations. The large SDs noted in the COP excursion area during the tibial nerve stimulation may provide evidence for CAI as a constraint to the sensorimotor system. Alternatively, the simplicity of the task did not considerably challenge the postural control system and resulted in highly variable responses. The double-leg stance is very stable, even with the perturbation applied. According to Brouwer et al, 30 dynamic balance tests that do not maximally challenge the postural control system result in high within-subjects and between-subjects variability in healthy young subjects. Poor reliability may be attributable to the large number of different response patterns that participants successfully used over all the trials. Fewer successful response strategies may be available with a more challenging task. 30
Our subjects also may have displayed inconsistent responses to the novel perturbation and exhibited high variability related to learning effects, not lesions, at the ankle joint. Other limitations include potential increased anxiety because of the stimulation, which may have increased between-trials variability. Additionally, the researcher who identified TTS was blinded to group membership, but bias was still possible. Reliability in identifying TTS was excellent, but observer error was still possible.
Although the tibial nerve stimulus appears to be more standardized than the single-leg jump landing used in previous TTS studies, it is not as valid externally. In addition, we and other investigators 6, 7 using TTS identified group differences primarily in the A/P direction. Differences in the M/L direction were noted only once. 11 The differences observed in the A/P direction may be solely attributable to the direction of the perturbation. Jumping or creating an external perturbation in the M/L direction may alter these findings and create differences in frontal-plane TTS. The ligaments involved in CAI limit motion in several planes, and testing TTS after perturbations in different directions may reveal previously unrecognized balance deficits. Finally, researchers and clinicians should note that the statistical power was low for almost all the variables. The small effect sizes and powers may be because of the stability of the double-leg stance or the variability observed in healthy subjects performing easy tasks such as this. 30
Future research should include a single-leg stance and kinematic and EMG analysis of the lower extremity during the tibial nerve stimulation. Rehabilitation programs targeting rapid and appropriate responses to external perturbation should be developed and tested to see if improvement in TTS can occur. The amount of variability in movement that the CAI group displays should be quantified and compared with the stable ankle group to see if increased variability increases TTS and places this group at greater risk for injury.
CONCLUSIONS
Dynamic balance measured by A/P TTS appears to be affected negatively in subjects with CAI during double-leg stance. Injury to the lateral ligaments may be a constraint that affects global sensorimotor responses, causing subjects with CAI to be less able to compensate and adjust after external perturbation. If the sensorimotor system is affected negatively and rapid reorganization cannot occur after external perturbation, individuals with CAI may be at greater risk for injury. Clinicians should focus on promoting rapid and appropriate stabilizations following external perturbation during rehabilitation.
Acknowledgments
We thank the University of North Carolina Injury Prevention Research Center Student Small Grants (Chapel Hill, NC) for providing funding, and we thank the research assistants in the Motor Control Laboratory for their help in data collection.
REFERENCES
- Injury Surveillance System. Sport specific injury data. Available at: http://www1.ncaa.org/membership/ed_outreach/health-safety/iss/Reports2003-04. Accessed April 3, 2004 .
- Willems T, Witvrouw E, Verstuyft J, Vaes P, De Clercq D. Proprioception and muscle strength in subjects with a history of ankle sprains and chronic instability. J Athl Train. 2002;37:487–493. [PMC free article] [PubMed] [Google Scholar]
- Yeung MS, Chan KM, So CH, Yuan WY. An epidemiological survey on ankle sprain. Br J Sports Med. 1994;28:112–116. doi: 10.1136/bjsm.28.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintermann B, Boss A, Schafer D. Arthroscopic findings in patients with chronic ankle instability. Am J Sports Med. 2002;30:402–409. doi: 10.1177/03635465020300031601. [DOI] [PubMed] [Google Scholar]
- Nakagawa L, Hoffman M. Performance in static, dynamic, and clinical tests of postural control in individuals with recurrent ankle sprains. J Sport Rehabil. 2004;13:255–268. [Google Scholar]
- Ross SE, Guskiewicz KM, Yu B, Garrett WE. Landing pattern difference between functionally stable and unstable ankles. Med Sci Sports Exerc. 2002;34:S173. [Google Scholar]
- Brown CN, Ross SE, Mynark R, Guskiewicz KM. Assessing functional ankle instability with joint position sense, time to stabilization, and electromyography. J Sport Rehabil. 2004;13:122–134. [Google Scholar]
- Rozzi SL, Lephart SM, Sterner R, Kuligowski L. Balance training for persons with functionally unstable ankles. J Orthop Sports Phys Ther. 1999;29:478–486. doi: 10.2519/jospt.1999.29.8.478. [DOI] [PubMed] [Google Scholar]
- Ross SE, Guskiewicz KM, Yu B. Single-leg jump-landing stabilization times in subjects with functionally unstable ankles. J Athl Train. 2005;40:298–304. [PMC free article] [PubMed] [Google Scholar]
- Tropp H, Odenrick P, Gillquist J. Stabilometry recordings in functional and mechanical instability of the ankle joint. Int J Sports Med. 1985;29:180–182. doi: 10.1055/s-2008-1025836. [DOI] [PubMed] [Google Scholar]
- Ross SE, Guskiewicz KM. Examination of static and dynamic postural stability in individuals with functionally stable and unstable ankles. Clin J Sport Med. 2004;14:332–338. doi: 10.1097/00042752-200411000-00002. [DOI] [PubMed] [Google Scholar]
- Bernier JN, Perrin DH, Rijke A. Effect of unilateral functional instability of the ankle on postural sway and inversion and eversion strength. J Athl Train. 1997;32:226–232. [PMC free article] [PubMed] [Google Scholar]
- Isakov E, Mizrahi J. Is balance impaired by recurrent sprained ankle? Br J Sports Med. 1997;31:65–67. doi: 10.1136/bjsm.31.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guskiewicz KM, Perrin DH. Research and clinical applications of assessing balance. J Sport Rehabil. 1996;5:45–63. [Google Scholar]
- Hoffman MA, Koceja DM. Dynamic balance testing with electrically evoked perturbation: a test of reliability. Arch Phys Med Rehabil. 1997;78:290–293. doi: 10.1016/s0003-9993(97)90036-8. [DOI] [PubMed] [Google Scholar]
- Caulfield BM, Garrett M. Functional instability of the ankle: differences in landing patterns of ankle and knee movement prior to and post landing in a single leg jump. Int J Sports Med. 2002;23:64–68. doi: 10.1055/s-2002-19272. [DOI] [PubMed] [Google Scholar]
- Colby SM, Hintermeister RA, Torry MR, Steadman JR. Lower limb stability with ACL impairment. J Orthop Sports Phys Ther. 1999;29:444–454. doi: 10.2519/jospt.1999.29.8.444. [DOI] [PubMed] [Google Scholar]
- Ross SE, Guskiewicz KM. Time to stabilization: a method for analyzing dynamic postural stability. Athl Ther Today. 2003;8(3):37–39. [Google Scholar]
- Davids K, Glazier P, Araujo D, Bartlett R. Movement systems as dynamical systems: the functional role of variability and its implications for sports medicine. Sports Med. 2003;33:245–260. doi: 10.2165/00007256-200333040-00001. [DOI] [PubMed] [Google Scholar]
- Scholz JP. Dynamic pattern theory: some implications for therapeutics. Phys Ther. 1990;70:827–843. doi: 10.1093/ptj/70.12.827. [DOI] [PubMed] [Google Scholar]
- Kurz MJ, Stergiou N. Applied dynamic systems theory for the analysis of movement. In: Stergiou N, ed. Innovative Analyses of Human Movement: Analytical Tools for Human Movements Research. Champaign, IL: Human Kinetics; 2003:93–119 .
- Scholz JP, Kelso JAS. A quantitative approach to understanding the formation and change of coordinated movement patterns. J Mot Behav. 1989;21:122–144. doi: 10.1080/00222895.1989.10735470. [DOI] [PubMed] [Google Scholar]
- Tropp H. Commentary: functional ankle instability revisited. J Athl Train. 2002;37:512–515. [PMC free article] [PubMed] [Google Scholar]
- Hoffman M, Schrader J, Applegate T, Koceja D. Unilateral postural control of the functionally dominant and nondominant extremities of healthy subjects. J Athl Train. 1998;33:319–322. [PMC free article] [PubMed] [Google Scholar]
- Delagi EF, Perotto A, Iazzetti J, Morrison D. Anatomic Guide for the Electromyographer. 2nd ed. Springfield, IL: Charles C. Thomas; 1980.
- Basmajian JV, Blumenstein R. Electrode Placement in EMG Biofeedback. Baltimore, MD: Williams & Wilkins; 1980.
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Wikstrom EA, Tillman MD, Borsa PA. Detection of dynamic stability deficits in subjects with functional ankle instability. Med Sci Sports Exerc. 2005;37:169–175. doi: 10.1249/01.mss.0000149887.84238.6c. [DOI] [PubMed] [Google Scholar]
- McKinley TO, Rudert MJ, Koos DC, Brown TD. Incongruity versus instability in the etiology of posttraumatic arthritis. Clin Orthop Relat Res. 2004;423:44–51. doi: 10.1097/01.blo.0000131639.89143.26. [DOI] [PubMed] [Google Scholar]
- Brouwer B, Culham EG, Liston RA, Grant T. Normal variability of postural measures: implications for the reliability of relative balance performance outcomes. Scand J Rehabil Med. 1998;30:131–137. doi: 10.1080/003655098444048. [DOI] [PubMed] [Google Scholar]
- van Emmerik RE, van Wegen EE. On the functional aspects of variability in postural control. Exerc Sport Sci Rev. 2002;30:177–183. doi: 10.1097/00003677-200210000-00007. [DOI] [PubMed] [Google Scholar]
- James CR, Dufek JS, Bates BT. Effects of injury proneness and task difficulty on joint kinetic variability. Med Sci Sports Exerc. 2000;32:1833–1844. doi: 10.1097/00005768-200011000-00004. [DOI] [PubMed] [Google Scholar]