Abstract
Stress responses during cocaine withdrawal likely contribute to drug relapse and may be intensified as a consequence of prior cocaine use. The present study examined changes in stressor‐induced activation of the hypothalamic‐pituitary‐adrenal (HPA) axis during acute withdrawal from chronic cocaine administration. Adult male Sprague Dawley rats received daily administration of cocaine (30 mg/kg, IP) or saline for 14 days. Twenty‐four hours after the last injection, rats in each group were sacrificed under stress‐free conditions or following 30 minutes of immobilization. Plasma corticosterone (CORT) was measured in trunk‐blood using radioimmunoassay, corticotropin‐releasing hormone (CRH) mRNA levels in the paraventricular nucleus (PVN) of the hypothalamus were measured using in situ hybridization and glucocorticoid receptor (GR) protein expression in the pituitary gland and dissected brain regions was measured using Western blot analysis. Basal CRH mRNA in the PVN was unaltered as a result of prior cocaine administration. However, a significant increase in CRH mRNA was observed 90 minutes following the termination of restraint in cocaine withdrawn, but not salinetreated, rats. Basal CORT was also unaffected by prior cocaine administration, but the CORT response measured immediately after restraint was significantly augmented in cocaine‐withdrawn rats. Differences in GR protein expression in number of regions implicated in negative feedback regulation of HPA function, including the hypothalamus, were not observed. These findings indicate that the HPA response to stressors is intensified during early withdrawal from cocaine administration and may be independent of changes in GR‐mediated negative feedback.
Keywords: Cocaine, stress, glucocorticoid receptor, CRF, HPA axis, corticosterone
INTRODUCTION
A growing body of evidence suggests that stress plays an important role in cocaine addiction [11, 28]. In addition to findings that stress promotes cocaine‐seeking behavior, it has been reported that stress‐related behavioral responses emerge and/or are exaggerated as a consequence of prior cocaine exposure, suggesting that the relationship between stress and cocaine abuse represents a self‐perpetuating cycle within which stress serves as both a precipitating factor for and a consequence of drug use.
The hypothalamic pituitary adrenal (HPA) axis is a critical mediator of physiological responses that enable organisms to adapt during times of stress. Such responses likely include changes in behavior that involve the same neurocircuitry responsible for illicit drug use and addiction. Accordingly, it has been reported that although stressor‐induced glucocorticoid secretion is not necessary for acute stressor‐induced cocaine‐seeking behavior [9], glucocorticoids play an important role in the effects of repeated stress on the addiction process as substrates through which chronic stressors increase cocaine self‐administration [20] and facilitators of addiction‐related neuroplasticity [19].
Like stressors, cocaine stimulates the HPA axis through a mechanism dependent on the release of the peptide corticotropin releasing hormone (CRH) from the terminals of parvocellular neurons originating in the paraventricular nucleus (PVN) of the hypothalamus [23, 24]. Repeated cocaine administration has been reported to produce long‐term alterations in basal HPA function [18, 26, 30, 32] that can also be observed in human addicts as a dysregulation of circadian HPA activity [7]. Less is known about how stressor‐induced HPA activation is altered as a consequence of prior cocaine use. Although there are reports that restraint‐induced corticosterone (CORT) secretion is unchanged following repeated psychomotor stimulant drug administration [17], others have found that HPA responses to stressors [2] and cocaine [27] are augmented, while still others have reported that individual differences in stressor‐induced CORT are eliminated following chronic cocaine administration, with the CORT response increasing in rats that were initially low CORT responders and decreasing in high responder rats [26].
Clinical studies have found that the HPA response to stressors is augmented in recovering cocaine abusers with a history of high frequency drug use [10] and that stressor‐induced cortisol and ACTH responses predict propensity towards drug relapse [29]. Thus, intensification of stressor‐induced HPA function following chronic cocaine administration could contribute to the addiction process by promoting further drug use.
The goal of this study was to examine the activity of the HPA axis under basal conditions and in response to a stressor, restraint, after 24 hours of withdrawal from 14 days of cocaine administration (30 mg/kg, IP daily). HPA function was assessed through determination of plasma CORT concentrations and CRH mRNA levels in the PVN measured using in situ hybridization. Additionally, since glucocorticoid receptors (GR) are known to play an inhibitor feedback role in HPA function [8], we also examined GR expression in pituitary and a number of brain regions using Western blot analysis.
METHODS
Subjects
Forty‐eight adult male Sprague‐Dawley rats (Harlan Laboratories, St. Louis, MO), approximately 90 days old were housed in pairs in a temperature‐ and humidity‐controlled, AAALAC‐accredited facility under a 12h/12h light/dark cycle (lights on at 7:00 am) and had access to food and water at all times. Experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (Publication No. 80‐23; rev. 1996).
Cocaine/Saline Injection Regimen
After a one‐week habituation period during which they received daily saline injections, rats were assigned to cocaine or saline treatment groups according to the ip injection regimen that they received for the next 14 days. Cocaine rats received daily ip injections of cocaine (30 mg/kg; NIDA Drug Supply Program) while saline rats received injections of 0.9% NaCl solution. Rats received injections at 10:00 AM.
Immobilization Stress and Sacrifice
All rats were sacrificed 24 hours after the final injection (at 10 AM) by decapitation under one of three conditions: 1) immediately after removal from the homecage for baseline CORT and CRH mRNA measurement; 2) immediately following 30 minutes of restraint for measurement of stressor‐induced increases in plasma CORT and regional glucocorticoid receptor (GR) protein expression; and 3) ninety minutes after the termination of restraint for measurement of stressor‐induced CRH mRNA expression and recovery of CORT levels following stress. During the 90‐min period prior to sacrifice, rats in this group were returned to their home cages. Restraint stress consisted of a 30‐min period during which rats were placed into acrylic restraining devices (21.6 cm length × 6.4 cm diameter). Following decapitation, trunk blood was collected for plasma CORT measurement and brain was rapidly excised and frozen in a −30° C isopentane solution. Pituitary gland was also removed and frozen on dry ice. Brain and pituitary were transferred to a −80° C freezer for storage.
Measurement of Plasma CORT
Plasma CORT was measured using a commercial radioimmunoassay kit (MP Biochemicals, Irvine, CA). Blood for CORT determination was collected into tubes containing heparin, stored on ice, and centrifuged to allow separation of plasma, which was frozen at −80° C.
In Situ Hybridization and Image Analysis
Analysis of hybridization signal involved sampling across the rostral‐caudal extent of the medial parvocellular PVN and measurements for individual rats were averaged to obtain one mean value. Data from the rostral‐most level of the PVN was excluded due to an inadequate number of sections. 35S‐labeled anti‐sense probes for CRH were generated from linearized cDNA encoding a 760bp segment spanning from exon 2 to the 3’ untranslated region. The specificity of this probe has been previously characterized [12]. Linearized DNA template was transcribed to anti‐sense riboprobe with T7 polymerase, followed by isolation of probe by high salt/ethanol precipitation. Slides with 16 µm cryosectioned coronal brain slices containing the PVN [21] were subjected to standard pre‐hybridization treatment (post‐fixation in 4% paraformaldehyde; glycine treatment; acetylation; dehydration/delipidationin ethanols/chloroform; air‐drying). Probe was diluted in commercial hybridization buffer (Amresco Solon, OH) with 100 mM DTT. Each slide was hybridized with 50µl of medium containing 106 cpm probe/50µl), coverslipped, and incubated overnight at 55°C in chambers humidified with 50% formamide. Following incubation, slides were post‐treated (rinses in 2X SSC; 200 µg/ml RNAse digestion of unbound probe; SSC rinses; 55°C hot bath in 0.2X SSC; dehydration in ethanols; air drying) and exposed to Kodak BioMAX MR autoradiographic film for 1 week. Densitometric analysis was performed using Scion Image 1.59 software. Data are presented as corrected gray level (gray level of sampled area minus gray level of non‐specifically labeled background region (white matter) in the same section).
PAGE Western Blot Analysis
Frozen brains were thawed on ice to permit dissection of medial prefrontal cortex, dorsomedial hypothalamus (including PVN), amygdala, dorsal hippocampus, and ventral subiculum. Brains were placed into a stainless steel brain block cooled to −20° C and slices (1–2 mm) were cut using a straight edge razor blade. Coronal slices from which tissues were dissected were defined according to coordinates from Paxinos and Watson [21]. Samples were homogenized by sonication on ice in a Tris buffer (50 mM Tris, pH 7.2) containing 10% (wt/vol) sucrose, 6 mM MgCl2 and protease inhibitors (1 mM EDTA, 1 mM PMSF, 3 mM benzamidine, 5 µg/ml leupeptin, 1 µg/ml pepstatin A, 1 µg/ml aprotinin, 5 µg/ml bestatin, 2 µg/ml E64). GR protein was measured in samples of whole cell tissue homogenate using polyacrylamide gel electrophoresis [15] followed by Western blot analysis with the polyclonal antibody, GR M‐20 (Santa Cruz Biotechnology, Santa Cruz, CA). Following protein determination using the bicinchoninic acid assay, samples were diluted in buffer (pH = 6.8; final concentration 0.25 M Tris‐HCl, 4 M urea, 10% glycerol, 1% SDS, 5% beta‐mercaptoethanol) and loaded onto a 7.5% polyacrylamide gel (30 µg protein/lane). Gel electrophoresis was performed using a Bio‐Rad Mini‐protean 3 system at 200 V for 20 min followed by 35‐40 min at 180 V. Samples were transferred onto Bio‐Rad Immun‐Blot™ PVDF membranes at 100 V for 50 min at 4° C using a Bio‐Rad Mini‐Transblot wet transfer system. Following repeated washes and blocking in a TBS solution containing 10% non‐fat dried milk and 0.5 µl/ml Tween‐20 solution for 90 minutes, membranes were incubated with GR M‐20 (1∶500) for 2 hours at room temperature and subsequently with a secondary horseradish peroxidase‐labeled antibody (donkey anti‐rabbit; Amersham Biosciences; 1∶5000) for 90 min at room temperature. An enhanced chemiluminescent detection system (Amersham Biosciences) was used to detect immunoreactive bands. Optical density readings were determined using a Kodak ImageStation 4000MM system. Raw optical density data were converted to percentages of the mean density for SAL samples on the same membrane.
Statistical Analyses
The statistical significance of differences between groups was examined using 2‐way ANOVA followed by post‐hoc analysis using the Fisher’s LSD test. A Bonferroni‐adjusted t‐test following one‐way ANOVA was used for planned comparisons. Statistical analyses were performed using SPSS 14.0 software. Statistical significance was defined as P < 0.05.
RESULTS
Plasma CORT
Figure 1 shows basal and restraint‐induced increases in plasma CORT in saline‐ and cocaine‐treated rats. Two‐way restraint condition x cocaine treatment group ANOVA examining plasma CORT immediately following restraint showed a significant effect of restraint (F1,26=117.52; P<0.0001) but not cocaine (P=0.063) and a significant restraint x cocaine interaction (F1,26=4.59; P<0.05). Restraint significantly increased plasma CORT in both cocaine‐ and saline‐treated rats (P<0.0001). However, plasma CORT following restraint was significantly higher in cocaine‐treated rats compared to saline controls (P<0.05). No differences were observed between groups 90 minutes after restraint.
Figure 1.
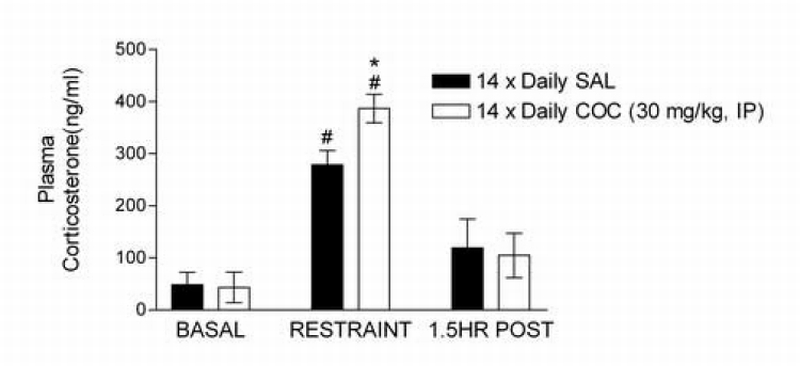
Plasma corticosterone (CORT) response to restraint in saline and cocaine treated rats. Data represent mean plasma CORT levels (ng/ml ± SE) under basal conditions (n=8 per group), immediately following 30 min of immobilization (n=7 per group), and after a 90‐min recovery period following immobilization (n=8 per group), 24 hours into withdrawal from daily cocaine (COC; 14 × 30 mg/kg, ip) or saline (SAL). The plasma CORT response to restraint was augmented in COC rats (#P < 0.0001 vs. basal; * P<0.05 vs SAL).
CRH mRNA in the PVN
CRH mRNA levels under basal conditions and 90 min after restraint in saline‐ and cocaine‐treated rats are shown in Figure 2. Two‐way restraint condition x cocaine treatment ANOVA examining the CRH mRNA in the PVN showed a significant overall effect of restraint (F1,23=5.30; P<0.05), but no significant effect of cocaine and no significant restraint x cocaine interaction despite a statistically non‐significant trend (P=0.08). However, a separate planned comparison of restraint‐induced increases in CRH mRNA using a Bonferroni corrected t‐test showed that restraint significantly increased CRH mRNA in cocaine‐treated rats, but not saline controls (P<0.05).
Figure 2.
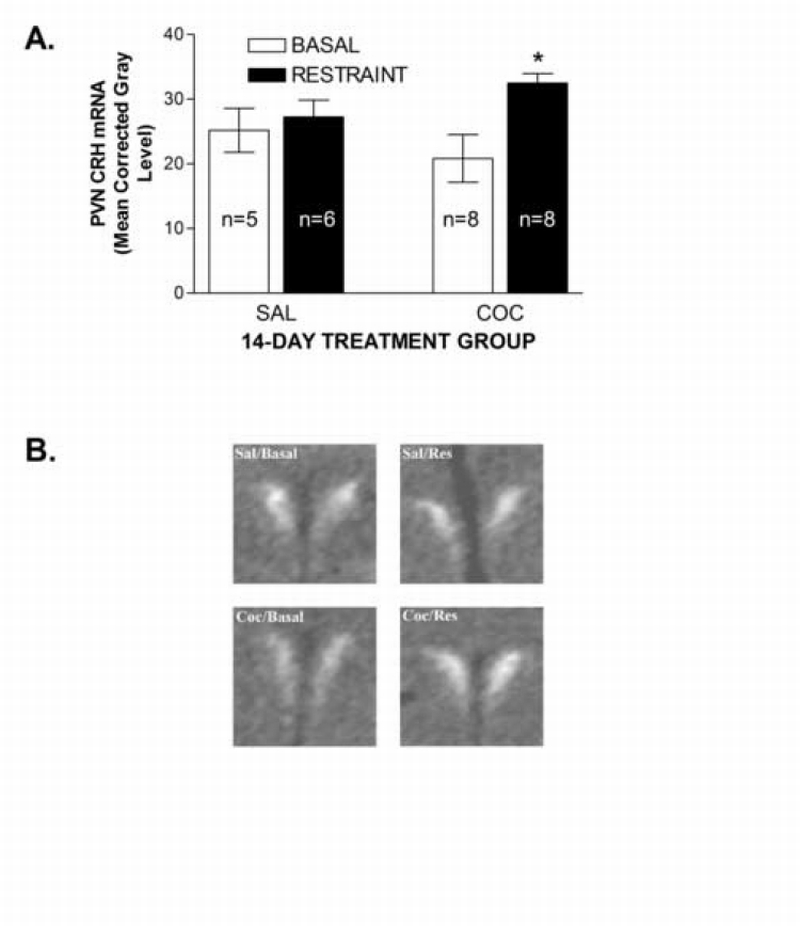
Effects of restraint on PVN CRH mRNA levels in cocaine‐ and saline‐treated rats. Data in Figure 2A represent the density of CRH mRNA in the medial rostral‐caudal level of the PVN in saline‐ (SAL) and cocaine‐ (COC) treated rats determined by in situ hybridization. Density was defined as the corrected gray level (gray level of PVN minus gray level of non‐specifically labeled background region (white matter) in the same section). A significant restraint‐induced increases in CRH mRNA was found in COC, but not SAL rats (*P<0.05 vs. SAL). Images in Figure 2B show representative CRH mRNA expression in the PVN from all 4 groups. Sal/Basal = saline/non‐stressed; Sal/Res = saline /restraint; Coc/Basal = cocaine/non‐stressed; Coc/Res = cocaine/restraint. Each image was digitally captured from films with NIH Image software and imported into Adobe Photoshop for assembly, with identical adjustments applied to all.
GR protein expression
Regional differences in GR protein expression between cocaine‐ and saline‐treated rats were analyzed using two‐tailed Student’s t‐tests and are shown in Table 1. No significant differences were observed in any region examined.
Table 1.
Effects of chronic cocaine administration on glucorticoid receptor (GR) protein expression.
| Region | GR Protein Levels (% Sal Control) |
|
|---|---|---|
| SAL | COC | |
| Amygdala | 100.0 ± 10.0 | 87.4 ± 10.3 |
| Dorsal hippocampus | 100.0 ± 7.1 | 96.0 ± 6.1 |
| Dorsomedial hypothalamus | 100.0 ± 8.5 | 98.7 ± 8.5 |
| Pituitary Gland | 100.0 ± 12.3 | 124.4 ± 5.9 |
| Ventral Subiculum | 100.0 ± 6.0 | 109.4 ± 14.2 |
Data represent the densities of GR immunoreactive bands presented as percentages of SAL controls (±S.E) in dissected brain regions and pituitary determined using Western blot analysis. No significant differences in GR protein levels were found.
DISCUSSION
The primary findings of the present study are that the hypothalamic CRH mRNA and CORT responses to a stressor, 30 min of restraint, are augmented during acute (24‐h) withdrawal from chronic cocaine administration (14 days of 30 mg/kg, ip). The findings differ from earlier reports that 7 days of 15 mg/kg cocaine failed to alter the CORT response to 20 minutes of immobilization 42 hours after the final injection [17] and reports of no changes in the CORT response to 60 minutes of immobilization 21 hours after the final injection of a 3‐ or 6‐week “binge’ cocaine regimen (3 × 15 mg/kg, daily) [26]. In the latter study, individual differences were found with increases in responsiveness in rats that initially displayed a lower CORT response to restraint and reductions in responsiveness in high CORT responders. The reasons for the discrepant findings are unclear, but a number of factors, including differences in duration of restraint and cocaine treatment, cocaine dose, time of day at which CORT was measured, strain of rat, and length of withdrawal, may have contributed. Our findings are consistent with reports that the CORT and ACTH responses to restraint were augmented in rats that received a 2‐week escalating single daily dose regimen of d‐amphetamine when measured 14 days into withdrawal [2] and with clinical reports that the HPA response to stressors is augmented in recovering cocaine abusers with a history of high frequency drug use [10]. Notably, similar discrepancies have been found when examining the effects of repeated drug exposure on the HPA response to cocaine with no changes in [5, 16], augmentation of [27], and attenuation of [30] the response reported, depending on the treatment parameters used.
The present study is, to our knowledge, the first to describe restraint‐induced increases in CRH mRNA during acute withdrawal. It is likely that the elevation of CRH mRNA in the PVN represents a replenishing of parvocellular neurons to compensate for earlier CRH peptide release, further suggesting that the restraint‐induced HPA response was augmented in cocaine‐treated rats. However, although the responses are presumably proportional, the precise relationship between the CRH peptide and mRNA responses is not entirely clear. Considering that restraint‐induced increases in CRH mRNA in the PVN have been previously reported in drug‐naïve rats [14], we were surprised at the lack of effect in our saline controls, especially since we observed a robust restraint‐induced increase in plasma CORT. Notably, very rapid post‐restraint increases in CRH mRNA in the PVN (i.e., less than one hour) have been reported [13]. Thus, we may have missed the peak CRH mRNA response in our saline rats, implying that chronic cocaine may change the duration as well as the magnitude of the CRH mRNA response. Alternatively, the repeated handling and saline injections in the control group may have dampened the HPA response in a manner that was surmountable by prior cocaine exposure.
Although the responses to restraint were augmented in cocaine‐treated rats, no differences in basal CORT and CRH mRNA levels in the PVN were observed. These findings are consistent with reports that hypothalamic CRH mRNA levels measured using a solution hydridization RNase protection assay are unchanged on day one of withdrawal from chronic “binge” pattern cocaine [31] and with reports that basal CORT levels are unaltered [4, 17, 32]. Zorilla et al [32] also reported that hypothalamic CRH‐like immunoreactivity was unaltered 24 hours into withdrawal. However, others have found that plasma CORT and/or ACTH is elevated following repeated cocaine administration when measured 21 hrs into withdrawal from either 3 or 6 weeks of chronic daily “binge‐pattern” (i.e., 3 × 15 mg/kg at 1‐h intervals) [26, 31] or 24 hours into withdrawal from one week of twice daily 10 mg/kg cocaine [1].
We also examined GR expression in the pituitary gland and several brain regions implicated in negative feedback regulation of the HPA axis and found no differences between cocaine‐ and saline‐withdrawn rats. Although these findings suggest that the augmented HPA response was independent of impaired GR‐mediated feedback inhibition, functional measures of feedback (e.g., sensitivity to dexamethasone suppression and closer scrutiny of the time‐course of the decline in plasma CORT following restraint) are necessary to clarify the contribution of altered negative feedback. Further investigation is required to determine the role of adaptations within the HPA axis that are independent of GR‐mediated feedback in the intensified stress response.
Importantly, this study examined the effects of investigator‐administered cocaine on the HPA axis. It has been demonstrated that the effects of cocaine on the HPA axis differ depending on if the drug is self‐administered or delivered in a response non‐contingent manner [6]. Determination of alterations in stressor‐responsiveness that are relevant to addiction will likely require the use of self‐administration protocols that simulate use patterns in human addicts.
To summarize, we demonstrate that stressor‐induced activation of the HPA axis is augmented during acute cocaine withdrawal. Along with previous reports that behavioral measures of anxiety [3, 25] and CRH activity in the amygdala [22, 32] are also augmented during acute withdrawal, our findings suggest that stressor responsiveness is intensified as a result of prior cocaine exposure. Considering the role of stress as a determinant of cocaine‐seeking behavior, one consequence of heightened stressor responsiveness could be a greater propensity to engage in further cocaine use. This assertion is supported the findings of Sinha et al [29] that stressor‐induced HPA responses predict the likelihood of drug relapse in recovering cocaine‐dependent subjects.
ACKNOWLEDGEMENTS
Supported by NIDA grant number DA15758 to JRM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Avila AH, Morgan CA, Bayer BM. Stress‐induced suppression of the immune system after withdrawal from chronic cocaine. J. Pharmacol. Exp. Ther. 2003;305:290–297. doi: 10.1124/jpet.102.045989. [DOI] [PubMed] [Google Scholar]
- [2].Barr AM, Hofmann CE, Weinberg J, Phillips AG. Exposure to repeated, intermittent damphetamine induces sensitization of HPA response to a subsequent stressor. Neuropsychopharmacol. 2002;26:286–294. doi: 10.1016/S0893-133X(01)00308-6. [DOI] [PubMed] [Google Scholar]
- [3].Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin‐releasing factor antagonist attenuates the “anxiogenic‐like” effect in the defensive burying paradigm but not in the elevated plus maze following chronic cocaine in rats. Psychopharmacol. 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- [4].Baumann MH, Milchanowski AB, Rothman RB. Evidence for alteration in alpha‐2 adrenergic receptor sensitivity in rats exposed to repeated cocaine administration. Neuroscience. 2004;125:683–690. doi: 10.1016/j.neuroscience.2004.02.013. [DOI] [PubMed] [Google Scholar]
- [5].Borowsky B, Kuhn CM. Chronic cocaine sensitizes behavioral but not neuroendocrine responses. Brain Res. 1991;543:301–306. doi: 10.1016/0006-8993(91)90041-s. [DOI] [PubMed] [Google Scholar]
- [6].Broadbear JH, Winger G, Cicero TJ, Woods JH. Effects of response contingent and noncontingent cocaineinjection on hypothalamic‐pituitary‐adrenal activity in rhesus monkeys. J. Pharmacol. Exp. Ther. 1999;290:393–402. [PubMed] [Google Scholar]
- [7].Contoreggi C, Herning RI, Koeppl B, Simpson PM, Negro PJ, Jr, Fortner‐Burton C, Hess J. Treatment‐seeking inpatient cocaine abusers show hypothalamic dysregulation of both basal prolactin and cortisol secretion. Neuroendocrinol. 2003;78:154–162. doi: 10.1159/000072797. [DOI] [PubMed] [Google Scholar]
- [8].de Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- [9].Erb S, Shaham Y, Stewart J. The role of corticotropin‐releasing factor and corticosterone in stress‐ and cocaine‐induced relapse to cocaine seeking in rats. J. Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fox HC, Talih R, Malison R, Anderson GM, Kreek MJ, Sinha R. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug‐related cues. Psychoneuroendocrinol. 2005;30:880–891. doi: 10.1016/j.psyneuen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- [11].Goeders NE. Stress and cocaine addiction. J. Pharmacol. Exp. Ther. 2002;301:785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- [12].Herman JP, Schafer MK, Thompson RC, Watson SJ. Rapid regulation of corticotropin‐releasing hormone gene transcription in vivo. Mol. Endocrinol. 1992;6:1061–1069. doi: 10.1210/mend.6.7.1324419. [DOI] [PubMed] [Google Scholar]
- [13].Hsu DT, Chen FL, Takahashi LK, Kalin NH. Rapid stress‐induced elevations in corticotropin‐releasing hormone mRNA in rat central nucleus and hypothalamic paraventricular nucleus: an in situ hybridization analysis. Brain Res. 1998;788:305–310. doi: 10.1016/s0006-8993(98)00032-8. [DOI] [PubMed] [Google Scholar]
- [14].Kalin NH, Takahashi LK, Chen FL. Restraint stress increases corticotropin‐releasing hormone mRNA content in the amygdale and paraventricular nucleus. Brain Res. 1994;656:182–186. doi: 10.1016/0006-8993(94)91382-x. [DOI] [PubMed] [Google Scholar]
- [15].Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- [16].Levy AD, Li Q, Alvarez Sanz MC, Rittenhouse PA, Kerr JE, Van de Kar LD. Neuroendocrine responses to cocaine do not exhibit sensitization following repeated cocaine exposure. Life Sci. 1992;51:887–897. doi: 10.1016/0024-3205(92)90396-7. [DOI] [PubMed] [Google Scholar]
- [17].Levy AD, Rittenhouse PA, Li Q, Yracheta J, Kunimoto K, Van de Kar LD. Influence of repeated cocaine exposure on the endocrine and behavioral responses to stress in rats. Psychopharmacol. 1994;113:547–554. doi: 10.1007/BF02245238. [DOI] [PubMed] [Google Scholar]
- [18].Mantsch JR, Yuferov V, Mathieu‐Kia AM, Ho A, Kreek MJ. Neuroendocrine alterations in a high‐dose, extended‐access rat self‐administration model of escalating cocaine use. Psychoneuroendocrinol. 2003;28:836–862. doi: 10.1016/s0306-4530(02)00088-4. [DOI] [PubMed] [Google Scholar]
- [19].Mantsch JR, Katz ES ES. Elevation of glucocorticoids is necessary but not sufficient for the escalation of cocaine self‐administration by chronic electric footshock stress in rats. Neuropsychopharmacol. 2006 Apr 12; doi: 10.1038/sj.npp.1301077. Advance online pub., doi: 10.1038/sj.npp.1301077. [DOI] [PubMed] [Google Scholar]
- [20].Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress, and psychostimulant drugs. Eur. J. Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- 21.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th Edition. San Diego: Academic Press; 1998. [Google Scholar]
- [22].Richter RM, Weiss F. In vivo CRF release in the rat amygdala is increased during cocaine withdrawal in self‐administering rats. Synapse. 1999;32:254–261. doi: 10.1002/(SICI)1098-2396(19990615)32:4<254::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- [23].Rivier C, Vale W. Cocaine stimulates adrenocorticotropin (ACTH) secretion through a corticotropin‐releasing factor (CRF)‐mediated mechanism. Brain Res. 1987;422:403–406. doi: 10.1016/0006-8993(87)90953-x. [DOI] [PubMed] [Google Scholar]
- [24].Sarnyai Z, Biro E, Penke B, Telegdy G. The cocaine‐induced elevation of plasma corticosterone is mediated by endogenous corticotropin‐releasing factor (CRF) in rats. Brain Res. 1993;589:154–156. doi: 10.1016/0006-8993(92)91176-f. [DOI] [PubMed] [Google Scholar]
- [25].Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G. Brain corticotrophin‐releasing factor mediates ‘anxiety‐like’ behavior induced by cocaine withdrawal in rats. Brain Res. 1995;675:89–97. doi: 10.1016/0006-8993(95)00043-p. [DOI] [PubMed] [Google Scholar]
- [26].Sarnyai Z, Dhabhar FS, McEwen BS, Kreek MJ. Neuroendocrine‐related effects of long‐term ‘binge’ cocaine administration: diminished individual differences in stress‐induced corticosterone secretion. Neuroendocrinol. 1998;68:334–344. doi: 10.1159/000054382. [DOI] [PubMed] [Google Scholar]
- [27].Schmidt ED, Tilders FJ, Janszen AW, Binnekade R, De Vries TJ, Scholffelmeer AN. Intermittent cocaine exposure causes delayed and long‐lasting sensitization of cocaine‐induced ACTH secretion in rats. Eur. J. Pharmacol. 1995;285:317–321. doi: 10.1016/0014-2999(95)00540-2. [DOI] [PubMed] [Google Scholar]
- [28].Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacol. 1999;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- [29].Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ BJ. Stress‐induced cocaine craving and hypothalamic‐pituitary‐adrenal responses are predictive of cocaine relapse outcomes. Arch. Gen. Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- [30].Zhou Y, Spangler R, LaForge KS, Maggos CE, Ho A, Kreek MJ. Corticotropinreleasing factor and type 1 corticotropin‐releasing factor receptor messenger RNAs in rat brain and pituitary during “binge”‐pattern cocaine administration and chronic withdrawal. J. Pharmacol. Exp. Ther. 1996;279:351–358. [PubMed] [Google Scholar]
- [31].Zhou Y, Spangler R, Schlussman SD, Ho A, Kreek MJ. Alterations in hypothalamicpituitary‐adrenal axis activity and in levels of proopiomelanocortin and corticotropin‐releasing hormone‐receptor 1 mRNAs in the pituitary and hypothalamus of the rat during chronic ‘binge’ cocaine and withdrawal. Brain Res. 964. 2003;964:187–199. doi: 10.1016/s0006-8993(02)03929-x. [DOI] [PubMed] [Google Scholar]
- [32].Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF‐like immunoreactivity and plasma corticosterone during protracted withdrawal in dependent rats. Psychopharmacol. 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]


