Abstract
Despite efforts spanning four decades, the therapeutic potential of thyroid hormone receptor (TR) agonists as lipid-lowering and anti-obesity agents remains largely unexplored in humans because of dose-limiting cardiac effects and effects on the thyroid hormone axis (THA), muscle metabolism, and bone turnover. TR agonists selective for the TRβ isoform exhibit modest cardiac sparing in rodents and primates but are unable to lower lipids without inducing TRβ-mediated suppression of the THA. Herein, we describe a cytochrome P450-activated prodrug of a phosphonate-containing TR agonist that exhibits increased TR activation in the liver relative to extrahepatic tissues and an improved therapeutic index. Pharmacokinetic studies in rats demonstrated that the prodrug (2R,4S)-4-(3-chlorophenyl)-2-[(3,5-dimethyl-4-(4′-hydroxy-3′-isopropylbenzyl)phenoxy)methyl]-2-oxido-[1,3,2]-dioxaphosphonane (MB07811) undergoes first-pass hepatic extraction and that cleavage of the prodrug generates the negatively charged TR agonist (3,5-dimethyl-4-(4′-hydroxy-3′-isopropylbenzyl)phenoxy)methylphosphonic acid (MB07344), which distributes poorly into most tissues and is rapidly eliminated in the bile. Enhanced liver targeting was further demonstrated by comparing the effects of MB07811 with 3,5,3′-triiodo-l-thyronine (T3) and a non-liver-targeted TR agonist, 3,5-dichloro-4-(4-hydroxy-3-isopropylphenoxy)phenylacetic acid (KB-141) on the expression of TR agonist-responsive genes in the liver and six extrahepatic tissues. The pharmacologic effects of liver targeting were evident in the normal rat, where MB07811 exhibited increased cardiac sparing, and in the diet-induced obese mouse, where, unlike KB-141, MB07811 reduced cholesterol and both serum and hepatic triglycerides at doses devoid of effects on body weight, glycemia, and the THA. These results indicate that targeting TR agonists to the liver has the potential to lower both cholesterol and triglyceride levels with an acceptable safety profile.
Keywords: cardiac sparing; hepatic steatosis; hyperlipidemia; (2R,4S)-4-(3-chlorophenyl)-2-[(3,5-dimethyl-4-(4′-hydroxy-3′-isopropylbenzyl)-phenoxy)methyl]-2-oxido-[1,3,2]-dioxaphosphonane (MB07811); thyroid hormone axis
Thyroid hormones (THs) affect growth, metabolism, and the physiological function of nearly all organs (1, 2). Biological activity arises from activation of nuclear hormone receptors [TH receptors (TRs)], which in turn modulate the expression of numerous target genes (3, 4). Two activities associated with TH action that appeal to the pharmaceutical industry are related to the ability of TH to markedly lower lipids and atherogenic lipoproteins associated with cardiovascular disease (5) and to induce weight loss through increased energy expenditure (6). Both activities are evident from the clinical profiles of patients with hyper- and hypothyroidism (7–9) and from studies in TR knockout mice (10). Unfortunately, despite efforts spanning nearly 40 years, neither activity could be achieved by using THs or related analogs without eliciting significant adverse effects on cardiac function and the thyroid hormone axis (THA) (11, 12).
One strategy with the potential to increase the therapeutic index (TI) of TR agonists that has received considerable attention over the past decade stems from studies demonstrating the existence of four different TR isoforms (TRα1, TRα2, TRβ1, TRβ2) and their differential expression across tissues (13). Studies in knockout mice suggest that TRβ, which is the predominant TR in the liver, is responsible for the cholesterol lowering (14), whereas TRα, which is the predominant TR in the heart, is responsible for most of the cardiovascular effects (15). Efforts to identify TRβ-specific agonists led to compounds (16, 17) with only modest TRβ-specificities (<20-fold) because of the high structural similarity between the ligand-binding domains for TRβ and TRα (18). Nevertheless, the TRβ selectivity achieved by these compounds resulted in an improved TI for lipid lowering relative to cardiac effects such as heart rate, cardiac hypertrophy, and contractility (19–21). However, no improvement was observed in the TI relative to THA effects, which are largely mediated by TRβ in the pituitary and result in thyroid-stimulating hormone (TSH) suppression.
Skeptical that compounds with a sufficient TI could be identified by using a TR isoform-selective approach, we focused on strategies capable of enhancing the activation of TRs in the liver relative to TRs in extrahepatic tissues. TR activation in the liver favorably affects plasma cholesterol and lipoprotein levels by multiple mechanisms, which may include increasing low-density lipoprotein clearance through increased expression of low-density lipoprotein receptors (LDLR) (22), increasing high-density lipoprotein uptake through SR-B1 (23), and increasing bile acid synthesis via cholesterol 7α-hydroxylase (CYP7A) (22). TR activation in extrahepatic tissues leads to altered cardiovascular function, THA suppression, muscle wasting, and bone loss. Theoretically, all of these detrimental effects could be avoided by reducing circulating levels of the TR agonist and/or its uptake by extrahepatic tissues. Herein, we report our discovery of (2R,4S)-4-(3-chlorophenyl)-2-[(3,5-dimethyl-4-(4′-hydroxy-3′-isopropylbenzyl)phenoxy)methyl]-2-oxido-[1,3,2]-dioxaphosphonane (MB07811) and studies in rodents demonstrating that MB07811 results in selective activation of liver TRs and an improved TI.
Results
Drug Design.
Phosphonic acids are negatively charged compounds at physiological pH that often differ with the corresponding carboxylic acid analogs in their interactions with proteins and their tissue distribution properties. Differences in binding affinity (24) and enzyme kinetics (25) likely reflect geometrical differences in structure and the inability of many binding sites to accommodate both a planar mononegatively charged carboxylate and a tetrahedral dinegatively charged phosphonate. In vivo, phosphonates exhibit a lower volume of distribution (26), presumably because of decreased cellular penetration arising from their greater negative charge or reduced recognition by the cellular transporters used to transport carboxylates.
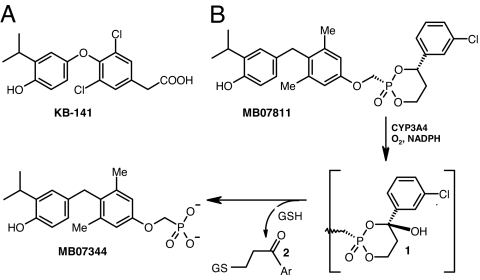
To test whether phosphonates bind to TRs, a variety of structurally diverse phosphonate-containing analogs were prepared, and the lead compound, (3,5-dimethyl-4-(4′-hydroxy-3′-isopropylbenzyl)phenoxy)methylphosphonic acid (MB07344), was compared with both 3,5,3′-triiodo-l-thyronine (T3) and the TRβ-selective agonist, 3,5-dichloro-4-(4-hydroxy-3-isopropylphenoxy)phenylacetic acid (KB-141) (16) (Fig. 1A). MB07344 exhibited reduced TR affinity and modest TRβ specificity based on its TRα Ki, TRβ Ki, and the TRα Ki/TRβ Ki ratio (35.2 ± 1.05 nM, 2.17 ± 0.41 nM, and 15.8, respectively) relative to T3 (0.22 ± 0.03 nM, 0.55 ± 0.05 nM, and 0.4) and KB-141 (7.18 ± 0.48 nM, 0.37 ± 0.03 nM, and 19.4).
Fig. 1.
Chemical structures and mechanism of MB07811 conversion to MB07344. (A) Chemical structure of KB-141. (B) Chemical structures of MB07811 and the products generated from the CYP3A-catalyzed cleavage reaction, namely MB07344 and the glutathione conjugate (2) produced from the addition of glutathione (GSH) to the aryl vinyl ketone byproduct. Ar, 3-chlorophenyl.
Prodrugs of MB07344 were prepared to enhance oral bioavailability, and the HepDirect prodrug MB07811 was selected based on its low affinity for TRs (TRβ Ki = 14.6 ± 0.5 μM; TRα Ki = 12.5 ± 0.6 μM) and its potential for liver targeting (27, 28). HepDirect prodrugs are aryl-substituted cyclic prodrugs that cleave to the active drug after an oxidation of the benzylic methine proton catalyzed by the cytochrome P450 (CYP) isoenzyme CYP3A (Fig. 1B). Oxidation results in rapid irreversible ring opening, leading to a negatively charged intermediate that undergoes a β-elimination reaction to produce the phosphonate and the glutathione conjugate (2) of the corresponding aryl vinyl ketone.
In Vitro Metabolism.
MB07811 was efficiently converted to MB07344 and the glutathione conjugate 2 by liver microsomes prepared from male Sprague–Dawley (SD) rats. The Vmax, Km, and CLint (intrinsic clearance = Vmax/Km) values were 2.74 ± 0.12 nmol·min−1·mg−1, 18.8 ± 3.06 μM, and 145 ± 24.5 μl·min−1·mg−1, respectively. Conversion was inhibited by clotrimazole (100% at 1 μM, Ki = 24 nM), suggesting that CYP3A is the predominant CYP responsible for prodrug conversion. Neither MB07811 nor MB07344 inhibited CYP3A at 10 μM.
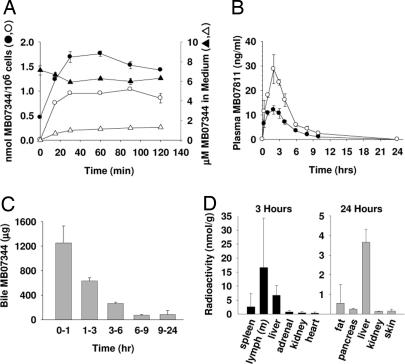
High intracellular levels of MB07344 were detected in freshly isolated rat hepatocytes incubated with MB07811 (Fig. 2A), indicating that MB07811 distributes readily into hepatocytes and is converted to MB07344 (Cmax = 1.03 ± 0.00 nmol per 106 cells; tmax = 1.5 h). Hepatocytes incubated with MB07344 also exhibited high intracellular MB07344 levels (1.77 ± 0.05 nmol per 106 cells; tmax = 1 h) (Fig. 2A), suggesting that MB07344, like other negatively charged phosphonates, may enter hepatocytes via organic anion transporters (29).
Fig. 2.
MB07811 metabolism, excretion, and tissue distribution. (A) Mean (±½ range) intracellular levels (circles) and levels in culture medium (triangles) of MB07344 from isolated, cultured rat hepatocytes incubated with 10 μM MB07811 (open symbols, n = 2) or 10 μM MB07344 (filled symbols, n = 2). (B) Mean (± SEM) systemic (filled circles, n = 5) and portal vein (open circles, n = 4) plasma concentrations of MB07811 after an oral dose of MB07811 (3 mg/kg) to male SD rats; (C) Mean (± SEM) MB07344 levels in bile samples collected over the indicated periods from bile duct-cannulated male SD rats (n = 3) treated with MB07344 (10 mg/kg, i.v.). (D) Approximate mean tissue concentration (total radioactivity) for the tissues with the highest concentration 3 and 24 h after an oral dose of [14C]-MB07811 (5 mg/kg) to male SD rats (n = 4). Tissues evaluated (liver, spleen, lymph, thyroid, testes, fat, bladder, prostate, pancreas, stomach, small and large intestine, adrenals, kidneys, thymus, heart, bone marrow, muscle, eye, brain, pituitary, skin, lung, and bone) but not listed in the figure (other than stomach and intestine) have <5% of the liver concentration at 3 h and <3% at 24 h.
Pharmacokinetic Studies.
Upon i.v. administration, MB07344 exhibited moderate clearance in the male SD rat (0.28 ± 0.00 liter·kg−1·h−1 with a distribution volume of 0.39 ± 0.05 liter/kg and a plasma half-life of 1.27 ± 0.26 h (Table 1). In contrast, MB07811 (SD rat, i.v.) was cleared rapidly (11.6 ± 1.9 liters·kg−1·h−1) with a distribution volume of 14.8 ± 4.0 liters/kg and a half-life of 1.23 ± 0.15 h. Higher relative oral bioavailability was demonstrated for MB07811 (39%) compared with MB07344 (<1%, data not shown). Oral administration of MB07811 resulted in significant first-pass hepatic extraction [≈55%; Fig. 2B and supporting information (SI) Appendix, Fig. 6].
Table 1.
Pharmacokinetic parameters for MB07344 and MB07811 in SD rats
| PK parameter | MB07344, 5 mg/kg, i.v. | MB07811, 3 mg/kg, i.v. |
MB07811, 3 mg/kg, per os |
||
|---|---|---|---|---|---|
| MB07811 | MB07344 | MB07811 | MB07344 | ||
| CL, liters·h−1·kg−1 | 0.28 | 11.6 | — | — | — |
| Vd, liters/kg | 0.39 | 14.8 | — | — | — |
| t1/2, h | 1.27 | 1.23 | 8.82 | 0.95 | 9.25 |
| tmax, h | — | — | 0.6 | 2.1 | 3.6 |
| Cmax, μg/ml | 40.4 | 0.60 | 0.09 | 0.012 | 0.018 |
| AUC0–24 h, mg·h/liter | 16.6 | 0.26 | 0.34 | 0.026 | 0.135 |
PK, pharmacokinetic; CL, clearance; Vd, volume of distribution; AUC, area under the curve.
SD rats administered a dose of 5 mg/kg [14C]-MB07811 per os excreted 28% and 93% of radioactivity after 3 and 24 h, respectively. Mass balance studies showed that MB07344 (2 mg/kg, i.v. bolus) was eliminated by the biliary system with only 2% of the total radioactivity recovered after 96 h in the urine compared with 98% in the feces. Bile samples collected from bile duct-cannulated rats administered MB07344 i.v. showed that MB07344 was excreted in the bile and that 50% of the dose was excreted within 1 h (Fig. 2C). Last, bile-diverted rats exhibited plasma MB07344 levels (area under the curve (AUC0–24 h) = 48.1 ± 13.7 mg·h/liter) similar to those seen in normal rats (AUC0–24h = 35.1 ± 8.5 mg·h/liter), suggesting that MB07344 is not subject to enterohepatic recirculation (SI Appendix, Fig. 7).
HPLC separation of the extractable radioactive metabolites indicated that 3 h after oral dosing with [14C]-MB07811, 63 ± 7% and 54 ± 5% of the radioactivity in the plasma and liver, respectively, coeluted with MB07344. Levels of MB07811 were below the limit of quantitation in both tissues. The highest tissue concentrations of radioactivity were associated with the stomach, small intestine, large intestine, mesenteric lymph nodes, and the liver, with lower concentrations found in spleen, adrenal, kidney, and heart tissues (Fig. 2D) and very low levels (<5% of the liver concentration) measured in bone† and 16 other tissues examined (SI Appendix, Table 3). After 24 h, the liver contained the highest concentrations of radioactivity followed by fat, pancreas, skin, and kidney, with all other nongastrointestinal tissues containing <3% of the liver concentration.
Effects on Gene Expression.
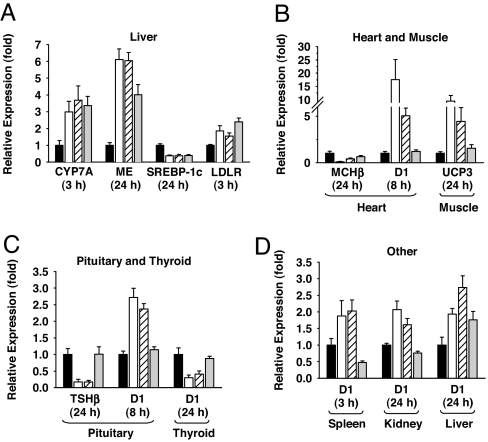
In cholesterol-fed (CF) SD rats, T3, KB-141, and MB07811 reduced total plasma cholesterol with ED50 values of 0.012 mg/kg, 0.05 mg/kg, and 0.40 mg/kg, respectively (see SI Appendix). To assess differences in tissue distribution, T3, KB-141, and MB07811 were administered to SD rats at 1-, 3-, and 10-fold their ED50 values, and mRNA levels for select T3-responsive genes (2) in liver and selected extrahepatic tissues were measured at 3, 8, and 24 h. As expected, T3, KB-141, and MB07811 exhibited similar time-dependent changes in liver mRNA levels of CYP7A (22), malic enzyme (30), sterol regulatory element-binding protein (SREBP)-1c (31), LDLR (22) (Fig. 3A), and type 1 iodothyronine deiodinase (D1) (32) (Fig. 3D). No significant changes were observed in the liver mRNA levels for SREBP-2, HMG-CoA reductase, or phosphoenolpyruvate carboxykinase at any time point (SI Appendix, Figs. 20–22). Changes in mRNA levels in heart (MHCβ and D1), muscle (uncoupling protein 3) (33), pituitary (TSHβ, D1), thyroid (D1), spleen (D1), and kidney (D1) were evident with T3 and to a lesser extent with KB-141, whereas MB07811 showed minimal to no change in mRNA levels even at the highest dose tested (Fig. 3 B–D).
Fig. 3.
Relative expression of mRNA in tissues of male SD rats (n = 6) treated with vehicle (black bar), T3 (white bar), KB-141 (white hatched bar), or MB07811 (gray bar) at 10× the CF rat ED50 (0.12, 0.5, and 4.0 mg/kg, respectively). Bar graphs are grouped by mRNA and represent the fold-change relative to vehicle at the time point (3, 8, or 24 h) associated with the largest fold-change for T3. Data from the other two time points and doses (1× and 3× ED50) are in SI Appendix, Figs. 8–19. (A) Liver mRNA: CYP7A, malic enzyme (ME), SREBP-1c, and LDLR. (B) Heart and skeletal muscle mRNA: MHCβ, deiodinase 1 (D1), and uncoupling protein 3 (UCP3). (C) Pituitary and thyroid mRNA: TSHβ and D1. (D) Other tissue mRNAs: D1 in spleen, kidney, and liver.
Cardiac Effects.
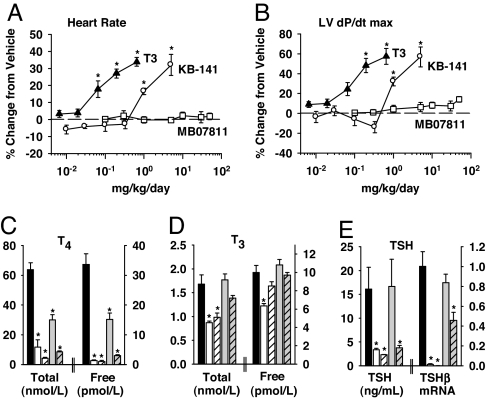
Oral administration of T3 (≥0.065 mg · kg−1 · day−1) or KB-141 (≥1 mg·kg−1·day−1) for 7 days to SD rats resulted in marked and statistically significant increases in heart rate (Fig. 4A), the first derivative of left ventricular pressure (LV dP/dt) (Fig. 4B), and systolic aortic pressure (data not shown). Rats treated with KB-141 at 1 mg·kg−1·day−1 (20× CF rat ED50) showed increased heart rate, LV dP/dt, and heart weight (13.2%), and significantly reduced body weight (BW) gain (61%) relative to vehicle-treated rats. In contrast, rats treated with MB07811 at 50 mg·kg−1·day−1 (125× CF rat ED50) exhibited no significant changes in heart rate, LV dP/dt, systolic and diastolic aortic pressures, heart weight, or BW gain.
Fig. 4.
Effects on SD rat cardiac function and THA. (A and B) Dose–response of T3 (▴, 6.5–650 μg·kg−1·day−1), KB-141 (○, 0.01–5 mg·kg−1·day−1), and MB07811 (□, 0.1–50 mg·kg−1·day−1) on heart rate (A) and LV dP/dt (B) in SD rats (n = 3–6 per dose) treated for 7 days. (C–E) Effects of KB-141 and MB07811 on total and free T4 (C), total and free T3 (D), and plasma TSH and pituitary TSHβ mRNA (E) (relative units) in SD rats (n = 5–6 per group) treated once daily for 28 days with vehicle (black bars), KB-141 at 0.1 mg/kg (white bars) and 1 mg/kg (white hatched bars), and MB07811 at 3 mg/kg (gray bars) and 30 mg/kg (gray hatched bars). The low and high doses for KB-141 are equivalent to 2× and 20×, respectively, the CF rat ED50. The doses for MB07811 are equivalent to 7.5× and 75× the CF rat ED50. *, P < 0.05.
THA Effects.
TR agonists suppress the THA by affecting production and metabolism of THs through modulation of pituitary TSH and pituitary/thyroid D1 expression (2, 32). KB-141 administered for up to 7 weeks to SD rats at 2× and 20× the CF rat ED50 (0.1 and 1 mg/kg) resulted in significant decreases in total 3,5,3′, 5′-tetraiodo-l-thyronine (T4) and free T4 (Fig. 4C), total T3 and free T3 (Fig. 4D), as well as serum TSH and pituitary TSHβ mRNA (Fig. 4E). Similar results were found for T3 (0.25−75× ED50) (SI Appendix, Table 5). In contrast, rats treated with MB07811 at 7.5× the CF rat ED50 (3 mg/kg) showed no significant changes in total T3, free T3, TSH, or TSHβ mRNA. At the higher dose of MB07811 (75× the ED50, 30 mg/kg), levels of TSH and TSHβ mRNA were reduced but remained above those observed for KB-141 dosed at 2× ED50, suggesting an enhancement in the TI relative to KB-141 of at least 38-fold. Total and free T4 levels were decreased by day 7 with both doses of MB07811 and remained constant over the subsequent 6 weeks of treatment (data not shown).
Glycemia.
SD rats treated with T3 (0.65 mg·kg−1·day−1, s.c., 54× the CF rat ED50) for 5 days showed increased fasting blood glucose (up to 25 mg/dl with no change in plasma insulin) and plasma fatty acid levels (by 540 μmol/liter) along with reduced BW (13%). In contrast, no changes in BW, fasting blood glucose, plasma insulin, or plasma free fatty acid levels were observed with MB07811 (50 mg·kg−1·day−1, 125× the CF rat ED50) (SI Appendix, Table 6).
Diet-Induced Obese (DIO) Mouse Study.
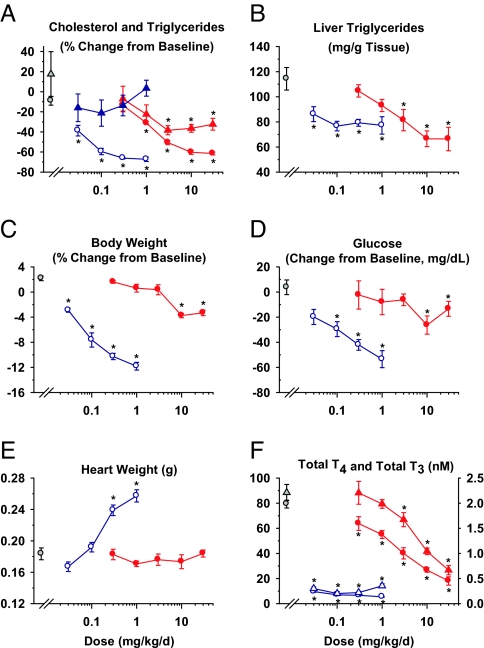
KB-141 or MB07811 reduced total plasma cholesterol in DIO mice after 2 weeks of treatment (Fig. 5A). Maximal cholesterol reductions achieved by KB-141 and MB07811 were not significantly different (67% and 61%, respectively). Similar to results reported for SD rats, hepatic LDLR mRNA levels were unchanged 24 h after MB07811 administration (SI Appendix, Fig. 23); however, in a separate study, they were increased at earlier time points after a single dose of either T3 (0.15 mg/kg, 2.04-fold, 3 h) or MB07811 (10 mg/kg, 2.01-fold, 8 h) (SI Appendix, Table 7). At the highest doses tested, MB07811 treatment reduced plasma TGs (40%) (Fig. 5A), liver TGs (42%) (Fig. 5B), and liver weight (42%) (data not shown), whereas KB-141 decreased liver TGs (33%) and liver weight (33%) but not plasma TGs. Significant differences were observed in several other indices. KB-141 reduced BW (maximum 12%) even at the lowest dose tested (0.03 mg/kg, 3% reduction), whereas MB07811 reduced BW only 3–4% at the highest dose tested (30 mg·kg−1·day−1) and had no effect on weight at a dose (3 mg·kg−1·day−1) that markedly reduced cholesterol (−51%; 84% of maximum), plasma TGs (−38%; 100% of maximum), and hepatic TGs (−29%; 69% of maximum) (Fig. 5C). Both KB-141 and MB07811 reduced blood glucose levels at doses associated with significant weight reduction (Fig. 5D). KB-141 increased heart weight, whereas MB07811 did not even at the highest dose (Fig. 5E). Finally, total T4 and total T3 were severely reduced by KB-141 at all doses, whereas MB07811 reduced each dose-dependently with the levels associated with the highest dose (30 mg·kg−1·day−1) still greater than those associated with the lowest dose of KB-141 (0.03 mg·kg−1·day−1) (Fig. 5F).
Fig. 5.
Dose–response curves in DIO mice (n = 8 per group) treated for 14 days with KB-141 (open symbols, blue) and MB07811 (filled symbols, red). Vehicle is denoted by gray-filled symbols ± SEM. (A, C, and D) Percent change from baseline for total plasma cholesterol (circles) and TGs (triangles) (A), BW (C), and blood glucose (D). (B, E, and F) Effects of KB-141 and MB07811 on liver TGs (B), heart weight (E), and serum total T4 (circles, left axis) and total T3 (triangles, right axis) (F). *, P < 0.05.
Discussion
Development of TR agonists for the treatment of hyperlipidemia has been impeded by various safety concerns stemming from activation of TRs in extrahepatic tissues such as heart, pituitary, muscle, and bone. Our results suggest that the TI is substantially improved by targeting (34, 35) TRβ-selective agonists to the liver, which is achieved by using a HepDirect prodrug of a phosphonate-containing TR agonist. Tissue distribution and pharmacokinetic studies provided the initial evidence for liver targeting. The results suggest that the HepDirect prodrug both enhances the low oral bioavailability of MB07344 (<1%) and limits its production to tissues that express CYP3A, i.e., liver and, to a lesser extent, the small intestine. Preferential conversion of MB07811 to MB07344 in the liver increases MB07344 exposure to the liver relative to extrahepatic tissues. Extrahepatic tissue exposure is also limited by the low circulating levels of MB07344 generated after oral administration of MB07811. The low plasma levels reflect excretion of a significant proportion of the MB07344 produced inside hepatocytes into the bile and the inability of biliary-excreted MB07344 to undergo enterohepatic recirculation. Levels in extrahepatic tissues may also be low because the amino acid transporter responsible for cellular uptake of T3 [i.e., monocarboxylate transporter-8 (36)] is unable to recognize and efficiently transport the phosphonate-containing TR agonist, MB07344.
Liver targeting was also demonstrated by monitoring the expression of TR agonist-responsive genes in liver and extrahepatic tissues. As expected, MB07811, T3, and KB-141 administered to rats at equivalent cholesterol-lowering doses resulted in qualitatively similar changes in liver gene expression. In contrast, gene expression changes in muscle, heart, pituitary, kidney, spleen, and thyroid were significantly greater in rats treated with either T3 or KB-141 relative to those treated with MB07811.
The pharmacological benefits of liver targeting are evident from the dose–response relationships generated in studies comparing MB07811, KB-141, and T3 in SD rats and DIO mice. No effects on heart rate, LV dP/dt, or heart weight were observed with MB07811 even at 125× the CF rat ED50, whereas the TIs for T3 and KB-141 were <6.7- and <20-fold, respectively. No TI was apparent for either T3 or KB-141 on the THA, whereas the TI for MB07811 was increased at least 38-fold based on 24 h pituitary TSHβ mRNA and circulating TSH levels. Both compounds decreased total T4, presumably by increasing hepatic D1 expression and correspondingly the metabolism of T4 to T3 and 3,3′,5′-triiodo-l-thyronine (reverse T3) (32, 37). Greater reductions were observed with KB-141, possibly because of its greater effects on TSH production in the pituitary. Accordingly, liver targeting appears to lessen the effects on the THA, which could be of long-term importance given the association of subclinical hyperthyroidism with increased risk of adverse events (38).
Liver targeting also appears to alter the pharmacological profile of TRβ agonists based on differences in BW and glycemia in MB07811- and KB-141-treated DIO mice. Unlike KB-141, MB07811 had no effect on BW at cholesterol-lowering doses (3 mg/kg) and only a modest effect at 10- and 30-fold higher doses. Consistent with these findings, BW was not reduced in monkeys dosed 28 days with MB07811 (data not shown), whereas monkeys dosed for only 7 days with KB-141 or another TR agonist exhibited a 4–7% reduction in BW (20, 21). BW reduction may reflect increased distribution of KB-141 into both fat and muscle and the subsequent enhancement in metabolism arising from increased expression of uncoupling proteins (7, 33) and enzymes controlling various futile cycles. Glucose lowering was also observed in DIO mice, but only in animals exhibiting reduced BW, suggesting that it may be secondary to the known beneficial effects of weight loss on insulin sensitivity. Overall, although both BW reduction and improved glycemia are desirable properties in much of the target hypercholesterolemic patient population, both effects likely reflect a wider tissue distribution by the TR agonist and consequently a greater long-term safety risk.
Limiting TR activation to the liver resulted in simultaneous reduction in cholesterol and both hepatic and plasma TG levels. Cholesterol lowering appeared to involve multiple mechanisms, including increased cholesterol metabolism via increased CYP7A and increased LDL clearance. Results supporting the latter mechanism include the consistent, although transient, 2-fold increase in hepatic LDLR mRNA levels observed in both euthyroid SD rats and DIO mice, the increased LDLR mRNA levels observed in thyroidectomized rats (SI Appendix, Table 8), and the failure of MB07811 to significantly lower cholesterol in CF LDLR−/− mice (SI Appendix, Table 9). The reduction in TGs contrasts with some studies in T3-treated rats (19) or hyperthyroid patients (39), wherein plasma and/or hepatic TGs (40) were either unchanged or increased. One reason for the difference may be related to T3-mediated activation of TRα in fat, which enhances catecholamine-stimulated lipolysis (41) and results in increased circulating fatty acid levels. Increased fatty acid supply to the liver favors TG production, which may counter the increased long-chain fatty acid oxidation induced by T3 in the liver via increased expression of carnitine palmitoyl transferase-1 (42).
Limiting TR activation to the liver may also avoid the reduced glycemic control commonly observed in diabetic patients treated with T3 (43) and attributed at least in part to increased gluconeogenesis. In the SD rat, T3, but not MB07811, increased fasting plasma glucose and free fatty acid levels. Neither T3 nor MB07811 increased hepatic expression of the rate-limiting gluconeogenic enzyme phosphoenolpyruvate carboxykinase (44), suggesting that the increased glucose levels associated with T3 are likely secondary to mobilization of free fatty acids and gluconeogenic substrates from peripheral tissues.
In summary, our results demonstrate that liver-targeted TR agonists lower cholesterol and TGs with decreased effects on extrahepatic tissues relative to T3 and non-liver-targeted TR agonists. The resulting improvement in the safety profile may enable the use of TR agonists in the treatment of patients with hyperlipidemia.
Materials and Methods
MB07811, MB07344, KB-141 (16), and the glutathione conjugate 2 (26) were synthesized at Metabasis Therapeutics (La Jolla, CA). [125I]-T3 was purchased from PerkinElmer (Boston, MA). T3 and clotrimazole were purchased from Sigma–Aldrich (St. Louis, MO). Male SD rats and male C57BL/6 mice were purchased from Harlan (San Diego, CA). Male thyroidectomized SD rats and male LDLR−/− mice were purchased from Charles River Laboratories (Wilmington, MA) and The Jackson Laboratory (Bar Harbor, ME), respectively. Lutrol F68 NF was purchased from BASF (Ludwigshafen, Germany).
In Vitro Assays.
Receptor binding affinities were determined by using recombinant TR/RXR heterodimers and a competition assay. Additional details are available in SI Appendix. Methods for determining prodrug activation kinetics and hepatocyte uptake and metabolism are described in SI Appendix and are based on those described in ref. 27.
Animal Care.
Rats and mice were housed under standard vivarium conditions (12 h light/dark cycle) with free access to chow and water unless otherwise indicated. All studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Rat Pharmacokinetics.
Pharmacokinetic parameters for MB07344 (i.v.) and MB07811 (i.v. and per os) in male SD rats were determined from the temporal profile of MB07811 and MB07344 in plasma, using the methods described in SI Appendix. First-pass hepatic extraction (EH) was determined by measuring MB07811 levels after oral administration of MB07811 (3 mg/kg) to catheterized male SD rats (n = 4–5 per group) and by using the equation EH = (AUCpv − AUCsys)/AUCpv, wherein AUCpv and AUCsys represent AUC values derived from the portal vein (pv) and carotid artery (sys) plasma concentration-time profiles. Biliary excretion and enterohepatic recirculation were assessed by measuring MB07344 levels in plasma and bile collected from naïve and bile duct-cannulated SD rats (n = 3 per group) administered MB07344 (10 mg/kg, i.v.). The tissue distribution of MB07811 was evaluated in male SD rats (n = 4 per group) administered [14C]-MB07811 (5 mg/kg, per os). Tissues harvested after killing the animals 3 and 24 h after dosing were processed and analyzed directly by liquid scintillation counting. Mass balance studies were conducted by administration of [3H]-MB07344 [2 mg/kg, 45.5 mCi (1 Ci = 37 GBq)/mmol] or [14C]-MB07811 (5 mg/kg, 20.6 mCi/mmol) to male SD rats (n = 6) and monitoring mean cumulative radioactivity excreted in urine, wash, and feces at 0, 12, 24, 48, 72, and 96 h. Detailed procedures and additional results for the above studies are reported in SI Appendix.
mRNA Expression Analysis.
Normal male SD rats (63–74 days old) were administered vehicle (water or CMC/Lutrol) or drug (T3, KB-141, or MB07811) at 1×, 3×, and 10× the CF rat ED50 (T3 in water at 0.012, 0.036, and 0.12 mg/kg; KB-141 in water at 0.05, 0.15, and 0.5 mg/kg; and MB07811 in CMC/Lutrol at 0.4, 1.2, and 4.0 mg/kg). Animals (n = 6 per group) were killed at 3, 8, and 24 h after oral dosing, and selected tissues were harvested under anesthetization with 2.5% isoflurane. Tissues were either removed and snap-frozen in liquid nitrogen (pituitary and thyroid gland) or freeze-clamped (liver, heart, soleus muscle, spleen, and kidney). mRNA expression was analyzed by using quantitative real-time PCR. Hepatic LDLR mRNA was measured in thyroidectomized male SD rats treated with T3 (0.5 mg/kg) or MB07811 (5 mg/kg). Detailed procedures are reported in SI Appendix.
Safety Pharmacology Studies.
In separate studies, normal male SD rats were treated daily with MB07811 to assess effects on cardiac function, glycemic control, and the THA. Procedures are briefly described below, with additional details found in SI Appendix.
Cardiac function.
MB07811 was administered daily in PEG400 to SD male rats (n = 6 per group) by oral gavage. Vehicle and KB-141 (1 mg/kg) groups were also included in the study. In separate experiments, T3 and KB-141 were administered by oral gavage (n = 6 per group). On day 7 after the start of dosing, animals were anesthetized with isoflurane, and the left ventricle was cannulated with a high fidelity catheter-tip transducer via the right carotid artery. Left ventricular pressure, its first derivative (LV dP/dt), and heart rate (via led I ECG) were digitally recorded. Systolic and diastolic aortic pressures were measured by retracting the catheter into the proximal aorta.
Glycemic control.
See SI Appendix.
THA.
Male SD rats (20 groups, n = 5–6 per group, and one group for baseline measurements, n = 8) were treated with vehicle (0.5% CMC/1% Lutrol F68), MB07811 (3 or 30 mg/kg), or KB-141 (0.1 or 1 mg/kg) once daily (per os). After 1, 2, 4, and 7 weeks of treatment, one group from each treatment cohort was weighed and then killed by decapitation without anesthesia to minimize stress effects on the THA. Serum obtained from trunk blood collected at killing was used for measurement of total T3, total T4, free T3, free T4 (Michigan State University, Lansing, MI), and TSH, which was measured by using the rat TSH [125I]-Biotrak assay system with magnetic separation (Amersham Biosciences, Piscataway, NJ).
Efficacy Studies.
DIO mice were derived from ≈4-week-old normal male C57BL/6 mice fed a high-fat diet [60% fat by kcal (1 cal = 4.18 J)] for ≈170 days and then assigned to 10 groups (n = 8 per group) such that all groups had comparable cholesterol levels and BW. Mice were dosed orally once daily with vehicle (0.5% CMC/1% Lutrol F68), MB07811 (0.3, 1, 3, 10, and 30 mg/kg), or KB-141 (0.03, 0.1, 0.3, and 1 mg/kg). Blood glucose and plasma cholesterol and TGs were measured at baseline from blood obtained via tail nick. After 2 weeks of treatment, animals were killed by cervical dislocation, and blood was collected by cardiac puncture for measurement of cholesterol, TGs, glucose, and THs. In addition, hepatic TGs were measured after removal and weighing of the liver. Additional details are described in SI Appendix, as are studies evaluating T3, KB-141, and MB07811 in the 24-h CF-rat assay and cholesterol-fed C57BL/6 and LDLR−/− mice.
Statistics.
Results are expressed as mean ± SEM unless otherwise indicated. All analyses were performed by using JMP 5.0–6.0 (SAS Institute, Cary, NC). Data obtained at multiple time points in the same animals were analyzed by using a two-way ANOVA with repeated measures on time. If a significant effect of either treatment or the interaction of treatment and time was found, data were analyzed by using the method for endpoint data. Endpoint data were analyzed by using a one-way ANOVA followed by a Dunnett's post hoc test with the vehicle-treated group as the control or an unpaired Student's t test as indicated. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Jian Li, Sergio Briones, Xuehong Song, Xiaohong Yang, Michael A. Insko, Don Reeder, Michael J. Estes, Michael Haughey, Patricia Frech, Eckhardt Schmidt, Bert Chi, Igor Belka, Rongrong Wu, and Cindy Phan for their technical assistance in these studies.
Abbreviations
- AUC
area under the curve
- BW
body weight
- CF
cholesterol-fed
- CYP
cytochrome P450
- D1
type 1 iodothyronine deiodinase
- DIO
diet-induced obese
- LDLR
low-density lipoprotein receptor
- LV dP/dt
first derivative of left ventricular (LV) pressure
- MB07344
(3,5-dimethyl-4-(4′-hydroxy-3′-isopropylbenzyl)phenoxy)methylphosphonic acid
- MB07811
(2R,4S)-4-(3-chlorophenyl)-2-[(3,5-dimethyl-4-(4′-hydroxy-3′-isopropylbenzyl)phenoxy)methyl]-2-oxido-[1,3,2]-dioxaphosphonane
- SD
Sprague–Dawley
- T3
3,5,3′-triiodo-l-thyronine
- KB-141
3,5-dichloro-4-(4-hydroxy-3-isopropylphenoxy)phenylacetic acid
- T4
3,5,3′,5′-tetraiodo-l-thyronine
- TG
triglyceride
- TH
thyroid hormone
- THA
thyroid hormone axis
- TI
therapeutic index
- TR
thyroid hormone receptor
- TSH
thyroid-stimulating hormone.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702759104/DC1.
The negligible levels in bone are consistent with the lack of effect of MB07811 on bone turnover in cynomolgus monkeys as assessed by plasma osteocalcin measurements after 1 week of treatment (E.E.C., personal communication).
References
- 1.Oppenheimer JH, Schwartz HL, Strait KA. In: The Thyroid: A Fundamental and Clinical Text. Braverman LE, Utiger RD, editors. New York: Lippincott-Raven; 1996. pp. 162–184. [Google Scholar]
- 2.Yen PM. Physiol Rev. 2001;81:1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- 3.Sap J, Munoz A, Damm K, Goldberg Y, Ghysdael J, Leutz A, Beug H, Vennstrom B. Nature. 1986;324:635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- 4.Weinberger C, Thompson CC, Ong ES, Lebo R, Gruol DJ, Evans RM. Nature. 1986;324:641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- 5.Hansson P, Valdemarsson S, Nilsson-Ehle P. Horm Metab Res. 1983;15:449–452. doi: 10.1055/s-2007-1018751. [DOI] [PubMed] [Google Scholar]
- 6.Moreno M, Lanni A, Lombardi A, Goglia F. J Physiol (London) 1997;505(Pt 2):529–538. doi: 10.1111/j.1469-7793.1997.529bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abrams JJ, Grundy SM. J Lipid Res. 1981;22:323–338. [PubMed] [Google Scholar]
- 8.Abrams JJ, Grundy SM, Ginsberg H. J Lipid Res. 1981;22:307–322. [PubMed] [Google Scholar]
- 9.Silva JE. Ann Intern Med. 2003;139:205–213. [PubMed] [Google Scholar]
- 10.Brent GA. Rev Endocr Metab Disord. 2000;1:27–33. doi: 10.1023/a:1010056202122. [DOI] [PubMed] [Google Scholar]
- 11.Underwood AH, Emmett JC, Ellis D, Flynn SB, Leeson PD, Benson GM, Novelli R, Pearce NJ, Shah VP. Nature. 1986;324:425–429. doi: 10.1038/324425a0. [DOI] [PubMed] [Google Scholar]
- 12.Yokoyama N, Walker GN, Main AJ, Stanton JL, Morrissey MM, Boehm C, Engle A, Neubert AD, Wasvary JM, Stephan ZF, et al. J Med Chem. 1995;38:695–707. doi: 10.1021/jm00004a015. [DOI] [PubMed] [Google Scholar]
- 13.Lazar MA. Endocr Rev. 1993;14:184–193. doi: 10.1210/edrv-14-2-184. [DOI] [PubMed] [Google Scholar]
- 14.Forrest D, Hanebuth E, Smeyne RJ, Everds N, Stewart CL, Wehner JM, Curran T. EMBO J. 1996;15:3006–3015. [PMC free article] [PubMed] [Google Scholar]
- 15.Wikstrom L, Johansson C, Salto C, Barlow C, Campos Barros A, Baas F, Forrest D, Thoren P, Vennstrom B. EMBO J. 1998;17:455–461. doi: 10.1093/emboj/17.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye L, Li YL, Mellstrom K, Mellin C, Bladh LG, Koehler K, Garg N, Garcia Collazo AM, Litten C, Husman B, et al. J Med Chem. 2003;46:1580–1588. doi: 10.1021/jm021080f. [DOI] [PubMed] [Google Scholar]
- 17.Chiellini G, Apriletti JW, Yoshihara HA, Baxter JD, Ribeiro RC, Scanlan TS. Chem Biol. 1998;5:299–306. doi: 10.1016/s1074-5521(98)90168-5. [DOI] [PubMed] [Google Scholar]
- 18.Wagner RL, Huber BR, Shiau AK, Kelly A, Cunha Lima ST, Scanlan TS, Apriletti JW, Baxter JD, West BL, Fletterick RJ. Mol Endocrinol. 2001;15:398–410. doi: 10.1210/mend.15.3.0608. [DOI] [PubMed] [Google Scholar]
- 19.Trost SU, Swanson E, Gloss B, Wang-Iverson DB, Zhang H, Volodarsky T, Grover GJ, Baxter JD, Chiellini G, Scanlan TS, et al. Endocrinology. 2000;141:3057–3064. doi: 10.1210/endo.141.9.7681. [DOI] [PubMed] [Google Scholar]
- 20.Grover GJ, Mellstrom K, Ye L, Malm J, Li YL, Bladh LG, Sleph PG, Smith MA, George R, Vennstrom B, et al. Proc Natl Acad Sci USA. 2003;100:10067–10072. doi: 10.1073/pnas.1633737100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grover GJ, Egan DM, Sleph PG, Beehler BC, Chiellini G, Nguyen NH, Baxter JD, Scanlan TS. Endocrinology. 2004;145:1656–1661. doi: 10.1210/en.2003-0973. [DOI] [PubMed] [Google Scholar]
- 22.Ness GC, Lopez D. Arch Biochem Biophys. 1995;323:404–408. doi: 10.1006/abbi.1995.0061. [DOI] [PubMed] [Google Scholar]
- 23.Johansson L, Rudling M, Scanlan TS, Lundasen T, Webb P, Baxter J, Angelin B, Parini P. Proc Natl Acad Sci USA. 2005;102:10297–10302. doi: 10.1073/pnas.0504379102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erion MD, van Poelje PD, Dang Q, Kasibhatla SR, Potter SC, Reddy MR, Reddy KR, Jiang T, Lipscomb WN. Proc Natl Acad Sci USA. 2005;102:7970–7975. doi: 10.1073/pnas.0502983102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erion MD, Walsh CT. Biochemistry. 1987;26:3417–3425. doi: 10.1021/bi00386a025. [DOI] [PubMed] [Google Scholar]
- 26.De Lombaert S, Erion MD, Tan J, Blanchard L, el-Chehabi L, Ghai RD, Sakane Y, Berry C, Trapani AJ. J Med Chem. 1994;37:498–511. doi: 10.1021/jm00030a009. [DOI] [PubMed] [Google Scholar]
- 27.Erion MD, Reddy KR, Boyer SH, Matelich MC, Gomez-Galeno J, Lemus RH, Ugarkar BG, Colby TJ, Schanzer J, van Poelje PD. J Am Chem Soc. 2004;126:5154–5163. doi: 10.1021/ja031818y. [DOI] [PubMed] [Google Scholar]
- 28.Erion MD, van Poelje PD, MacKenna DA, Colby TJ, Montag AC, Fujitaki JM, Linemeyer DL, Bullough DA. J Pharmacol Exp Ther. 2005;312:554–560. doi: 10.1124/jpet.104.075903. [DOI] [PubMed] [Google Scholar]
- 29.van Montfoort JE, Hagenbuch B, Groothuis GM, Koepsell H, Meier PJ, Meijer DK. Curr Drug Metab. 2003;4:185–211. doi: 10.2174/1389200033489460. [DOI] [PubMed] [Google Scholar]
- 30.Towle HC, Mariash CN, Schwartz HL, Oppenheimer JH. Biochemistry. 1981;20:3486–3492. doi: 10.1021/bi00515a028. [DOI] [PubMed] [Google Scholar]
- 31.Hashimoto K, Yamada M, Matsumoto S, Monden T, Satoh T, Mori M. Endocrinology. 2006;147:4292–4302. doi: 10.1210/en.2006-0116. [DOI] [PubMed] [Google Scholar]
- 32.Zavacki AM, Ying H, Christoffolete MA, Aerts G, So E, Harney JW, Cheng SY, Larsen PR, Bianco AC. Endocrinology. 2005;146:1568–1575. doi: 10.1210/en.2004-1392. [DOI] [PubMed] [Google Scholar]
- 33.Lanni A, Beneduce L, Lombardi A, Moreno M, Boss O, Muzzin P, Giacobino JP, Goglia F. FEBS Lett. 1999;444:250–254. doi: 10.1016/s0014-5793(99)00061-7. [DOI] [PubMed] [Google Scholar]
- 34.Tomlinson E. Adv Drug Delivery Rev. 1987;1:87–198. [Google Scholar]
- 35.Erion MD. In: Prodrugs: Challenges and Rewards, Part II. Stella VJ, Borchardt RT, Hageman M, Oliyai R, Maag H, Tilley J, editors. New York: Springer; 2006. pp. 529–560. [Google Scholar]
- 36.Friesema EC, Jansen J, Visser TJ. Biochem Soc Trans. 2005;33:228–232. doi: 10.1042/BST0330228. [DOI] [PubMed] [Google Scholar]
- 37.Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Endocr Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 38.Parle JV, Maisonneuve P, Sheppard MC, Boyle P, Franklyn JA. Lancet. 2001;358:861–865. doi: 10.1016/S0140-6736(01)06067-6. [DOI] [PubMed] [Google Scholar]
- 39.Cachefo A, Boucher P, Vidon C, Dusserre E, Diraison F, Beylot M. J Clin Endocrinol Metab. 2001;86:5353–5357. doi: 10.1210/jcem.86.11.7981. [DOI] [PubMed] [Google Scholar]
- 40.Klion FM, Segal R, Schaffner F. Am J Med. 1971;50:317–324. doi: 10.1016/0002-9343(71)90220-8. [DOI] [PubMed] [Google Scholar]
- 41.Liu YY, Schultz JJ, Brent GA. J Biol Chem. 2003;278:38913–38920. doi: 10.1074/jbc.M306120200. [DOI] [PubMed] [Google Scholar]
- 42.Mynatt RL, Park EA, Thorngate FE, Das HK, Cook GA. Biochem Biophys Res Commun. 1994;201:932–937. doi: 10.1006/bbrc.1994.1791. [DOI] [PubMed] [Google Scholar]
- 43.Dimitriadis GD, Raptis SA. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S225–S239. doi: 10.1055/s-2001-18584. [DOI] [PubMed] [Google Scholar]
- 44.Hartong R, Wiersinga WM, Lamers WH. Endocrinology. 1987;120:2460–2467. doi: 10.1210/endo-120-6-2460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.