Introduction
Public concern regarding environmental hazards is perhaps greatest when potential exposures are related to fetal development, pregnancy loss and/or reproductive health. An increasing body of evidence reveals associations between various therapeutic/environmental compounds that act as endocrine disrupting substances (EDS) and many sex hormone sensitive diseases/disorders (Colborn and Clement, 1992; Guillette, 2006; Massart et al., 2006). Exposure to these EDS can result in reduced fecundity, abnormal fetal development, delayed onset of puberty, disruption of ovarian function, abnormal lactation, early onset of reproductive senescence and cancer (Sharpe and Irvine, 2004; Buck Louis et al., 2006; Darbre, 2006; Guillette, 2006; Maffini et al., 2006). These adverse effects appear to be mediated largely through their ability to interfere with sex steroid action. In some cases, the etiologies of these conditions are believed to be environmental in origin as a result of persistent contaminations (Lipworth, 1995). More importantly, EDS may pose species specific risks that are difficult to investigate because they also often act silently with severe latent adverse effects (Fenton, 2006).
A large pool of literature exists for EDS with estrogenic potential and a large number of environmental hazards with estrogenic properties have been identified and classified (Guillette, 2006). While there are similar health concerns regarding androgenic EDS that can reduce sperm production, alter genital development and contribute to neurological syndromes in males, the identification and classification of these putative health hazards have progressed slowly. Recent reports of several non-steroidal compounds that have the ability to bind and activate the androgen receptor (AR) are of particular concern because many of these xenobiotics are ubiquitous in daily life and some are present as high production volume (HPV) compounds that are manufactured or imported into the United States in millions of pounds per year. The presence of these compounds in the environment has stimulated new interest in the identification of environmental contaminants that may act as selective AR modulators (SARMs) (Yin et al., 2003; Bohl et al., 2004; Chen et al., 2005).
Preservatives and/or antimicrobial agents are commonly used in food, soap, detergent, toothpaste, disinfectant, cosmetic and pharmaceutical products (Cabana et al., 2006; Darbre, 2006; Lakeram et al., 2006). These compounds are continually produced and are rapidly becoming prevalent at detectable concentrations: 1) environmentally in ground water and soil; and 2) in human blood, breast milk, and tissue (Hovander et al., 2002; Kolpin et al., 2002; Darbre, 2006; Dayan, 2006; Heidler et al., 2006; Nakada et al., 2006; Dayan, 2007). This knowledge has lead to growing public concern over the possible impacts on human health (Daughton and Ternes, 1999; Darbre, 2006). While some of these compounds have demonstrated varying estrogenic potencies, their androgenic properties remain poorly characterized. This study investigates the androgenic potential of selected antimicrobial and preservative compounds to which humans are exposed daily. This report specifically focuses on alkyl hydroxyl benzoate (parabens), triclosan and thymol through the application of a recently developed bioassay for human AR ligands (Chen et al., 2006). This cell-based AR-mediated bioassay assesses both androgenic and antiandrogenic properties of natural and synthetic compounds and its application provides new information on potential environmental EDS. The antimicrobial and preservative agents tested in this report are small non-steroidal structures containing a phenolic moiety. The data presented demonstrate that some widely used antimicrobial compounds have antiandrogenic properties and warrant further investigation to fully understand their potential impact on human reproductive health.
Methods
Chemicals
Butyl 4-hydroxybenzoate, methyl 4-hydroxybenzoate, propyl 4-hydroxybenzoate, p-hydroxybenzoic acid (≥99%), thymol (≥99%), triclosan(≥97%), flutamide and vinclozolin were purchased from Sigma-Aldrich (St. Louis, MO, USA). 17(beta)-hydroxy-4-androsten-3-one (testosterone, T) was purchased from Steraloids (Newport, RI, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). The testosterone was dissolved in absolute ethyl alcohol while all other compounds were dissolved in dimethylsulfoxide (DMSO).
Cell culture/bioassay reagents
Human embryonic kidney (HEK 293) cells were obtained from American Type Culture Collection (ATCC). Phenol-red free Dulbecco's Modified Eagle Medium (DMEM), fetal bovine serum (FBS), blasticidin and neomycin (G418) were obtained from Invitrogen (Carlsbad, CA, USA). Dextran-coated charcoal-treated (DCC) FBS was purchased from Hyclone (Logan, UT, USA). Cell lysis buffer was purchased from Promega (Madison, WI, USA).
Cell-based human AR-mediated bioassay
A full detailed description of the development and application of the cell-based human AR-mediated bioassay has been published by Chen et al. (Chen et al., 2006). Briefly, the bioassay system employs human embryonic kidney (HEK) 293 cells that lack critical steroid metabolizing enzymes. The cells are stably transfected with PCDNA6-hAR and an MMTV-Luc.neo plasmid containing a luciferase reporting gene (Chen et al., 2006). The cells (designated as 2933Y) are highly responsive to endogenous steroids as well as synthetic compounds. The signal induction is stable for more than 60 passages under double antibiotic selection conditions (Chen et al., 2006).
To evaluate the androgenic/antiandrogenic activity of the test compounds, the 2933Y cells were cultured in DMEM with 10% FBS. When cells reached 80% confluence, cells were trypsinized (0.05% trypsin-EDTA) and dispersed well in phenolred free DMEM supplemented with 10% DCC-FBS. Suspended cells (50 μL/well; density = 25,000 cells/50 μL) were placed in 96-well tissue culture plates containing 150 μL/well of phenol-red free DMEM supplemented with 10% DCC-FBS. The total volume of the medium in each well was 200 μL. On the following day, the media in each well was removed and replaced with 200 μL of phenol-red free DMEM supplemented with 10% DCC-FBS. On day 3, media was again removed and replaced with 200 μL phenol red-free DMEM supplemented with 10% DCC-FBS and containing 20 μL (10%; v/v) of the test compound alone, testosterone alone or a combination of testosterone and test compound at the designated concentrations. To compensate for any organic solvent effects, the final content of ethyl alcohol in the assay system was 0.1% (v/v) for all studies and the total DMSO concentration in the final culture media was no more than 0.2% (v/v). The total concentration of organic solvent (v/v) was maintained at the same level for both controls and test compounds. The cells with T and/or test compounds in 96-well plates were further cultured for 16 h. The media was then removed and 100 μL of cell lysis buffer was added to each well and allowed to incubate for 30 minutes. Cell lysates (30 μL) were then transferred to 96-well Microfluor II plates (Fisher Scientific, Santa Clara, CA). Luciferin substrate was then injected into each well and the luciferase activity induced by the test compounds or T was measured by a Veritas Luminometer (Turner Biosystems, Sunnyvale, CA, USA) (Chen et al., 2006). Luciferase catalyzes luciferin oxidation and the chemical energy of this reaction produces a light which is measured by the luminometer. The intensity of the light is expressed as relative light units (RLU) and is directly proportional to the induced luciferase activity. The lower limit of detection of this assay was 15 pM T in cell culture medium (blank +3 SD) with intra- and inter-assay coefficients of variation of 7.4 and 7.5% at 0.25 nM T and 4.9 and 6.4% at 0.03 nM T, respectively (Chen et al., 2006).
A relatively low concentration of T (0.125 nM) was selected to test interactions of compounds in this report. The dominant circulating androgen in most mammalian systems is testosterone. The mean circulating concentration of T in humans ranges from less than 3.5 to 35 nM (Williams and Larsen, 2003). Approximately 2-3% of circulating T is free, non-SHBG bound and is considered bioactive (Siiteri et al., 1982; Pardridge, 1988). Thus, the selected dose of 0.125 nM T is physiologically relevant and enhances the likelihood of antagonist recognition at lower concentrations of the natural ligand since the stronger the effect of the androgen control, the greater the competition (higher concentration) of the antiandrogen required. In addition, the selected dose of 0.125 nM T can induce significant luciferase activity above the vehicle controls (20-fold induction) with a relatively small variance (coefficient of variance of 5-6% of relative light units). Use of a low dose of T also avoids the large variance and potential “hook effects” associated with the use of higher concentrations of T in the assay. Furthermore, the lower concentration allows for the observation of higher responses when testing compounds that augment or enhance the effects of T.
MTT assay
The MTT assay for cell proliferation or cytotoxicity testing under varying concentrations of test compounds was performed according to the manufacturer's instructions (ATCC, Catalog Number 30-1010K, USA). Briefly, the 2933Y cells were plated at the same density and cultured by the same procedure as previously described for each 96-well plate. After 16 hours of treatment, 20 μl of MTT was added to each well and the plate was incubated at 37°C for 4 h. The yellow tetrazolium MTT was reduced by metabolically active cells, in part by the action of dehydrogenase enzymes. The resulting intracellular purple formazan was solubilized by adding 100 μL of detergent reagent and incubating the plate at room temperature in darkness for 2 hours. At the end of this period, the absorbance was measured at a test wavelength of 570 nm and a reference wavelength of 650 nm using an EMax Spectrophotometer (Molecular Devices, Sunnyvale, CA, USA).
Statistical analysis
The values shown are mean ± SD from three independent experiments for each dose tested. Data were analyzed by one-way analysis of variance (ANOVA), followed by multiple comparisons test when appropriate, using Sigmstat (Systat Software, San Jose, CA). The level of significance was set at p<0.05. To test for agonist properties, treatments were compared to the negative control group containing vehicle only. For androgen antagonist properties, treatments were compared to the testosterone positive control group.
Results
Cell proliferation and cytotoxicity testing
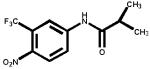
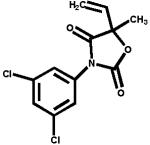
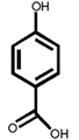
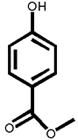
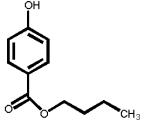
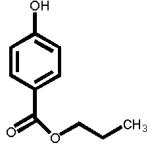
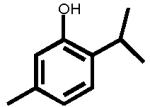
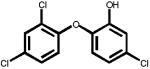
The structures of two known antiandrogens and test antimicrobial compounds are shown in Table 1. The common structural component of the test antimicrobial compounds is the phenolic moiety. As measured by the MTT assay, none of the test compounds (parabens, triclosan and thymol) exhibited cytotoxicity when tested alone at 10 μM or in combination with 0.125 nM of testosterone (Figure 1). No cytotoxicity was observed when testosterone was tested alone. Vehicle-treated groups and chemical-treated groups did not demonstrate statistically significant differences with respect to cell proliferation.
Table 1.
The chemical structures of tested compounds
Figure 1.
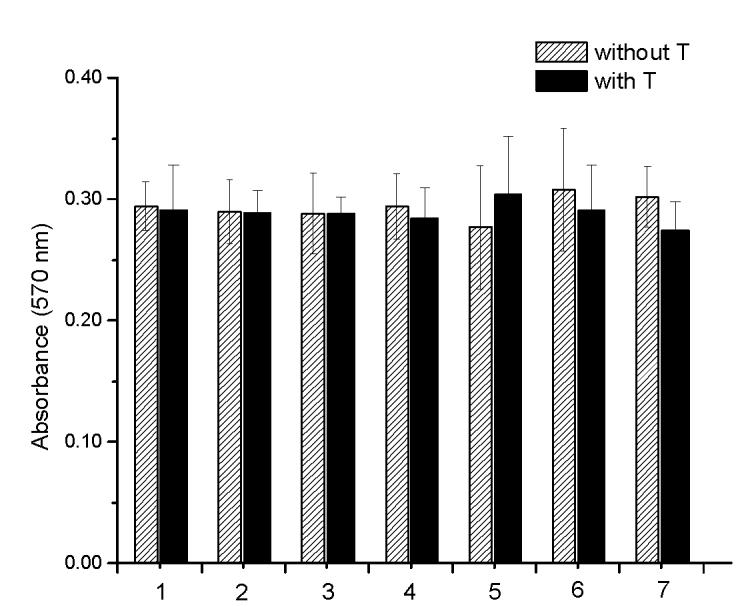
Cell proliferation and cytotoxicity evaluation in MTT assay. No significant cytotoxicity or cell proliferation was observed for parabens, triclosan or thymol either tested alone or in combination with T. There was no significant cell proliferation or cytotoxicity observed in the T treated cells when compared to the vehicle control. 1: control with and without T; 2: p-hydroxybenzoic acid; 3: butyl-4-hydroxybenzoate; 4: methyl-4-hydroxybenzoate; 5: propyl-4-hydroxybenzoate; 6: triclosan and 7: thymol.
Response of 2933Y to the AR antagonists, flutamide and vinclozolin
Given the known strong antiandrogenic activities of flutamide and vinclozolin, five concentrations of each compound were tested with 1.0 nM T in order to ensure comparable information was obtained over the entire range of the dose-response curve. As expected, flutamide and vinclozolin were potent antiandrogenic compounds that significantly inhibited the luciferase activity induced by 1.0 nM T. Flutamide inhibited a 1.0 nM T induced signal by 90% at 10 μM, 41% at 1.0 μM and 11% at 0.1 μM while vinclozalin inhibited the same T induced signal by 94% at 10 μM, 81% at 1.0 μM and 32% at 0.1 μM (Figure 2). Flutamide exhibited no androgenic properties at the concentrations tested when compared to the vehicle control. In contrast, vinclozolin was slightly androgenic at the highest concentration tested (10 μM) when compared to the vehicle control (Figure 2).
Figure 2.
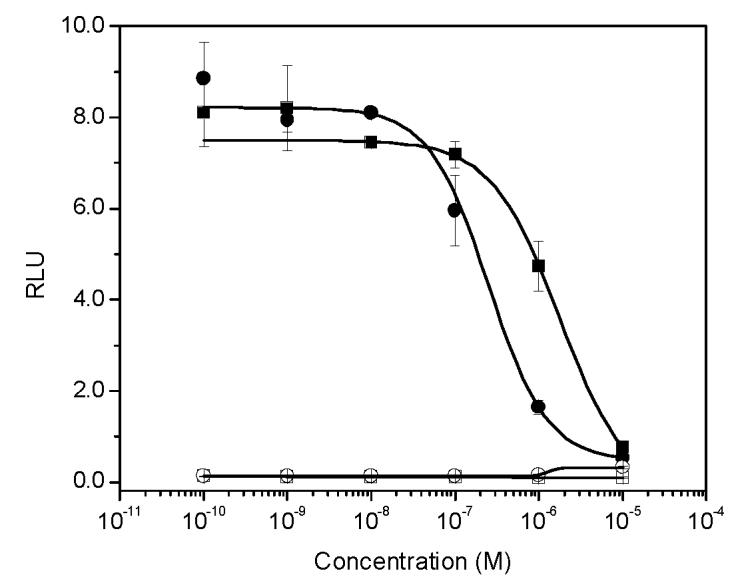
The effect of strong antiandrogens on AR-mediated transcriptional activity induced by 1.0 nM T. Open circle: vinclozolin alone; closed circle: vinclozolin in the presence of 1.0 nM T. Open square: flutamide alone; solid square: flutamide in the presence of 1.0 nM T.
The androgenic/antiandrogenic activity of parabens, triclosan and thymol
At concentrations between 10−3 and 10 μM (1.0 nM to 10 μM), p-hydroxybenzoic acid and its derivatives revealed no androgenicity. In contrast, at the highest concentrations tested (10 μM), methyl-, butyl- and propyl-4-hydroxybenzoate significantly inhibited the transcriptional activity of testosterone by 40%, 33% and 19%, respectively (P<0.05). No statistically significant inhibition was detected for p-hydroxybenzoic acid, the major paraben metabolite (Figure 3).
Figure 3.
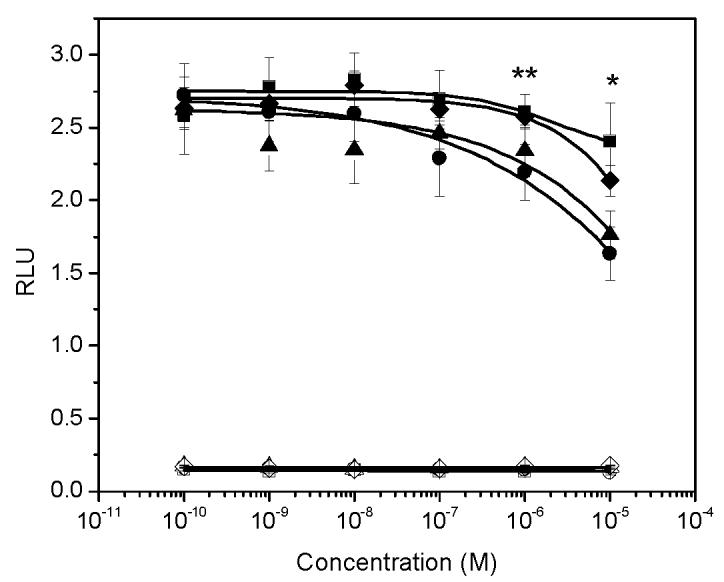
The effect of parabens on AR-mediated transcriptional activity induced by 0.125 nM T. Open square: p-hydroxybenzoic acid alone; closed square: p-hydroxybenzoic acid in the presence of 0.125 nM T. Open diamond: butyl-4-hydroxybezoate alone; closed diamond: butyl-4-hydroxybenzoate in the presence of 0.125 nM T. Open triangle: propyl-4-hydroxybenzoate; closed triangle: propyl-4-hydroxybenzoate in the presence of 0.125 nM T. Open circle: methyl-4-hydroxybenzoate alone; closed circle: methyl-4-hydroxybenzoate in the presence of 0.125 nM T. *: significant decrease of T induced transcriptional activity by butyl, methyl and propyl-4-hydroxybenzoate at 10 μM; **: significant decrease of T induced transcriptional activity by methyl and propyl-4-hydroxybenzoate at 1.0 μM.
Triclosan inhibited transcriptional activity of 0.125 nM T by more than 92% at a concentration of 10 μM, and 38.8% at a concentration of 1.0 μM (P<0.05). In contrast, 34% of the transcriptional activity induced by 0.125 nM T was inhibited by thymol at 10 μM (P<0.05) and an 11% inhibition was observed at a concentration of 1.0 μM (Figure 4). Neither of these compounds exhibited androgenic activities at concentrations up to 10 μM when evaluated without testosterone (Figure 4).
Figure 4.
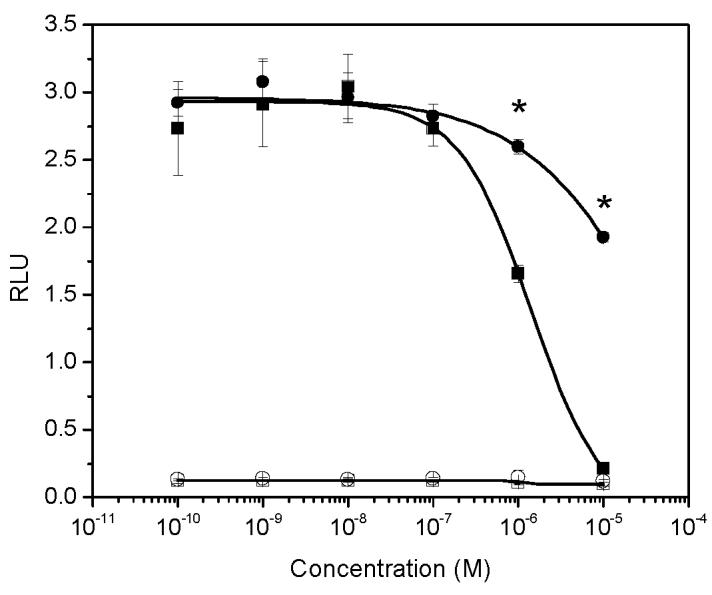
The effect of thymol and triclosan on AR-mediated transcriptional activity induced by 0.125 nM T. Open circle: thymol alone; closed circle: thymol in the presence of 0.125 nM T. Open square: triclosan alone; solid square: triclosan in the presence of 0.125 nM T. *: significant decrease of T induced transcriptional activity by thymol and triclosan at concentrations of 1.0 μM and 10 μM.
Discussion
An important approach to identify and assess the potential impact of environmental EDS has been the development of cell-based bioassays. The AR-mediated assay utilizes a stably transfected cell line does not express endogenous 5(alpha)-reductase activity, 17(beta)-hydroxysteroid dehydrogenase and 3(beta)-hydroxysteroid dehydrogenase activities, enzymes that could potentially metabolize test steroids or environmental EDS (Chen et al., 2006). This assay has been shown to be predicatively responsive to natural and synthetic compounds with a wide range of biopotencies including flutamide and vinclozolin (Figure 2). The observed androgenic activity of vinclozolin in this assay at high concentrations is supported by similar findings in the literature (Wong et al., 1995; Nellemann et al., 2003).
Alkyl esters of p-hydroxybenzoic acid (parabens) have been reported to be weakly estrogenic with different potencies in vitro and in vivo (Pugazhendhi et al., 2006a). The presence of carboxylesterase in skin is reportedly responsible for the hydrolysis of dermally applied paraben esters to p-hydroxybenzoic acid, a metabolite with no or very low EDS capacity (Lobemeier et al., 1996; Pugazhendhi et al., 2005). The debate remains as to whether the level of carboxylesterase is sufficient to hydrolyze all dermally applied parabens and, in particular, whether high consumer use of cosmetics, individual variations in carboxylesterase levels or exposure to esterase inhibitor could result in incomplete hydrolysis (Harvey and Darbre, 2004; Pugazhendhi et al., 2005). The recent detection of unmetabolized parabens in human breast cancer tissue warrants further screening and testing of parabens in order to assess their potential activity as EDS.
Methyl- and propyl-4-hydroxybenzoate are among the most widely used esters with the highest detectable concentrations in more than 96% of tested subjects (Ye et al., 2006). Parabens have been shown to produce male reproductive disorders in animal models with a dose-dependent decrease of absolute and relative weights of epididymis, ventral prostates, as well as reduction in sperm reserve and daily production in rats (Oishi, 2002a; Oishi, 2002b). However, the underlying mechanism for these effects are largely unknown and has been speculated to be estrogen receptor mediated. The present data indicate that methyl-, propyl- and butyl-4-hydroxybenzoate are antiandrogens and some of these parabens can inhibit the T induced transcriptioanl activity by as much as 40% at a concentration of 10 μM. This observation provides a plausible and complementary explanation to the observed male reproductive disorders associated with these compounds (Oishi, 2002a; Oishi, 2002b) since the role of androgens in the development of the male reproduction tract, sexual accessory organs, spermatogenesis and maturation of spermatozoa is critical (Desjardins, 1978). It is, therefore, possible that some of these adverse effects are the result of lowered circulating androgen action by EDS which impedes androgen signaling. The lack of a statistically significant inhibitory effect of p-hydroxybenzoic acid in this report, however, indicates low, if any, antiandrogenic potency of this paraben metabolite. To our knowledge, this is the first report regarding the androgenic/antiandrogenic potencies of any paraben compound in vitro.
Triclosan and thymol are also commonly used in cosmetic and personal care products (Nakada et al., 2006) and previous studies report that most estrogenic and antiandrogenic EDS contain a phenolic moiety (Kuiper et al., 1998; Kitamura et al., 2005; Xu et al., 2005). As predicted by the structural similarities of these compounds, the present data demonstrate that parabens containing a methyl, butyl or propyl moiety, triclosan and thymol are antiandrogenic with varying potencies.
Data on human and wildlife exposure to parabens, triclosan and thymol are limited and the toxic effects are largely unknown or under debate (Harvey and Darbre, 2004; Harvey and Everett, 2004; Houtman et al., 2004; Golden et al., 2005; Ye et al., 2006). Urinary concentrations of total parabens have been reported with levels as high as 680 ng/mL in adults. Concentrations of triclosan have been found in fish bile at levels of approximately 14 to 80 μg/mL (48 to 275 μM) indicating that these compounds are present in aquatic environments at relatively high concentrations (Houtman et al., 2004). Concentrations of parabens tested in most in vitro studies ranged from 0.001 to 100 μM (Byford et al., 2002; Darbre et al., 2002; Darbre et al., 2003; Pugazhendhi et al., 2005; Pugazhendhi et al., 2006b) and reported concentrations of triclosan tested in vitro were 8 to 68 μM (Liu et al., 2002). This information supports the selection of 10 μM as the highest dose for the compounds tested in this report.
The highest concentration of 10 μM tested was approximately 100,000 fold in excess of the T concentration used. It is clear that the relative binding efficiencies, if any, of the test compounds for the AR are orders of magnitude below that of the natural ligands. For this reason, a competitive receptor binding assay was not conducted because the relative effect of the test compounds is the primary and critical issue. In addition, many EDS seem to be less potent than the natural ligands in both in vitro and in vivo assays, however, comparable effects were observed when these compounds were administered at critical time points at doses that were several orders of magnitude lower (Soto et al., 2006). This lack of correlation between the deleterious developmental effects and relative ligand-receptor binding affinity underscores the need for further investigation (Strunck et al., 2000).
In summary, given the recognized wide-spread human exposure to antimicrobial EDS, both the mechanism(s) of endocrine action and the structure-activity relationships (SARs) of these compounds should be fully investigated. It is also important that exposure levels be determined by direct measurements in the near future. Further investigation with adequate screening systems and in vivo confirmation is urgently needed to fully appreciate the spectrum of these endocrine-disrupting properties.
Acknowledgements
We are grateful to Dr. Dan Chang (University of California, Davis) for critical review of the manuscript. This research was supported by the NIEHS Superfund Basic Research Program (P42ES04699) and NIEHS (P01ES06198, R37ES02710 and P30ES005707).
Footnotes
The authors declare they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bohl CE, Chang C, Mohler ML, Chen J, Miller DD, Swaan PW, Dalton JT. A ligand-based approach to identify quantitative structure-activity relationships for the androgen receptor. J. Med. Chem. 2004;47:3765–3776. doi: 10.1021/JM0499007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Lynch CD, Cooney MA. Environmental influences on female fecundity and fertility. Semin. Reprod. Med. 2006;24:147–155. doi: 10.1055/s-2006-944421. [DOI] [PubMed] [Google Scholar]
- Byford JR, Shaw LE, Drew MG, Pope GS, Sauer MJ, Darbre PD. Oestrogenic activity of parabens in MCF7 human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2002;80:49–60. doi: 10.1016/s0960-0760(01)00174-1. [DOI] [PubMed] [Google Scholar]
- Cabana H, Jiwan JL, Rozenberg R, Elisashvili V, Penninckx M, Agathos SN, Jones JP. Elimination of endocrine disrupting chemicals nonylphenol and bisphenol A and personal care product ingredient triclosan using enzyme preparation from the white rot fungus Coriolopsis polyzona. Chemosphere. 2007;67:770–778. doi: 10.1016/j.chemosphere.2006.10.037. [DOI] [PubMed] [Google Scholar]
- Chen J, Hwang DJ, Bohl CE, Miller DD, Dalton JT. A selective androgen receptor modulator for hormonal male contraception. J. Pharmacol. Exp. Ther. 2005;312:546–553. doi: 10.1124/jpet.104.075424. [DOI] [PubMed] [Google Scholar]
- Chen J, Sowers MR, Moran FM, McConnell DS, Gee NA, Greendale GA, Whitehead C, Kasim-Karakas SE, Lasley BL. Circulating bioactive androgens in midlife women. J. Clin. Endocrinol. Metab. 2006;91:4387–4394. doi: 10.1210/jc.2006-0284. [DOI] [PubMed] [Google Scholar]
- Colborn T, Clement C. Chemically-induced alterations in sexual and functional development : the wildlife/human connection. Princeton Scientific Publishing Co.; Princeton: 1992. [Google Scholar]
- Darbre PD. Environmental oestrogens, cosmetics and breast cancer. Best Pract. Res. Clin. Endocrinol. Metab. 2006;20:121–143. doi: 10.1016/j.beem.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Darbre PD, Byford JR, Shaw LE, Hall S, Coldham NG, Pope GS, Sauer MJ. Oestrogenic activity of benzylparaben. J. Appl. Toxicol. 2003;23:43–51. doi: 10.1002/jat.886. [DOI] [PubMed] [Google Scholar]
- Darbre PD, Byford JR, Shaw LE, Horton RA, Pope GS, Sauer MJ. Oestrogenic activity of isobutylparaben in vitro and in vivo. J. Appl. Toxicol. 2002;22:219–226. doi: 10.1002/jat.860. [DOI] [PubMed] [Google Scholar]
- Daughton CG, Ternes TA. Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ. Health Perspect. 1999;107(Suppl 6):907–938. doi: 10.1289/ehp.99107s6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan AD. Response to: Ethics, data and the pharmaceutical physician. Int. J. Clin. Pract. 2006;60:1009. doi: 10.1111/j.1742-1241.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- Dayan AD. Risk assessment of triclosan [Irgasan((R))] in human breast milk. Food Chem. Toxicol. 2007;45:125–129. doi: 10.1016/j.fct.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Desjardins C. Endocrine regulation of reproductive development and function in the male. J. Anim. Sci. 1978;47(Suppl 2):56–79. [PubMed] [Google Scholar]
- Fenton SE. Endocrine-disrupting compounds and mammary gland development: early exposure and later life consequences. Endocrinology. 2006;147(Suppl 6):S18–24. doi: 10.1210/en.2005-1131. [DOI] [PubMed] [Google Scholar]
- Golden R, Gandy J, Vollmer G. A review of the endocrine activity of parabens and implications for potential risks to human health. Crit. Rev. Toxicol. 2005;35:435–458. doi: 10.1080/10408440490920104. [DOI] [PubMed] [Google Scholar]
- Guillette LJ., Jr. Endocrine disrupting contaminants-beyond the dogma. Environ. Health Perspect. 2006;114(Suppl 1):9–12. doi: 10.1289/ehp.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PW, Darbre P. Endocrine disrupters and human health: could oestrogenic chemicals in body care cosmetics adversely affect breast cancer incidence in women? J. Appl. Toxicol. 2004;24:167–176. doi: 10.1002/jat.978. [DOI] [PubMed] [Google Scholar]
- Harvey PW, Everett DJ. Significance of the detection of esters of phydroxybenzoic acid (parabens) in human breast tumours. J. Appl. Toxicol. 2004;24:1–4. doi: 10.1002/jat.957. [DOI] [PubMed] [Google Scholar]
- Heidler J, Sapkota A, Halden RU. Partitioning, persistence, and accumulation in digested sludge of the topical antiseptic triclocarban during wastewater treatment. Environ. Sci. Technol. 2006;40:3634–3639. doi: 10.1021/es052245n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtman CJ, Van Oostveen AM, Brouwer A, Lamoree MH, Legler J. Identification of estrogenic compounds in fish bile using bioassay-directed fractionation. Environ. Sci. Technol. 2004;38:6415–6423. doi: 10.1021/es049750p. [DOI] [PubMed] [Google Scholar]
- Hovander L, Malmberg T, Athanasiadou M, Athanassiadis I, Rahm S, Bergman A, Wehler EK. Identification of hydroxylated PCB metabolites and other phenolic halogenated pollutants in human blood plasma. Arch. Environ. Contam. Toxicol. 2002;42:105–117. doi: 10.1007/s002440010298. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Suzuki T, Sanoh S, Kohta R, Jinno N, Sugihara K, Yoshihara S, Fujimoto N, Watanabe H, Ohta S. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol. Sci. 2005;84:249–259. doi: 10.1093/toxsci/kfi074. [DOI] [PubMed] [Google Scholar]
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999-2000: a national reconnaissance. Environ. Sci. Technol. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Lakeram M, Lockley DJ, Sanders DJ, Pendlington R, Forbes B. Paraben Transport and Metabolism in the Biomimetic Artificial Membrane Permeability Assay (BAMPA) and 3-Day and 21-Day Caco-2 Cell Systems. J. Biomol. Screen. 2006;12:84–91. doi: 10.1177/1087057106295383. [DOI] [PubMed] [Google Scholar]
- Lipworth L. Epidemiology of breast cancer. Eur. J. Cancer Prev. 1995;4:7–30. doi: 10.1097/00008469-199502000-00002. [DOI] [PubMed] [Google Scholar]
- Liu B, Wang Y, Fillgrove KL, Anderson VE. Triclosan inhibits enoylreductase of type I fatty acid synthase in vitro and is cytotoxic to MCF-7 and SKBr-3 breast cancer cells. Cancer Chemother. Pharmacol. 2002;49:187–193. doi: 10.1007/s00280-001-0399-x. [DOI] [PubMed] [Google Scholar]
- Lobemeier C, Tschoetschel C, Westie S, Heymann E. Hydrolysis of parabenes by extracts from differing layers of human skin. Biol. Chem. 1996;377:647–651. doi: 10.1515/bchm3.1996.377.10.647. [DOI] [PubMed] [Google Scholar]
- Maffini MV, Rubin BS, Sonnenschein C, Soto AM. Endocrine disruptors and reproductive health: the case of bisphenol-A. Mol. Cell. Endocrinol. 2006;254-255:179–186. doi: 10.1016/j.mce.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Massart F, Parrino R, Seppia P, Federico G, Saggese G. How do environmental estrogen disruptors induce precocious puberty? Minerva Pediatr. 2006;58:247–254. [PubMed] [Google Scholar]
- Nakada N, Tanishima T, Shinohara H, Kiri K, Takada H. Pharmaceutical chemicals and endocrine disrupters in municipal wastewater in Tokyo and their removal during activated sludge treatment. Water Res. 2006;40:3297–3303. doi: 10.1016/j.watres.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Nellemann C, Dalgaard M, Lam HR, Vinggaard AM. The combined effects of vinclozolin and procymidone do not deviate from expected additivity in vitro and in vivo. Toxicol. Sci. 2003;71:251–262. doi: 10.1093/toxsci/71.2.251. [DOI] [PubMed] [Google Scholar]
- Oishi S. Effects of propyl paraben on the male reproductive system. Food Chem. Toxicol. 2002a;40:1807–1813. doi: 10.1016/s0278-6915(02)00204-1. [DOI] [PubMed] [Google Scholar]
- Oishi S. Effects of butyl paraben on the male reproductive system in mice. Arch. Toxicol. 2002b;76:423–429. doi: 10.1007/s00204-002-0360-8. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Selective delivery of sex steroid hormones to tissues by albumin and by sex hormone-binding globulin. Oxf. Rev. Reprod. Biol. 1988;10:237–292. [PubMed] [Google Scholar]
- Pugazhendhi D, Pope GS, Darbre PD. Oestrogenic activity of p-hydroxybenzoic acid (common metabolite of paraben esters) and methylparaben in human breast cancer cell lines. J. Appl. Toxicol. 2005;25:301–309. doi: 10.1002/jat.1066. [DOI] [PubMed] [Google Scholar]
- Pugazhendhi D, Sadler AJ, Darbre PD. Comparison of the global gene expression profiles produced by methylparaben, n-butylparaben and 17beta-oestradiol in MCF7 human breast cancer cells. J. Appl. Toxicol. 2006;27:67–77. doi: 10.1002/jat.1200. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Irvine DS. How strong is the evidence of a link between environmental chemicals and adverse effects on human reproductive health? BMJ. 2004;328:447–451. doi: 10.1136/bmj.328.7437.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siiteri PK, Murai JT, Hammond GL, Nisker JA, Raymoure WJ, Kuhn RW. The serum transport of steroid hormones. Recent Prog. Horm. Res. 1982;38:457–510. doi: 10.1016/b978-0-12-571138-8.50016-0. [DOI] [PubMed] [Google Scholar]
- Soto AM, Maffini MV, Schaeberle CM, Sonnenschein C. Strengths and weaknesses of in vitro assays for estrogenic and androgenic activity. Best Pract. Res. Clin. Endocrinol. Metab. 2006;20:15–33. doi: 10.1016/j.beem.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Strunck E, Stemmann N, Hopert A, Wunsche W, Frank K, Vollmer G. Relative binding affinity does not predict biological response to xenoestrogens in rat endometrial adenocarcinoma cells. J. Steroid Biochem. Mol. Biol. 2000;74:73–81. doi: 10.1016/s0960-0760(00)00092-3. [DOI] [PubMed] [Google Scholar]
- Williams RH, Larsen PR. Williams textbook of endocrinology. Saunders; Philadelphia: 2003. [Google Scholar]
- Wong C, Kelce WR, Sar M, Wilson EM. Androgen receptor antagonist versus agonist activities of the fungicide vinclozolin relative to hydroxyflutamide. J. Biol. Chem. 1995;270:19998–20003. doi: 10.1074/jbc.270.34.19998. [DOI] [PubMed] [Google Scholar]
- Xu LC, Sun H, Chen JF, Bian Q, Qian J, Song L, Wang XR. Evaluation of androgen receptor transcriptional activities of bisphenol A, octylphenol and nonylphenol in vitro. Toxicology. 2005;216:197–203. doi: 10.1016/j.tox.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Bishop AM, Needham LL, Calafat AM. Quantification of the urinary concentrations of parabens in humans by on-line solid phase extraction-high performance liquid chromatography-isotope dilution tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006;844:53–59. doi: 10.1016/j.jchromb.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Yin D, He Y, Perera MA, Hong SS, Marhefka C, Stourman N, Kirkovsky L, Miller DD, Dalton JT. Key structural features of nonsteroidal ligands for binding and activation of the androgen receptor. Mol. Pharmacol. 2003;63:211–223. doi: 10.1124/mol.63.1.211. [DOI] [PubMed] [Google Scholar]