Abstract
Hearing loss can be caused by primary degeneration of spiral ganglion neurons or by secondary degeneration of these neurons after hair cell loss. The replacement of auditory neurons would be an important step in any attempt to restore auditory function in patients with damaged inner ear neurons or hair cells. Application of β-bungarotoxin, a toxin derived from snake venom, to an explant of the cochlea eradicates spiral ganglion neurons while sparing the other cochlear cell types. The toxin was found to bind to the neurons and to cause apoptotic cell death without affecting hair cells or other inner ear cell types as indicated by TUNEL staining, and, thus, the toxin provides a highly specific means of deafferentation of hair cells. We therefore used the denervated organ of Corti for the study of neuronal regeneration and synaptogenesis with hair cells and found that spiral ganglion neurons obtained from the cochlea of an untreated newborn mouse reinnervated hair cells in the toxin-treated organ of Corti and expressed synaptic vesicle markers at points of contact with hair cells. These findings suggest that it may be possible to replace degenerated neurons by grafting new cells into the organ of Corti.
Keywords: beta-bungarotoxin, apoptosis, spiral ganglion, transplantation
Sensorineural hearing loss can be caused by a loss of hair cells, the sound transducing sensory cells of the cochlea, or by loss of afferent neurons, the spiral ganglion neurons, that connect the hair cells to the cochlear nucleus in the brainstem. Degeneration of afferent neurons can occur secondarily to the loss of hair cells that normally supply trophic support to these neurons (Liberman and Kiang, 1978; Keithley and Croskrey, 1990) or from primary neuronal loss involving degeneration of neurons in the absence of hair cell degeneration (Keithley and Feldman, 1979; Suzuka and Schuknecht, 1988). This condition is termed auditory neuropathy when it occurs in the presence of functional hair cells in humans (Starr et al., 2000). Disruption of function studies in mice have shown that survival of spiral ganglion neurons is dependent on a number of genes such as neurotrophins (Ernfors et al., 1995; Hossain et al., 2002), components of the erbB pathway (Stankovic et al., 2004), and cAMP-dependent protein kinase (Bok et al., 2003). Other studies have shown that neuronal degeneration can be pronounced during aging in animals with mutations in the gene encoding the β2 subunit of the acetylcholine receptor (Bao et al., 2005) and in an age-related hearing loss gene, which is most likely cadherin 23 (White et al., 2000; Noben-Trauth et al., 2003).
After neuronal degeneration in the auditory system, unlike peripheral neurons of the motor system and some sensory systems, the auditory neurons do not regenerate to any clinically significant extent (Carnicero et al., 2002; Sekiya et al., 2003). However, the possibility that transplanted neurons could regenerate new connections to hair cells has not been explored. Transplantation of dorsal root ganglion neurons and neuronal stem cells demonstrated survival of neurons in the cochlea (Hu et al., 2005a, 2005b), but the processes from these cells extended to other neurons not to hair cells (Hu et al., 2005a). During development, the bipolar spiral ganglion neurons of the cochlea connect to the cochlear nucleus in the CNS and to the sensory cells of the organ of Corti through central and peripheral processes emanating from cell bodies that form the auditory ganglion. Two types of spiral ganglion neurons innervate the cochlear sensory epithelium: type I neurons synapse with single inner hair cells through unbranched processes and type II neurons synapse with several outer hair cells via branched processes. Some aspects of a peripheral process leading to a hair cell are similar to a dendrite, as the electrical signal from the hair cell is transmitted via this postsynaptic process to the spiral ganglion cell body, but other aspects of the peripheral process are more like an axon, as the process is myelinated, has nodes, and developmentally grows much like an axon to the hair cell target. Functional repair of the auditory nerve after injury would be possible only if the transplanted neurons reconstructed both the peripheral and the central connections.
We sought to test the ability of spiral ganglion neurons to grow processes and form connections with hair cells in a postnatal cochlea to determine whether the signals that guide the neurons to the hair cells in the embryo are intact. To do this we induced the degeneration of the spiral ganglion neurons in an organ of Corti explanted from one animal and then isolated neurons from a second animal and transferred them into the explanted organ of Corti. To induce the degeneration of the neurons we treated the organ of Corti explant with β-bungarotoxin, a toxin that was previously found to destroy the auditory neurons in chick embryos (Hirokawa, 1977).
METHODS
Organ of Corti In Vitro Culture
The cochlea was dissected using sterile conditions under a Zeiss Stemi 2000 dissection microscope. Organotypic explant cultures were prepared from the cochleas of 1 to 3 day postnatal C57BL/6 mice or Atoh1-nGFP transgenic mice that express green fluorescent protein under the control of the Atoh1 enhancer (Lumpkin et al., 2003). We used the methods described in detail previously (Sobkowicz et al., 1975) with some modifications, using four to five explants per condition. The head was bisected midsagittally and the cochlea removed and placed in ice cold Hank’s balanced salt solution (HBSS) (Invitrogen). Using two forceps the organ of Corti and the spiral ganglion tissue were gently freed from the capsule and separated from the stria vascularis. The organ of Corti was transferred using a wide-mouth pipette containing a small amount of HBSS from the dissection dish into a 4-well dish (Greiner Labortechnik) coated with fibronectin (BD Bioscience). The tissue was oriented so that the apical surfaces of the hair cells were pointing up and the basilar membrane was directed toward the fibronectin substrate. Excess medium was removed by aspiration. The explanted tissue was allowed to attach to the fibronectin substrate for 12–24 h in a 37°C incubator with 5% CO2 in a minimum volume of HBSS while avoiding drying of the tissue. Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen) and F12 (100 μL, mixed 1:1; Invitrogen), supplemented with N2, B27 (both from Invitrogen), and ampicillin (50 μg/mL), was deposited gently at the side of the tissue. After the tissue was attached, the plate was again placed in the 37°C incubator and the cultures were kept for up to 2 weeks in a humidified chamber with changes of medium every 2 days.
When dissection of the organ of Corti was performed using neurogenin (ngn) 1−/− mice (Ma et al., 2000), newborn animals were used (these mice do not survive for more than a day). The heterozygous mice (provided by Q. Ma, Dana Farber Cancer Center) were bred to obtain homozygotes that were identified by genotyping by polymerase chain reaction (PCR). All handling of animals was according to the National Institutes of Health Guide for the Care and Use of Laboratory of Animals.
Treatment of Organ of Corti with β-Bungarotoxin
For the selective elimination of the spiral ganglion neurons, medium for culture of the organ of Corti was supplemented with β-bungarotoxin (Biotium, Inc.) for 24–48 h at concentrations from 0.01 nM to 2 μM. The organ of Corti was fixed and examined by immunohistochemistry. Quantification of the outer hair cells, inner hair cells, and spiral ganglion neurons for four organs of Corti divided in segments of 100 μm was performed with Axiovision 4.3 software and the data subjected to analysis of variance (ANOVA) with significance inferred at a p value <0.01.
Binding of β-Bungarotoxin to Cells of the Organ of Corti
The toxin was labeled with tetramethylrhodamine-5-(and 6)-isothiocyanate (TRITC; Sigma) prior to addition to the tissue. The β-bungarotoxin (1 mg) was diluted in 200 μL of conjugation buffer (100 mM carbonate/bicarbonate buffer, pH 9), and the toxin was mixed with 35 μL of TRITC in dimethylsulfoxide in a 1.5 mL tube protected from light. After 2 h at room temperature, excess or hydrolyzed TRITC was removed by dialysis in a Slide-A-Lyzer dialysis cassette (Pierce) overnight. Dilutions from 200 to 10,000 of a solution of 212.7 μM TRITC-labeled β-bungarotoxin were added to the organ of Corti from Atoh1-nGFP transgenic mice at P0–P2. We assessed binding of TRITC-labeled β-bungarotoxin at intervals of 5 min to 2 h after labeling. Organotypic cultures were rinsed with PBS and fixed with 4% paraformaldehyde in PBS for 15 min at 4°C. After washing with PBS, the organ of Corti was examined with a fluorescence microscope.
TUNEL Assay for Cell Death in Organ of Corti Explants
The organ of Corti from a C57BL/6 mouse at P0 was treated with 0.5 μM β-bungarotoxin for 24 h. The tissue was washed with PBS and fixed with 4% paraformaldehyde in PBS for 10 min. DNA strand breaks were labeled by terminal deoxynucleotidyl transferase with TUNEL label mix (Roche Molecular Biochemicals). After two washes with PBS, 50 μL of the TUNEL reaction mixture was added to the sample. Negative controls were performed by adding 50 μL of the label solution (without terminal transferase) instead of the TUNEL reaction mixture and by TUNEL staining of the untreated organ of Corti. The organ of Corti was incubated in a 37°C incubator for 60 min and the reaction was stopped by three washes with PBS and analyzed by fluorescence microscopy.
Immunohistochemistry
Organs of Corti were fixed with 4% paraformaldehyde in PBS for 10 min. After three washes with PBS we permeabilized and blocked nonspecific binding sites with PBT1 solution [0.1% Triton X-100, 1% BSA (w/v), and 5% heat-inactivated goat serum in PBS] for 20 min. Fixed and permeabilized organs of Corti were incubated with primary antibodies overnight in antiserum diluted in PBT1. Dilutions used were 1:500 for mouse monoclonal TuJ antibody (β-III tubulin; Covance), 1:200 for polyclonal rabbit anti-neurofilament M (145 kD) (Chemicon International), 1:2000 for polyclonal rabbit antibody to parvalbumin 3, 1:500 for polyclonal rabbit anti-synapsin antibody (Chemi-con), and 1:500 for mouse monoclonal antibody to SV2 (Developmental Studies Hybridoma Bank). Specimens were washed three times for 15 min each with PBS. FITC, TRITC, and Cy5-conjugated anti-rabbit and anti-mouse secondary antibodies (Jackson ImmunoResearch) were used to detect primary antibodies. F-actin was labeled with TRITC-conjugated phalloidin (Sigma), and cell nuclei were stained by exposure to 4,6-diamidino-2-phenylindole with Vecta-shield (Vector Laboratories). Staining was visualized with epifluorescence microscopy (Axioskop 2 Mot Axiocam; Zeiss) or confocal microscopy (TCD; Leica).
Spiral Ganglion Neuron Isolation and Coculture with the In Vitro Organ of Corti
Cochlear cartilage was removed with fine forceps and the spiral ganglion tissue was separated from four to five organs of Corti and transferred to ice-cold HBSS. The neurons were from C57BL/6 mice or thy1-CFP mice in which the CFP gene is under the control of thy1 regulatory elements (Feng et al., 2000) resulting in neuronal expression. The tissue was used directly for coculture with the organ of Corti explant or was first dissociated to obtain the neurons. For this dissociation, the tissue was digested with trypsin in a 37°C incubator for 20 min (25 μL/cochlea). After the trypsinization was stopped by addition of DMEM/F12 containing 10% fetal bovine serum (25 μL/cochlea), the spiral ganglion tissue was triturated with a P200 micropipettor set at 180 μL by pipetting up and down 20–30 times gently. DMEM/F12 (1:1) supplemented with N2, B27, and ampicillin was added and the cell suspension was centrifuged at 800 rpm for 5 min. The supernatant was removed and medium (100 μL) containing NT3 (50 μg/mL) and BDNF (100 μg/mL) (both from Chemicon) was added to the cells.
The spiral ganglion neurons obtained from P0–P2 C57/BL6 mice or thy1-CFP mice (donors) were added to the denervated organ of Corti explant (recipient) in 100 μL of medium. The recipient organ of Corti was from P0–P2 mice, treated to eliminate spiral ganglion neurons and to leave the hair cells intact either by β-bungarotoxin treatment for 48 h, or from the ngn 1−/− mouse. The co-culture of cells and organ of Corti was carried out in a 4-well dish in a 37°C incubator for 2 days. Immunohistochemistry was performed to trace the donor cells after coculture.
RESULTS
Removal of Spiral Ganglion Neurons with Neurotoxin
The dissected organ of Corti retained its basic structure in vitro for periods of up to 2 weeks and innervation of the hair cells by the radial afferent processes from the spiral ganglion neurons remained intact as detected by immunohistochemistry using antibodies to β-III tubulin and parvalbumin 3 [Fig. 1(A)]. Increasing concentrations of β-bungarotoxin were tested to determine whether it was possible to destroy spiral ganglion neurons while sparing hair cells so that we could use the cochlear explant as an in vitro system for neural regeneration [Fig. 1(B–G)]. In these experiments we observed a dose-dependent induction of cell death by the toxin. At the lowest concentrations of the toxin tested (0.5 nM) most of the neurons at the base of the cochlea were destroyed, but the afferent processes at the apex of the cochlea remained intact after 48 h [Fig. 1(C)]. Neurons at the base of the cochlea were completely eliminated when the concentration was increased to 50 nM, but there were surviving neurons in the apex, indicating only partial destruction of neurons [Fig. 1(D)]. Treatment of the organ of Corti with a concentration of 0.5 μM β-bungarotoxin destroyed the spiral ganglion neurons throughout the cochlea, but did not affect the hair cells as assessed using hair cells that expressed GFP (Atoh1-nGFP mouse) and immunohistochemistry for β-III tubulin [Fig. 1(E)]. The spiral ganglion neurons were eliminated at all concentrations above 1 μM [Fig. 1(F,G)] but the number of outer hair cells was also decreased. As shown in Figure 1(F) and (G), the surviving hair cells continued to show green fluorescence from nGFP (Atoh1-nGFP mouse). These hair cells appeared intact in the absence of innervation for periods as long as 2 weeks in cultures treated with β-bungarotoxin. These data corroborate the finding that hair cell survival is independent of the innervation by spiral ganglion neurons (Hirokawa, 1977; Fritzsch et al., 1997).
Figure 1.
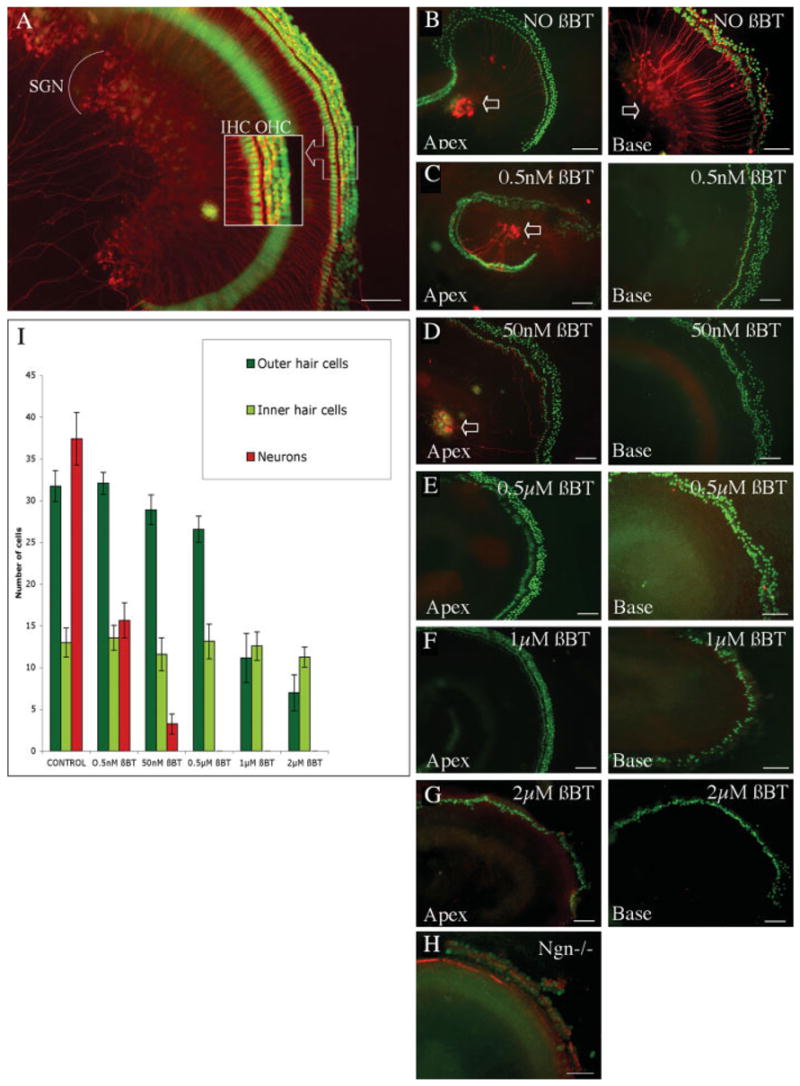
Organ of Corti explants and treatment of explants with β-bungarotoxin. Organ of Corti explant of a C57BL/6 mouse after 3 days in culture (A). Outer hair cells (OHC) and inner hair cells (IHC) were visualized with an antibody to parvalbumin 3 and secondary FITC-labeled antibody (shown in green), and spiral ganglion neurons (SGNs) were detected with an antibody to β-III tubulin and TRITC-labeled secondary antibody (shown in red). Inset shows a higher magnification of the hair cells. After treatment for 2 days (B–G) in increasing concentrations of toxin (β-BT) organs of Corti of Atoh1-nGFP transgenic mice were immunostained with an antibody to β-III tubulin detected with a secondary TRITC-labeled antibody (shown in red) and hair cell nuclei were detectable by nuclear green fluorescence. The apical turns of the cochlea (Apex) are shown in the left column and basal turns (Base) are shown in the right column. (B) Untreated control explants showing intact hair cells and spiral ganglion neurons. Neurons stained for β-III tubulin (indicated by arrows). (C) Treatment with 0.5 nM β-bungarotoxin. Note that neurons at the base of the organ of Corti were eliminated but the neurons of the apex were not lost. (D) Treatment with 50 nM β-bungarotoxin. Note complete elimination of neurons at the base and partial survival in the apical turns. (E) Treatment with 0.5 μM β-bungarotoxin. There was no evidence of neuronal survival whereas the outer and inner hair cells were intact. (F) Treatment with 1 μM β-bungarotoxin. At this concentration there was significant loss of outer hair cells in the base of the cochlea and complete elimination of neurons. (G) Treatment with 2 μM β-bungarotoxin. Note that outer hair cells were nearly completely eliminated but inner hair cells were retained. Neurons were completely absent. (H) Organ of Corti explant of a newborn ngn 1 knock-out mouse. Immunostaining as above for neurons and with parvalbumin 3 using a FITC-labeled secondary antibody (shown in green) and phalloidin labeled with TRITC (shown in red) for hair cells. There was a complete lack of spiral ganglion neurons and a reduced number of hair cells. Scale bars in (A–H) are 100 μm. (I) Quantification of surviving outer hair cells, inner hair cells, and neurons from organ of Corti of Atoh1-nGFP transgenic mouse in culture for 48 h with different concentrations of β-bungarotoxin. Data are expressed as the mean number of cells ± SD per 100 μm of explant length. Higher concentrations of the neurotoxin decreased the number of outer hair cells. Beta-bungarotoxin (0.5 μM) for 2 days eliminated the neurons without significant alteration in the number of hair cells. [Color figure can be viewed in the online issue, which is available at http://www.interscience.wiley.com.]
The cell survival, measured using expression of nGFP, decreased with increasing concentrations of β-bungarotoxin and cell counting indicated that the spiral ganglion neurons were markedly decreased even at concentrations of 0.5 nM, whereas inner hair cells were only slightly decreased at a concentration up to 2 μM and outer hair cells were not significantly decreased (ANOVA, p < 0.01) at concentrations up to 0.5 μM β-bungarotoxin [Fig. 1(I)]. The decrease in spiral ganglion neurons was statistically significant (p < 0.01). A concentration of 0.5 μM yielded an organ of Corti without detectable neuronal cell bodies and radial fibers but with complete survival of hair cells. This concentration was therefore selected for subsequent experiments.
The innervation of cochlear hair cells was completely lacking in newborn ngn 1 knock-out mice [Fig. 1(H)]. Like the β-bungarotoxin treated organ of Corti, there were no detectable neuronal cell bodies and radial fibers in the ngn 1−/− organ of Corti. However, this mouse was not used for our replacement studies, because there was an overall reduction in size of the inner ear, the modiolus was nearly absent, and the hair cells were reduced in number compared to the wild-type mouse.
Binding of β-Bungarotoxin by Spiral Ganglion Neurons
Beta-bungarotoxin binds to potassium channels on the surface of neurons (Herkert et al., 2001) and leads to cell death by increasing intracellular Ca++ concentrations. We labeled β-bungarotoxin with TRITC and tested the ability of the cochlear cell types to bind or take up the labeled molecule. TRITC-labeled β-bungarotoxin was rapidly and specifically associated with spiral ganglion neurons, and labeled the cell bodies of the neurons without evident distribution into neurites. At the lowest concentration used, extensive labeling of spiral ganglion cell bodies was observed after 30 min (Fig. 2). Controls without β-bungarotoxin showed no staining [Fig. 2(C,D)]. No binding of the labeled toxin was observed in hair cells, Schwann cells, or supporting cells during the labeling period.
Figure 2.
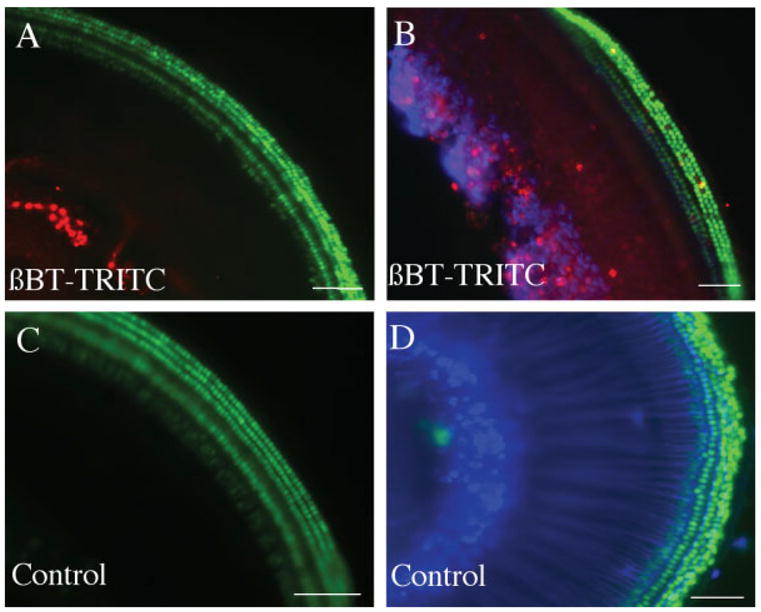
Treatment of newborn mouse organ of Corti with TRITC-labeled β-bungarotoxin. Organs of Corti of Atoh1-nGFP transgenic mouse were treated with 50 nM β-bungarotoxin labeled with TRITC (shown in red) for 30 min (A,B). The controls were treated with medium without β-bungarotoxin but containing TRITC (C,D). Tissue was fixed without further staining (A,C) or immunostained with antibody to β-III tubulin followed by a Cy5-labeled secondary antibody [blue, (B,D)]. Note labeling by β-bungarotoxin-TRITC in the spiral ganglion neurons. Scale bars are 100 μm. [Color figure can be viewed in the online issue, which is available at http://www.interscience.wiley.com.]
Apoptosis of Spiral Ganglion Neurons after β-Bungarotoxin Treatment
The organ of Corti after treatment with 0.5 μM β-bungarotoxin for 24 h was examined for cell death by TUNEL staining. Extensive staining of apoptotic nuclei was observed in neurons [Fig. 3(A)] after a 24 h treatment, but hair cells were not affected by the toxin. In a control without the terminal transferase, no staining was observed in the organ of Corti [Fig. 3(B)]. In a control without β-bungarotoxin treatment, neurons were not labeled by the TUNEL reagent [Fig. 3(C)].
Figure 3.
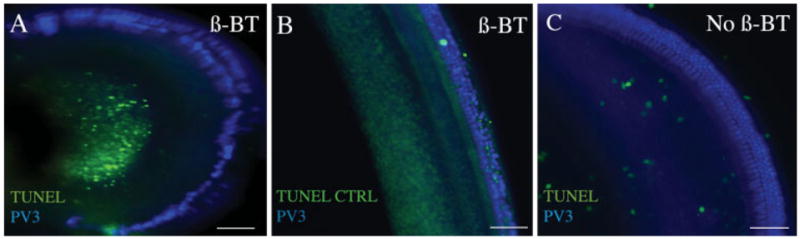
Organ of Corti explants treated with β-bungarotoxin and subjected to TUNEL assay. Organ of Corti of C57BL/6 mouse was treated with 0.5 μM β-bungarotoxin for 24 h (A). The treated sample was fixed and tested for TUNEL staining using FITC-labeled dUTP (green) and immunostained with parvalbumin 3 antibody and Cy5-conjugated secondary antibody (blue). (B) Organ of Corti of C57BL/6 mouse treated with control label solution (TUNEL CTRL) lacking terminal deoxynucleotidyl transferase, immunostained with parvalbumin 3 and Cy5-conjugated secondary antibody (blue). (C) A C57BL/6 mouse organ of Corti without β-bungarotoxin treatment was stained by the TUNEL procedure with FITC-labeled dUTP (green) and immunostained with parvalbumin 3 antibody (blue). The β-bungarotoxin treatment (A) caused neuronal cell death without death of the other cell types in the organ of Corti. Scale bars are 100 μm. [Color figure can be viewed in the online issue, which is available at http://www.interscience.wiley.com.]
Spiral Ganglion Neurons from Newborn Mice Send Out Neurites that Contact Hair Cells
The destruction of neurons and survival of hair cells in the organ of Corti after β-bungarotoxin treatment allowed us to test for reinnervation of hair cells by spiral ganglion neurons. Intact spiral ganglia from P1 mice were placed in close proximity to the organ of Corti that had been treated with β-bungarotoxin. The neurons sent out new processes that grew toward hair cells [Fig. 4(A)]. The new processes grew from the side or from the distal region of the organ of Corti and not in the radial direction taken by the original neurons that had been removed by the toxin. This finding was consistent and suggested a repulsion of the processes by the remaining tissue in the spiral lamina after removal of the spiral ganglion neurons. Dissociated spiral ganglion neurons transferred onto the deafferented organ of Corti also sent out neurites toward hair cells and formed terminal swellings once they had established contact with hair cells [Fig. 4(B)]. We found approximately 50 neurons making an average of 10 contacts with hair cells from the isolated neurons from one ear of a donor mouse. Similar connections between neurons from a donor mouse and hair cells in an explanted organ of Corti were found when neurons were taken from the thy1-CFP mouse. Staining of the neurons by both CFP and TuJ showed that the neurons had to originate from the donor mice [Fig. 4(C)].
Figure 4.
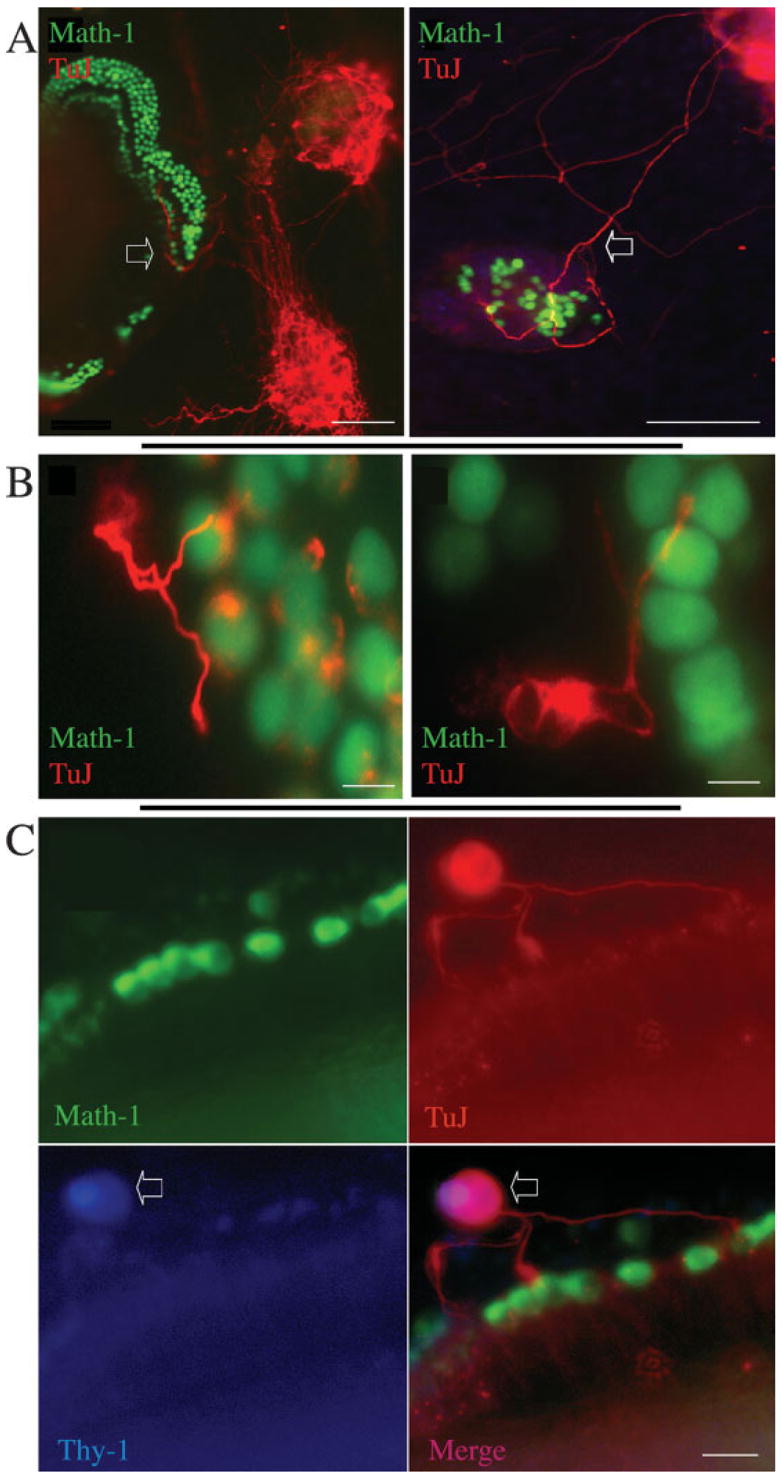
Coculture of spiral ganglion or dissociated neurons with the denervated organ of Corti. The organ of Corti of an Atoh1-nGFP transgenic mouse was treated with 0.5 μM β-bungarotoxin for 2 days followed by addition of spiral ganglion tissue dissected at P1 from a C57BL/6 mouse (A) and cultured for 24 h. Immunostaining was with a β-III tubulin antibody followed by a TRITC-labeled secondary antibody (red). Left: spiral ganglion neurons extended processes into the organ of Corti (indicated by the arrow). Right: some processes of the spiral ganglion neurons (arrow) displayed growth toward the hair cells and made contacts after growing across a space between the hair cells and the neurons. (B) The organ of Corti of an Atoh1-nGFP transgenic mouse was treated with 0.5 μM β-bungarotoxin for 2 days followed by addition of dissociated spiral ganglion dissected from a C57BL/6 mouse at P1. Immunostaining was with a β-III tubulin antibody followed by a TRITC-labeled secondary antibody (shown in red). Hair cells are shown in green expressing nGFP. Left: spiral ganglion neurons sent processes into the organ of Corti where they contacted hair cells. Right: a neurite from a spiral ganglion neuron formed a terminal swelling at a point of contact with a hair cell. (C) The organ of Corti from an Atoh1-nGFP mouse was treated with 0.5 μM β-bungarotoxin for 2 days followed by addition of dissociated spiral ganglion neurons from a thy1-CFP mouse. Hair cells were visualized by expression of nGFP (Math1), and cultures were immunostained with an antibody to β-III tubulin (TuJ) followed by a TRITC-labeled secondary antibody (shown in red) and an antibody to CFP followed by a Cy5-conjugated secondary antibody (Thy1). Arrows show a neuron from the thy1-CFP mouse (stained for β-III tubulin and CFP) whose processes contacted hair cells. Scale bars are 100 μm in (A); 15 μm in (B); 20 μm in (C). [Color figure can be viewed in the online issue, which is available at http://www.interscience.wiley.com.]
The occurrence of synaptic proteins in contacts between the spiral ganglion neurons and hair cells was evaluated using an antibody to synapsin (Fig. 5). The neurons that sent out processes into the deafferented organ of Corti had synapsin-positive swellings at the connections to hair cells, indicating the presence of synaptic vesicles forming at a presynaptic site [Fig. 5(C)]. Staining was not seen in hair cells under these conditions and was restricted to the terminal swellings of the neurons [Fig. 5(D)]. Some of the processes were branched and there was clear directional growth of each of these branches to individual hair cells. We observed that neurons contacting the outer hair cells branched to contact numerous targets, whereas branching in the inner hair cell area was more restricted; this pattern recapitulates one of the basic differences between the normal afferent innervation of inner and outer hair cells by type I and type II spiral ganglion cells, respectively (Berglund and Ryugo, 1987). The synaptic endings at the hair cells, furthermore, displayed SV2 immunoreactivity. Again, the staining was closely associated with hair cells, but this was due to the close apposition to the hair cell of the neuron and its terminal swelling [Fig. 6(C,D), open arrow]. The synaptic vesicle protein was almost exclusively found at points of contact with hair cells, and hair cells that had no synaptic contacts were not labeled [Fig. 6(D), closed arrow].
Figure 5.
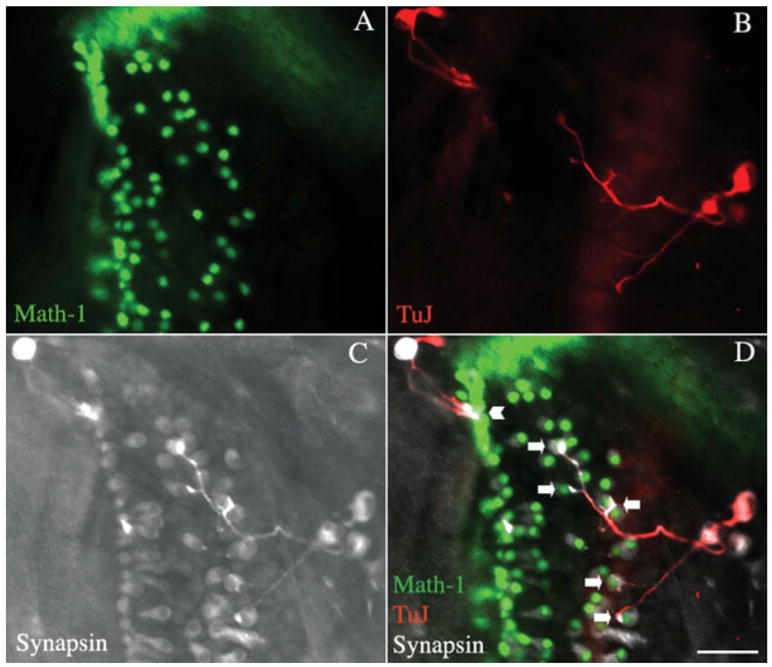
Assessment of synaptic markers at points of contact between spiral ganglion neurons and hair cells in a recipient organ of Corti. The organ of Corti of an Atoh1-nGFP transgenic mouse was treated with β-bungarotoxin (A), and after 2 days, spiral ganglion neurons obtained at P1 by dissociation of the tissue from a C57BL/6 mouse were added to the organ of Corti for 2 days. Hair cells visualized by endogenous fluorescence (green). (B) Staining with an antibody to β-III tubulin followed by a TRITC-labeled secondary antibody (shown in red). (C) Staining with antibody to synapsin detected with a Cy5-labeled secondary antibody (shown in white). (D) Merged image. The contacts between transplanted neurons and hair cells were immunopositive for synapsin. Neurons made several contacts with outer hair cells (arrows), while they made single contacts with inner hair cells (arrowhead). Scale bar is 30 μm. [Color figure can be viewed in the online issue, which is available at http://www.interscience.wiley.com.]
Figure 6.
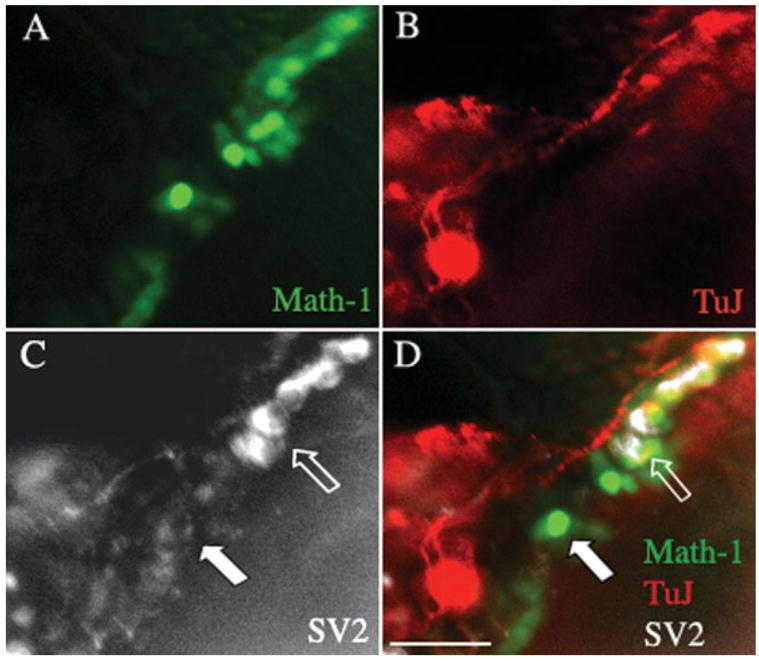
Staining of synaptic markers in neurons cocultured with a β-bungarotoxin treated organ of Corti. An organ of Corti from an Atoh1-nGFP transgenic mouse was treated with β-bungarotoxin (A) and dissociated spiral ganglion tissue from a P1 mouse was added for a 2-day culture. Hair cells were positive for endogenous GFP. (B) Staining with an antibody to β-III tubulin followed by a TRITC-labeled secondary antibody (shown in red). (C) SV2 antibody detected with a Cy5-labeled secondary antibody (shown in white). (D) Merged image. Strong staining for SV2 was seen at points of contact between neurons from the C57BL/6 mouse and hair cells from the Atoh1-nGFP mouse (open arrow). No staining was seen in hair cells that were not in contact with a neuron (solid arrow). Scale bar is 30 μm. [Color figure can be viewed in the online issue, which is available at http://www.interscience.wiley.com.]
DISCUSSION
We show here that spiral ganglion neurons placed into a cochlea that had been treated with a neurotoxin to remove afferent neurons could grow processes that reinnervated hair cells. Replacement of both hair cells and auditory neurons is an important goal in protocols attempting to regenerate an auditory response after cell degeneration in the inner ear, as sensorineural hearing loss can result from degeneration of hair cells, neurons, or both. Selective removal of cochlear afferent neurons with the toxin provides an ideal system in which to better understand auditory nerve regeneration after engraftment of new neurons. Although several specific means for killing hair cells are available, it has been difficult to examine the regeneration of the sensory neuron until a system was available in which the spiral ganglion neurons were lost and hair cells remained intact.
The need for a model system in which auditory neurons could form new connections with hair cells required that we destroy the afferent innervation and spare the hair cells. Spiral ganglion neurons have been reported to be destroyed by cisplatin (Ding et al., 1999; Lee et al., 2003), but this also results in damage to hair cells (Ding et al., 1999). Aminoglyco-side antibiotics destroy spiral ganglion neurons but this loss is secondary to the killing of hair cells (McFadden et al., 2004) and thus is not a useful model for nerve regeneration to hair cells. Oubain has been used to kill spiral ganglion neurons in a model of auditory nerve degeneration (Schmiedt et al., 2002; Lang et al., 2005) but the restricted killing of spiral ganglion neurons in the in vivo model requires that the toxin be infused directly onto the auditory nerve at some distance from the hair cells and the toxin would probably affect hair cells if it had access (Hamada and Kimura, 1999). Acetylsalicylic acid has also been reported to kill spiral ganglion neurons while sparing hair cells (Zheng and Gao, 1996). Mice with targeted deletions of genes that are needed for development of the sensory ganglia are potential models for an in vitro system for hair cell innervation, but some of these animals such as the trkB, trkC, NT-3, BDNF, Brn3a, and NeuroD knock-outs are not useful for these studies because, despite defects in formation or targeting of these neurons, they retain partial innervation of hair cells (Farinas et al., 1994; Ernfors et al., 1995; Schimmang et al., 1995; Huang et al., 2001; Kim et al., 2001), whereas others, such as the Brn3c knock-out, are not useful because they have incomplete development of functional hair cells (Xiang et al., 1997). Mice with a targeted deletion of the transcription factor ngn 1 (Ma et al., 2000) do not develop auditory neurons and are potentially useful as models for auditory nerve replacement, but, as we show here, the cochlea from this animal is not normal even at P0. Even if the hair cells were completely intact, the lack of development of auditory nerves as compared to loss after maturation of the neurons may be less representative of the human condition involving neuronal loss and, moreover, degenerating neurons may provide cues for the cells that would not be present in an ear that had never been innervated.
These studies of neuronal regeneration were possible because we were able to induce apoptosis in the afferent neurons with β-bungarotoxin. This toxin, from the Taiwanese banded krait, was described in the earliest studies as a toxin that poisoned the neuro-muscular junction by depleting presynaptic vesicles in the motor neuron, and these studies focused on its presynaptic activity on motor neuron endings. It has thus been considered a presynaptic toxin (Montecucco and Rossetto, 2000). The toxin consists of two polypeptides linked by disulfide bonds: subunit A, which is a Ca++-dependent phospholipase A2, and subunit B, which is a potassium channel binding subunit homologous to Kunitz domain protease inhibitors. The toxin is thought to act by binding to potassium channels and inducing an increase in intracellular Ca++ that leads to cell death (Herkert et al., 2001; Shakhman et al., 2003). We have found that spiral ganglion neurons are killed by β-bungarotoxin by a mechanism that involves specific binding to the neurons and induction of apoptosis. As the β-bungarotoxin is thought to bind to voltage-gated potassium channels (Herkert et al., 2001) the binding by spiral ganglion neurons indicates that these ion channels are expressed in these cells at the time of birth. The reason that hair cells were spared by the β-bungarotoxin is not clear, although we speculate that the receptors for the toxin are at a lower concentration on the surface of these cells in the early postnatal cochlea, before complete development of the hair cell synapse to afferent neurons. Hair cells express voltage-gated potassium channels that change their conductance characteristics in the time interval between birth and the onset of hearing (Kros et al., 1998) and therefore we expected some cell death in these cells as well. However, we found that β-bungarotoxin did not bind to hair cells or cause cell death under conditions in which it did destroy the neurons.
Uptake of β-bungarotoxin by receptor-meditated endocytosis after binding to potassium channels or to NMDA receptors (Tseng and Lin-Shiau, 2003) causes an increase in intracellular Ca++ concentration and generation of reactive oxygen species (Shakhman et al., 2003). In previous studies it had been noted that β-bungarotoxin destroyed the auditory ganglion neurons of the chick (Hirokawa, 1977). The peripheral process of the spiral ganglion neuron is the postsynaptic side of the hair cell synapse, and thus the toxin is acting on the postsynaptic side of the afferent synapse in the cochlea. These cells undergo apoptotic cell death, presumably due to an increase in intracellular Ca++ and an increase in oxygen radicals. The β-bungarotoxin model of spiral ganglion cell death is useful because apoptosis appears to be the mechanism by which the neurons are lost in vivo.
Formation of new synaptic connections in the in vitro situation that we have described requires that the neurons be able to find their targets and undergo synaptogenesis. The formation of new synaptic connections is the key step in regeneration of the auditory afferent system and will depend upon the ability of these neurons to recognize and connect to hair cells. We have shown in this study that afferent neurons connect with hair cells in this model of regeneration and that, at points of contact with hair cells at these nascent synapses, the neurons express synapsin and SV2. Whether these are functional synapses will have to be determined in future studies by testing the electrophysiological response of the neurons to hair cell stimulation. The route taken by the spiral ganglion neurons that contacted outer hair cells was branched, in contrast to the direct, unbranched path taken by spiral ganglion neurons to inner hair cells, and this suggested some specificity in the pathfinding as these patterns are similar to the pattern observed in animals. Our observation of connections between these cells and apparent synapse formation is novel, because it has not been shown that neurons can form new synapses with hair cells that had lost their afferent innervation, and it does suggest, moreover, the possibility of regeneration of the primary afferent neurons of this sensory system by implantation of new neurons. Little regeneration occurs spontaneously after the death of spiral ganglion neurons (Carnicero et al., 2002; Sekiya et al., 2003), and even damage to the peripheral processes of these neurons, which have limited capacity for regrowth, can lead to hearing loss (Nadol, 1997; Puel et al., 1997).
The processes from the spiral ganglion neurons that contact hair cells appear to have some properties of presynaptic fibers, including synapsin staining and SV2 staining. The nature of the neuronal process that extends to the hair cells is of interest because the process is a dendrite based on the conductance of the signal from the hair cells to the central nervous system, and yet, during development the process extends to the hair cell much like a peripheral axon, which it resembles histologically. Previous work has indicated that the processes of vestibular ganglion neurons (Scarfone et al., 1991) stain for synapsin and synaptophysin during development, and it is possible that the identity of this peripheral process is only acquired after it is connected to the hair cell. Initial outgrowth of neurites from other neurons takes place without specifying their identity as axon or dendrite until one process takes on an axonal identity and expresses axonal markers (Goslin and Banker, 1989). The establishment of polarization appears to be controlled by the inactivation of GSK-3β in axons but not dendrites (Jiang et al., 2005), and by activation of the small GTPases Rap1 and Cdc42 (Schwamborn and Puschel, 2004). The auditory neurons likely had not acquired their full bipolar character when we transplanted them and therefore may express axonal specializations in the growing neurites, and these may only become afferent peripheral processes when they make a connection to the hair cell. The pathfinding ability of the neurons may depend on use of guidance signals that are used by growing axons so that the neural process can respond to cues from the hair cell. In future experiments we plan to investigate whether these processes lose their synaptic vesicles as they become the postsynaptic side of a functional synapse with the hair cell and we will assess the ability of stem cell-derived neurons to replace damaged spiral ganglion neurons.
Acknowledgments
The authors thank Qiufu Ma for the neurogenin 1 knock-out mice and Jane E. Johnson for the Atoh1-nGFP mice.
Contract grant sponsor: Programa nacional para la movilidad de profesores de universidad e investigadores, Secretaria de Estado de Educacion y Universidades, Spain.
Contract grant sponsor: National Institutes of Health; contract grant number: F33 DC006789; contract grant number: R01 DC007174; contract grant number: R01 DC006167; contract grant number: P30 DC05209.
Contract grant sponsor: Hamilton H. Kellogg and Mildred H. Kellogg Charitable Trust.
References
- Bao J, Lei D, Du Y, Ohlemiller KK, Beaudet AL, Role LW. Requirement of nicotinic acetylcholine receptor subunit beta2 in the maintenance of spiral ganglion neurons during aging. J Neurosci. 2005;25:3041–3045. doi: 10.1523/JNEUROSCI.5277-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund AM, Ryugo DK. Hair cell innervation by spiral ganglion neurons in the mouse. J Comp Neurol. 1987;255:560–570. doi: 10.1002/cne.902550408. [DOI] [PubMed] [Google Scholar]
- Bok J, Zha XM, Cho YS, Green SH. An extra-nuclear locus of cAMP-dependent protein kinase action is necessary and sufficient for promotion of spiral ganglion neuronal survival by cAMP. J Neurosci. 2003;23:777–787. doi: 10.1523/JNEUROSCI.23-03-00777.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicero E, Knipper M, Tan J, Alonso MT, Schimmang T. Herpes simplex virus type 1-mediated transfer of neurotrophin-3 stimulates survival of chicken auditory sensory neurons. Neurosci Lett. 2002;321:149–152. doi: 10.1016/s0304-3940(01)02501-0. [DOI] [PubMed] [Google Scholar]
- Ding DL, Wang J, Salvi R, Henderson D, Hu BH, McFadden SL, Mueller M. Selective loss of inner hair cells and type-I ganglion neurons in carboplatin-treated chinchillas. Mechanisms of damage and protection. Ann NY Acad Sci. 1999;884:152–170. doi: 10.1111/j.1749-6632.1999.tb08640.x. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Van De Water T, Loring J, Jaenisch R. Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron. 1995;14:1153–1164. doi: 10.1016/0896-6273(95)90263-5. [DOI] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Backus C, Wang XY, Reichardt LF. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369:658–661. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Farinas I, Reichardt LF. Lack of neurotrophin 3 causes losses of both classes of spiral ganglion neurons in the cochlea in a region-specific fashion. J Neurosci. 1997;17:6213–6225. doi: 10.1523/JNEUROSCI.17-16-06213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin K, Banker G. Experimental observations on the development of polarity by hippocampal neurons in culture. J Cell Biol. 1989;108:1507–1516. doi: 10.1083/jcb.108.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M, Kimura RS. Morphological changes induced by administration of a Na+, K+-ATPase inhibitor in normal and hydropic inner ears of the guinea pig. Acta Otolaryngol. 1999;119:778–786. doi: 10.1080/00016489950180423. [DOI] [PubMed] [Google Scholar]
- Herkert M, Shakhman O, Schweins E, Becker CM. Beta-bungarotoxin is a potent inducer of apoptosis in cultured rat neurons by receptor-mediated internalization. Eur J Neurosci. 2001;14:821–828. doi: 10.1046/j.0953-816x.2001.01699.x. [DOI] [PubMed] [Google Scholar]
- Hirokawa N. Disappearance of afferent and efferent nerve terminals in the inner ear of the chick embryo after chronic treatment with beta-bungarotoxin. J Cell Biol. 1977;73:27–46. doi: 10.1083/jcb.73.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain WA, Brumwell CL, Morest DK. Sequential interactions of fibroblast growth factor-2, brain-derived neurotrophic factor, neurotrophin-3, and their receptors define critical periods in the development of cochlear ganglion cells. Exp Neurol. 2002;175:138–151. doi: 10.1006/exnr.2002.7872. [DOI] [PubMed] [Google Scholar]
- Hu Z, Ulfendahl M, Olivius MP. NGF stimulates extensive neurite outgrowth from implanted dorsal root ganglion neurons following transplantation into the adult rat inner ear. Neurobiol Dis. 2005a;18:184–192. doi: 10.1016/j.nbd.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Hu Z, Wei D, Johansson CB, Holmstrom N, Duan M, Frisen J, Ulfendahl M. Survival and neural differentiation of adult neural stem cells transplanted into the mature inner ear. Exp Cell Res. 2005b;302:40–47. doi: 10.1016/j.yexcr.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Liu W, Fritzsch B, Bianchi LM, Reichardt LF, Xiang M. Brn3a is a transcriptional regulator of soma size, target field innervation and axon pathfinding of inner ear sensory neurons. Development. 2001;128:2421–2432. doi: 10.1242/dev.128.13.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Guo W, Liang X, Rao Y. Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3beta and its upstream regulators. Cell. 2005;120:123–135. doi: 10.1016/j.cell.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Croskrey KL. Spiral ganglion cell endings in the cochlear nucleus of young and old rats. Hear Res. 1990;49:169–177. doi: 10.1016/0378-5955(90)90103-v. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Feldman ML. Spiral ganglion cell counts in an age-graded series of rat cochleas. J Comp Neurol. 1979;188:429–442. doi: 10.1002/cne.901880306. [DOI] [PubMed] [Google Scholar]
- Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, et al. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128:417–426. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kros CJ, Ruppersberg JP, Rusch A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature. 1998;394:281–284. doi: 10.1038/28401. [DOI] [PubMed] [Google Scholar]
- Lang H, Schulte BA, Schmiedt RA. Ouabain Induces Apoptotic Cell Death in Type I Spiral Ganglion Neurons, but not Type II Neurons. J Assoc Res Otolaryngol. 2005;6:63–74. doi: 10.1007/s10162-004-5021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Nakagawa T, Kim TS, Iguchi F, Endo T, Dong Y, Yuki K, et al. A novel model for rapid induction of apoptosis in spiral ganglions of mice. Laryngoscope. 2003;113:994–999. doi: 10.1097/00005537-200306000-00015. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Kiang NY. Acoustic trauma in cats. Cochlear pathology and auditory-nerve activity. Acta Otolaryngol Suppl. 1978;358:1–63. [PubMed] [Google Scholar]
- Lumpkin EA, Collisson T, Parab P, Omer-Abdalla A, Haeberle H, Chen P, Doetzlhofer A, et al. Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expr Patterns. 2003;3:389–395. doi: 10.1016/s1567-133x(03)00089-9. [DOI] [PubMed] [Google Scholar]
- Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1:129–143. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Jiang H, Salvi RJ. Time course of efferent fiber and spiral ganglion cell degeneration following complete hair cell loss in the chinchilla. Brain Res. 2004;997:40–51. doi: 10.1016/j.brainres.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Montecucco C, Rossetto O. How do presynaptic PLA2 neurotoxins block nerve terminals? Trends Biochem Sci. 2000;25:266–270. doi: 10.1016/s0968-0004(00)01556-5. [DOI] [PubMed] [Google Scholar]
- Nadol JB., Jr Patterns of neural degeneration in the human cochlea and auditory nerve: implications for cochlear implantation. Otolaryngol Head Neck Surg. 1997;117:220–228. doi: 10.1016/s0194-5998(97)70178-5. [DOI] [PubMed] [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet. 2003;35:21–23. doi: 10.1038/ng1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel JL, d’Aldin C, Ruel J, Ladrech S, Pujol R. Synaptic repair mechanisms responsible for functional recovery in various cochlear pathologies. Acta Otolaryngol. 1997;117:214–218. doi: 10.3109/00016489709117773. [DOI] [PubMed] [Google Scholar]
- Scarfone E, Dememes D, Sans A. Synapsin I and Synaptophysin expression during ontogenesis of the mouse peripheral vestibular system. J Neurosci. 1991;11:1173–1181. doi: 10.1523/JNEUROSCI.11-05-01173.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmang T, Minichiello L, Vazquez E, San Jose I, Giraldez F, Klein R, Represa J. Developing inner ear sensory neurons require TrkB and TrkC receptors for innervation of their peripheral targets. Development. 1995;121:3381–3391. doi: 10.1242/dev.121.10.3381. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA, Okamura HO, Lang H, Schulte BA. Ouabain application to the round window of the gerbil cochlea: a model of auditory neuropathy and apoptosis. J Assoc Res Otolaryngol. 2002;3:223–233. doi: 10.1007/s1016200220017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwamborn JC, Puschel AW. The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity. Nat Neurosci. 2004;7:923–929. doi: 10.1038/nn1295. [DOI] [PubMed] [Google Scholar]
- Sekiya T, Shimamura N, Yagihashi A, Suzuki S. Effect of topically applied basic fibroblast growth factor on injured cochlear nerve. Neurosurgery. 2003;52:900–907. doi: 10.1227/01.neu.0000053509.98561.16. [DOI] [PubMed] [Google Scholar]
- Shakhman O, Herkert M, Rose C, Humeny A, Becker CM. Induction by beta-bungarotoxin of apoptosis in cultured hippocampal neurons is mediated by Ca(2+)-dependent formation of reactive oxygen species. J Neurochem. 2003;87:598–608. doi: 10.1046/j.1471-4159.2003.02035.x. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, Bereman B, Rose JE. Organotypic development of the organ of Corti in culture. J Neurocytol. 1975;4:543–572. doi: 10.1007/BF01351537. [DOI] [PubMed] [Google Scholar]
- Stankovic K, Rio C, Xia A, Sugawara M, Adams JC, Liberman MC, Corfas G. Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J Neurosci. 2004;24:8651–8661. doi: 10.1523/JNEUROSCI.0733-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr A, Sininger YS, Pratt H. The varieties of auditory neuropathy. J Basic Clin Physiol Pharmacol. 2000;11:215–230. doi: 10.1515/jbcpp.2000.11.3.215. [DOI] [PubMed] [Google Scholar]
- Suzuka Y, Schuknecht HF. Retrograde cochlear neuronal degeneration in human subjects. Acta Otolaryngol Suppl. 1988;450:1–20. doi: 10.3109/00016488809098973. [DOI] [PubMed] [Google Scholar]
- Tseng WP, Lin-Shiau SY. Activation of NMDA receptor partly involved in beta-bungarotoxin-induced neurotoxicity in cultured primary neurons. Neurochem Int. 2003;42:333–344. doi: 10.1016/s0197-0186(02)00118-3. [DOI] [PubMed] [Google Scholar]
- White JA, Burgess BJ, Hall RD, Nadol JB. Pattern of degeneration of the spiral ganglion cell and its processes in the C57BL/6J mouse. Hear Res. 2000;141:12–18. doi: 10.1016/s0378-5955(99)00204-x. [DOI] [PubMed] [Google Scholar]
- Xiang M, Gan L, Li D, Chen ZY, Zhou L, O’Malley BW, Jr, Klein W, et al. Essential role of POU-domain factor Brn-3c in auditory and vestibular hair cell development. Proc Natl Acad Sci USA. 1997;94:9445–9450. doi: 10.1073/pnas.94.17.9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Differential damage to auditory neurons and hair cells by ototoxins and neuroprotection by specific neurotrophins in rat cochlear organotypic cultures. Eur J Neurosci. 1996;8:1897–1905. doi: 10.1111/j.1460-9568.1996.tb01333.x. [DOI] [PubMed] [Google Scholar]


