Abstract
This study examined top-down and bottom-up control of attention in a group of 24 patients with schizophrenia and 16 healthy volunteers. Participants completed a visual search task in which they reported whether a target oval contained a gap. The target was accompanied by 5, 11, or 17 distractors. On some trials, the target was identified by a highly salient feature that was shared by only 2 distractors, causing this feature to “pop out” from the display. This feature provided strong bottom-up information that could be used to direct attention to the target. On other trials, half of the distractors contained this distractor, making these distractors no more salient than the other distractors requiring greater use of top-down control to restrict processing to items containing this feature. Patient visual search efficiency closely approximated control performance in the first trial type. In contrast, patients demonstrated significant slowing of search in the second trial type, which required top-down control. These results suggest schizophrenia does not impair the ability to implement the selection of a target when attention can be guided by bottom-up information, but it does impair the ability to use top-down control mechanisms to guide attention. These results extend prior studies that have focussed on aspects of executive control in complex tasks and suggest that a similar underlying deficit may also impact the performance of perceptual systems.
Keywords: schizophrenia, attention, visual search, executive control
1. Introduction
The idea that schizophrenia involves a compromise of attention has been one of the guiding notions of the field, beginning with the original clinical descriptions of the disorder, and has motivated hundreds of experimental studies. Cumulative progress has been slow because the concept of attention has been used to refer to a wide array of behavioral phenomenon in the clinical literature (Mirsky et al., 1995; Zubin, 1975). In the present study, we sought to assess impairments in a specific aspect of attention, namely the top-down control of visual selective attention, which has been extensively studied in the cognitive literature and can be isolated in the context of visual search tasks.
In search tasks, an observer searches for a predefined target in an array containing distracter objects, and the primary dependent variable is the slope of the function relating reaction time (RT) to the number of items in the display (the set size). This function reflects the increment in RT caused by adding an additional item to the stimulus array. Search is called efficient if this slope is near zero, because this means that the individual can rapidly determine whether each added item is a target or a distractor; it is called inefficient if the slope is steep, which means that additional distractors cannot easily be rejected. Any factors that influence processes before the search process (e.g., low-level sensory encoding) or after the search processes (e.g., response selection) influence the y-intercept of this function, so the slope provides a pure measure of the efficiency of the search process.
There is broad agreement that search becomes efficient when the target-distractor similarity is low and distractor-distractor similarity is high (Duncan and Humphreys, 1989; Wolfe, 1998). When the distractors are identical or similar to each other and the target is highly distinctive (e.g., a red target among blue distractors), the target appears to “pop-out” from the display, allowing attention to be rapidly directed to the target with minimal scanning of the distractors, yielding a shallow search slope
In contrast, when the target and distractors are so similar that they cannot be distinguished without focused attention, RTs increase appreciably with each additional item added to the search array, resulting in a steep slope (typically between 25–100 ms/item). In this case, attention may shift at random from object to object until the target is found (Woodman and Luck, 1999; Woodman and Luck, 2003). Because the search is random, top-down control is even less important under these conditions than it is for pop-out targets.
In a third variety of search, shown in Figure 1, the target is defined by two highly discriminable features (e.g., color and orientation), and the target shares one of these features with each of the distractors. In this case, the target does not contain a distinctive feature that allows it to pop out from the background. However, if subjects can restrict search to the items that contain one of the target’s features (e.g., searching just the red items or just the horizontal items), search need not be completely random, and the search slope can be fairly shallow (Treisman and Sato, 1990; Wolfe, 1994; Wolfe et al., 1989). In this case, all the distractors have equivalent levels of bottom-up salience, but strong top-down control can make search efficient.
Figure 1.
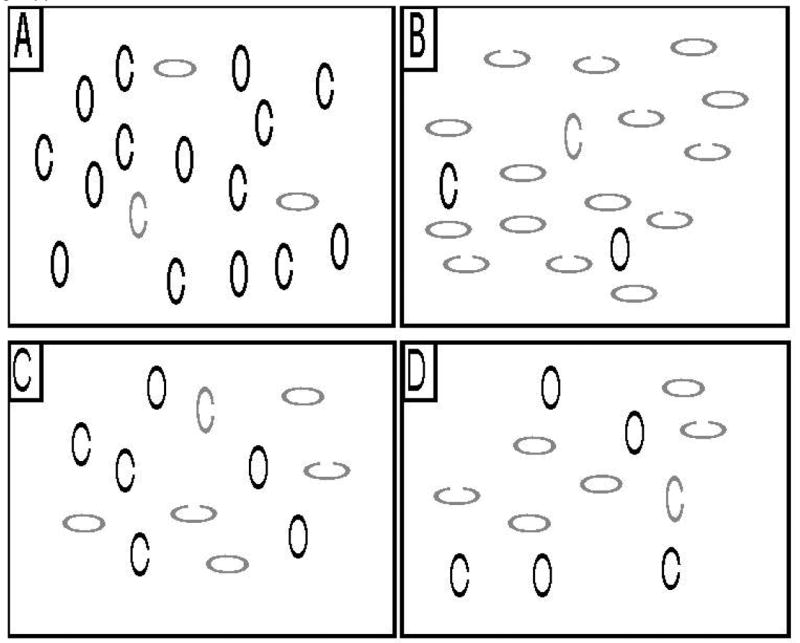
In each panel the target is the red (gray) vertical oval. Subjects indicated the presence or absence of a gap in the target. In panels A and C, participants were instructed to search the red (gray) items. In panels B and D, participants were instructed to search the vertical items. Panels A and B are examples of a 3-attended trials. Panels C and D are examples of half-attended trials.
Previous studies of visual search in schizophrenia have yielded mixed results. Some have found impairments in efficient “pop out” tasks (Carr et al., 1998; Lieb et al., 1994) as well as in inefficient feature conjunction tasks (Carr et al., 1998), while others have failed to find deficits in either type of task (Hess et al., 1992; Mori et al., 1996). Other evidence suggestive of search abnormalities has come from a variety of paradigms involving very brief stimulus presentations such as Span of Apprehension (Asarnow et al., 1991) and studies stressing perceptual organization or latent inhibition (Knight et al., 2000; Lubow, 2005; Lubow et al., 2000; Place and Gilmore, 1980; Silverstein et al., 2000; Wells and Leventhal, 1984). However, the integrity of visual search is difficult to isolate given the cognitive complexity of these paradigms.
We recently reported that that the timing and behavioral efficacy of an initial shift of attention, measured both behaviorally and electrophysiologically, was surprisingly normal in patients with schizophrenia when searching for a highly salient target item (Luck et al., 2006). That is, strong bottom-up signals led to equally rapid shifts of attention in patients and control subjects, indicating that the basic mechanisms for implementing shifts of attention are intact in patients. In another study, we found evidence that top-down control of attention is impaired (Fuller et al., 2006). Specifically, patients demonstrated the greatest proportional impairment relative to controls in a task that required subjects to search for a well-defined feature that was relatively low in salience, which presumably required top-down control. In contrast, patients were less impaired (proportionally) in a task in which the target and distractors were so similar that a random search was necessary. The pattern of results across studies suggests that the locus of schizophrenia impairment is in the top-down control of attention rather than in the actual operation of attention once a location has been selected. That is, it appears that patients are able to shift attention and filter distractors effectively when guided by bottom-up information (i.e., when attention is guided by relatively automatic processes), but have difficulty limiting their search to the most likely target item when bottom-up cues are not sufficiently salient (i.e., when attention is guided by controlled processes).
The present experiment was designed to provide a direct test of this formulation by using a single task in which a subtle trial-to-trial manipulation of the stimuli leads to variations in the relative contributions of bottom-up and top-down control. The two task conditions, shown in Figure 1, were adapted from the study of Egeth et al. (1984). On every trial, observers were asked to decide whether a target oval did or did not have a gap: a target was present in each array to minimize the speed-accuracy tradeoffs that occur in target present-absent search tasks. Unlike the Span of Apprehension, the search display remained visible until the subject responded. The target was defined by a combination of color and orientation (e.g., red horizontal), and each distractor contained one of these two feature values (e.g., red vertical or blue horizontal). For one kind of trials (called 3-attended trials; Figure 1A and 1B), the target item was defined by a highly salient feature value (e.g., red) that was shared by only 2 distractors, regardless of the set size. Thus, an ideal observer would restrict search to the 3 red items. The set size was manipulated by varying the number of distractors that did not contain this value (e.g., 3 red items were accompanied by 3, 9, or 15 blue items). The 3-attended trials are a variant of a pop-out task, with 3 rather than 1 item defined by a salient feature. Even with a severe impairment of attentional control, the bottom-up salience of these three items should cause them to attract attention rapidly and should lead to a shallow search slope. That is, the salience of these items should minimize the need to use top-down control mechanisms to direct attention to the appropriate subset of items. However, it will still be necessary to implement attentional suppression of the unattended items. Thus, performance in this condition is largely a reflection of the effectiveness of attention once it has been directed to a set of items, with minimal contributions from attentional control.
In the other trials (called half-attended trials), half of the items shared a salient feature with the target (see Figure 1C and 1D). With equal numbers of relevant items (e.g., red) and irrelevant items (e.g., blue), there is no bottom-up or salience advantage that would lead to preferential processing of the relevant items. To perform efficiently in these trials, observers must use top-down control to restrict attention to the items containing the relevant feature in order to make the gap discrimination and avoid searching the other items. Given the equal bottom-up salience of potential targets and distractor items in the half-attended trials, this condition demands far more top-down control than the attend-3 trials. An increased search slope for half-attended trials but not for 3-attended trials would indicate that attentional control is impaired (leading to a deficit on half-attended trials) but that the implementation of attentional selection is intact (leading to spared performance on 3-attended trials).
2. Method
2.1 Participants
The participants included 24 patients meeting Diagnostic and Statistical Manual of Mental Disorders (4th ed., DSM–IV) (American Psychiatric Association, 1994) criteria for schizophrenia (9 paranoid and 12 undifferentiated type) or schizoaffective disorder (3), along with 16 healthy control subjects. Diagnosis was established for each patient using a best estimate approach combining information from past medical records, collateral informants, and the results of a Structured Clinical Interview for DSM-IV (SCID) (First et al., 1997a, 1997b). The patients were clinically stable outpatients, receiving the same medication, at the same dose, for at least 4 weeks prior to study participation: 18 were receiving second generation antipsychotic medication and 6 were receiving traditional antipsychotic medication.
Healthy controls were recruited from the community via newspaper advertisements, word of mouth, or random digit dialing and were screened using the complete SCID-I (SCID I: First et al., 1997a) and SCID-II (SCID II: First et al., 1997b). The controls were free of a current or past history of major psychiatric illness and denied a family history of psychotic disorders.
Demographic features are shown in Table 1. The two groups did not differ on demographic variables other than Wide Range Achievement Test (WRAT 3: Wilkinson, 1993) scores. All subjects provided written informed consent and were compensated for participating.
Table 1.
Subject Demographics
| Patients | Controls | |
|---|---|---|
| Age | 44.9 (8.1) | 45.4 (11.6) |
| Education1 | 13.0 (2.8) | 14.5 (2.3) |
| Paternal Education2 | 14.6 (3.8) | 13.5 (4.3) |
| WRAT 3 † | 93.1 (15.9) | 104.15 (5.4) |
| Race | ||
| African American | 7 | 4 |
| Caucasian | 15 | 11 |
| Hispanic | 1 | 0 |
| Native American | 0 | 1 |
| Other | 1 | 0 |
| Handedness (L: R) | 2:22 | 1:15 |
| Sex (Male: Female) | 18:6 | 9:7 |
data missing for one patient.
data missing for one patient and one control.
p < 0.05
L = left hand, R = right hand, WRAT = Wide Range Achievement Test (WRAT 3: Wilkinson, 1993)
2.2 Stimuli and Procedure
Six, twelve or eighteen stimuli were presented on a video monitor with a gray background viewed at a distance of 57 cm. Stimuli were horizontal or vertical outlined ovals that were red or blue and had a gap (0.30°) in one side or did not have a gap. The ovals subtended 1.51° × 0.57° with a line thickness of 0.15°. Stimuli were positioned randomly within a 12.10° × 12.10° region, with a minimum center-to-center distance of 2.27°. The target was defined by a particular combination of color and orientation (e.g., blue horizontal); the specific combination was assigned at random to each participant. Each display contained this target along with 5, 11, or 17 distractors. Each distracter contained either the same color or the same orientation as the target, and the other dimension differed from the target (i.e., blue vertical and red horizontal). Subjects searched for their assigned target and determined whether it contained a gap (which was present on 50% of trials). Subjects indicated the presence or absence of a gap by pushing one of two buttons on a game controller. Each array remained on the screen until a response was made, and a 500-ms blank interval was interposed between the response and the onset of the next array.
The experiment was divided into attend-color and attend-orientation blocks, tested in random order. Subjects were instructed to restrict their search to items that shared the to-be-attended feature. Within these blocks, 3-attended and half-attended trials were randomly intermixed. On the 3-attended trials, three of the stimuli (including the target) shared the attended feature value, regardless of set size. In the half-attended trials, in contrast, half of the items contained the attended feature value and half contained the other value. In this design, participants are motivated to attend to the instructed feature value because it facilitates performance on 3-attended trials by serving to restrict search to the most likely target locations, thereby avoiding most of the distractors. It also allows the participants to avoid half of the distractors on the half-attended trials.
Extensive explicit instructions and demonstration displays were provided prior to each condition, followed by a practice block. Instructions for each condition were identical save for which dimensions were described as being to-be-attended and to-be-ignored. For each feature condition (attend-color and attend-orientation), there were 2 blocks containing 156 trials each.
2.3 Data Analysis
Mean RTs (for correct trials only) were computed for each subject after removing outlier trials using the method of Van Selst & Jolicoeur (1994). To measure the efficiency of the search process independently of any between-group differences in overall RTs, the RTs across the three set sizes for a given condition were converted into slope values (using the slope of the least-squares best-fit linear function). These slope values were analyzed using a group (patients vs. controls) by trial type (3-attended vs. half-attended) ANOVA, collapsed across the attend-orientation and attend-color conditions (analyses revealed that the orientation vs. color factor did not have a significant main effect; F= 1, 38=.90, p=.40; nor was there any evidence of an interaction with trial type; F= 1, 38 1.15, p=.22, or with the group by dimension by trial type interaction; F 1, 38 =.05, p=.83). The goodness of fit of the linear slopes was quite high on half-attended trials and was nearly identical for the patient group (mean r2 = .88) and the control group (mean r2 = .91). The goodness of fit was lower on the 3-attended trials due to the low slopes in this condition, but was still adequate and similar across the patient and control groups (mean r2 = .54 and .60, respectively).
3. Results
3.1 Reaction Time and Accuracy
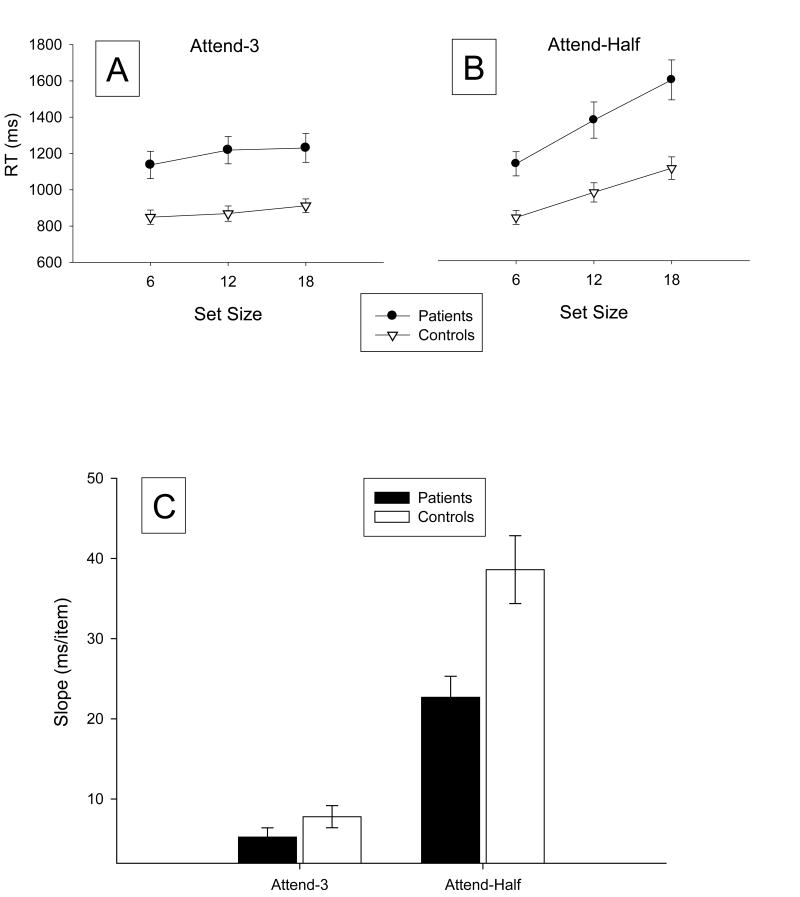
As shown in Figure 2 patients were consistently slower than controls across tasks and array sizes, and both groups were slower on half-attended trials than on 3-attended trials. On 3-attended trials, RTs in both groups were nearly flat across set sizes, evidence of highly efficient search. In contrast, RTs on half-attended trials increased in a linear fashion as set size increased in both groups, with a larger effect seen in the patient group. Mean accuracy was very high in both the patient group (97.4% correct) and the control group (98.2% correct), and the groups did not differ significantly (p > .25). Accuracy did not increase in the conditions with long reaction times, ruling out speed-accuracy tradeoffs.
Figure 2.
Mean reaction times observed for the 3-attended (A) and half-attended trials (B), along with mean slope values (C). Error bars show the standard error of the mean.
3.2 Search Slopes
The slopes (see Figure 2C) were very shallow for 3-attended trials but were fairly steep for half-attended trials, leading to a significant main effect of trial type (F (1,38) = 85.89, p<.0001) in the overall ANOVA(model included factors for group, dimension, trial type and all interaction terms). In addition, overall slopes were steeper for patients than for controls, leading to a significant main effect of group (F (1,38) = 7.79, p<.01). Of greatest interest was a significant interaction between group and trial type (F (1,38) = 6.60, p<.02). This interaction reflects the fact that both groups searched efficiently on 3-attended trials, whereas the efficiency of search was substantially more impaired for patients than for controls on the half-attended trials. The mean slope on 3-attended trials was 7.8 ms/item for patients and 5.3 ms/item for control subjects. This was a modest difference in terms of the absolute size (2.5 ms/item), in terms of the percent difference across groups (40%), and in terms of the effect size (d = 0.23), and it did not reach significance in a post hoc t-test (t=1.31, df=38, p=0.20). In contrast, the mean slope on half-attended trials was 38.6 ms/item for patients and 22.7 ms/item for control subjects. This difference was larger in terms of the absolute size (an increase of 15.9 ms/item), in terms of the percent change (a 70% increase in patient slopes compared to control slopes), and in terms of the effect size (d = 0.81), and it was significant in a post hoc t-test (t= 2.83, df = 38, p=0.007). Thus, by any measure, the patient deficit was greater on half-attended trials than on 3-attended trials, which is consistent with a deficit in the top-down control of attention.
4. Discussion
Since the time of William James (1890), researchers have stressed the notion that the role of attention is to select a subset of the perceptually available information for further processing. Visual search paradigms stress this form of selection by presenting observers with crowded arrays, and attention serves to focus perceptual processing on likely target items and to suppress processing of distracters. The patient deficit documented on the half-attended trials in the present study reflects impairment in this defining function of attention. The increase in slope suggests that patients have difficulty efficiently restricting processing to goal-relevant items. This increase in slope cannot be explained on the basis of perceptual difficulty because the same items were used in the 3-attended trials, where patients demonstrated relatively normal performance.
How then to understand the relatively intact patient performance in the 3-attended trials? The shallow slopes observed in these trials are in the range expected from a single target pop-out. Search in this condition is highly efficient, supported by the strong bottom-up salience of the feature cues, and the finding of no significant patient impairment on these trials is consistent with our previous electrophysiological and behavioral evidence that the rate of attentional shifting is normal in patients with schizophrenia when the shift is triggered by a highly salient cue (Luck et al., 2006). Further, the very shallow slope observed in this condition is powerful evidence that patients were able to restrict their attention to the relevant items as the addition of distractors had minimal impact on their search performance. In contrast, the patient impairment emerged when the bottom-up saliency was equated among all of the distractors and strong top-down control was therefore needed to guide selection.
The slopes observed on half-attended trials were an order of magnitude greater than those on 3-attended condition and were typical for serial search tasks using easily discriminable stimuli (Wolfe, 1998). The patient deficit in this condition, a 70% increase over the slopes observed in controls, is substantial, and consistent with previous findings from our group and others (Fuller et al., 2006)
This pattern of results is consistent with a deficit in the control of attention rather than in the implementation of selection. The distinction between control and implementation may be illustrated through the use of a flashlight metaphor (Posner et al., 1980). The control of attention has to do with where the attentional flashlight is pointed, and the question is whether the light is pointed at the most likely relevant location. In contrast, the implementation of selection has to do with how bright the light is, and the critical question is whether the light is strong enough to allow accurate perception. Our data suggest that the brightness of the attentional “light” is normal in schizophrenia. If the light were not bright enough, then the presence of additional distractors at higher set sizes should have led to elevated slopes on both the 3-attended and the half-attended trials. However, the patients were able to effectively filter out the distractors on the 3-attended trials, indicating that the implementation of selection is unimpaired. Thus, the impairment observed on the half-attended trials is best explained by a deficit in the top-down control of attention, resulting in the light being directed to irrelevant locations on a portion of trials, thereby slowing the search process. If we had included only the half-attended condition, it would not be possible to determine if the deficit arose at the level of control or selection. However, the fact that search was intact when guided by salient bottom-up information in the 3-attended condition, but was significantly slowed in the absence of such bottom-up support in the half-attended condition, strongly implicates a failure in top-down control.
These results are consistent with the broader notion that schizophrenia involves a compromise of executive control, but they indicate that such deficits are not limited to complex problem-solving tasks, (such as the Wisconsin Card Sorting Test, Milner, 1964)) or tasks that involve extensive response inhibition (such as the Stroop Test, Stroop, 1935), which have been the major focus of research on executive control in schizophrenia. In each instance, the role of top down control is to resolve competition between competing stimuli that could be processed (as in visual search), between possible competing responses (as in the Stroop), or between competing rules to guide action selection (as in the Wisconsin Card Sorting Test). In each of these instances, we hypothesize that the patient impairment results from a failure of top down control in the face of strong bottom up competition.
This study has two limitations that require comment. First, we studied relatively small samples, resulting in limited statistical power to reliably detect small-to-medium effect sizes. This is most important for interpretation of the 3-attended trials. We are not asserting a null effect in this condition (or asserting that deficits might not be observed in other tasks that involve bottom-up control) but instead are suggesting that the impairment in this condition is substantially less than the impairment in the attend half condition (a 2.5 ms difference vs. a 15.9 ms difference in slope across groups). While the effect size in the 3-attended condition is not trivial, it would be very difficult to provide sufficient power to detect a 2.5-ms slope difference, and one might also question the theoretical and functional significance of such a small difference. However, the 70% increase in slope on the half-attended trials could accumulate into a functionally important deficit if it means that patients make several unnecessary shifts of attention every time they use top-down control to find an object in the environment.
It is also possible to question whether our results are a psychometric artifact. There is no question that the attend-half condition was more difficult, as reflected in much higher search slopes, and the greater effect variance observed in this condition should enhance the discriminating power of the task. However, the pattern of results that we have documented across visual search experiments is not consistent with a differential “difficulty” explanation. In a prior report, we found no significant slope difference between patients and controls in a perceptually challenging search condition that yielded steeper slopes than those observed in the half-attended trials of the present study but minimized the role for attentional control. In contrast, the previous study demonstrated a significant group difference in a search task that yielded shallow slopes but required precise top-down control (Fuller et al., 2006). Here, we show the opposite pattern of between group difference and slope magnitudes. This overall pattern of results cannot be explained on the basis of psychometric artifact, but can be explained by a deficit in the operation of top-down control mechanisms.
A second limitation is that the patients in this study were all taking antipsychotic medication. While unlikely, we cannot rule out the possibility that the impairment documented here is secondary to drug effects. Deficits in related tasks (Span of Apprehension and CPT) have been documented in unmedicated at-risk and patient populations (Chen et al., 2004; Mirsky et al., 1992; Nestor et al., 1991; Weickert et al., 2003). However, in the absence of direct evidence, we cannot completely eliminate the possibility that the impairment documented here is a secondary treatment effect.
In summary, patients with schizophrenia demonstrate impaired visual search performance when required to use top-down goals to limit search to relevant items. In contrast, patient performance approximates that of healthy controls when salient bottom-up perceptual cues are sufficient to restrict search to relevant items. The present results suggest that executive control deficits impact very elementary aspects of attention and are therefore likely detectable in a wide array of cognitive operations if sensitive methods are used.
Acknowledgments
We gratefully acknowledge the contributions of our patient and healthy volunteers. We also acknowledge the statistical advice of Robert McMahon, and data collection efforts of Pablo Diego.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asarnow RF, Granholm E, Sherman T. Span of apprehension in schizophrenia. In: Steinhauer SR, Gruzelier JH, editors. Neuropsychology, psychophysiology, and information processing. Handbook of schizophrenia. Vol. 5. Amsterdam, Netherlands: Elsevier Science Publishing Co, Inc; 1991. pp. 335–370. [Google Scholar]
- Association AP. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: 1994. [Google Scholar]
- Carr VJ, Dewis SAM, Lewin TJ. Preattentive visual search and perceptual grouping in schizophrenia. Psychiatry Research. 1998;79(2):151–162. doi: 10.1016/s0165-1781(98)00035-3. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Chang CH, Liu SK, Hwang TJ, Hwu HG. Sustained attention deficits in nonpsychotic relatives of schizophrenic patients: a recurrence risk ratio analysis. Biol Psychiatry. 2004;55(10):995–1000. doi: 10.1016/j.biopsych.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Duncan J, Humphreys GW. Visual search and stimulus similarity. Psychol Rev. 1989;96(3):433–458. doi: 10.1037/0033-295x.96.3.433. [DOI] [PubMed] [Google Scholar]
- Egeth HE, Virzi RA, Garbart H. Searching for conjunctively defined targets. Journal of Experimental Psychology: Human Perception and Performance. 1984;10:32–39. doi: 10.1037//0096-1523.10.1.32. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) American Psychiatric Press, Inc; Washington, D.C.: 1997a. [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Structured Clinical Interview for DSM-IV Personality Disorders (SCID-II) American Psychiatric Press, Inc; Washington, D.C.: 1997b. [Google Scholar]
- Fuller RL, Luck SJ, Braun EL, Robinson BM, McMahon RP, Gold JM. Impaired control of visual attention in schizophrenia. J Abnorm Psychol. 2006;115(2):266–275. doi: 10.1037/0021-843X.115.2.266. [DOI] [PubMed] [Google Scholar]
- Hess R, Schu U, Muller P, Schuttler R. Preattentive perception? Limited capacity channel system? What is different in schizophrenic information processing. In: Spitzer M, Uehlein FA, Schwartz MA, Mundt C, editors. Phenomenology, Language and Schizophrenia. New York: Springer- Verlag; 1992. pp. 290–302. [Google Scholar]
- James W. The Prinicples of Psychology. H. Holt and Company; New York: 1890. [Google Scholar]
- Knight RA, Manoach DS, Elliott DS, Hershenson M. Perceptual organization in schizophrenia: the processing of symmetrical configurations. J Abnorm Psychol. 2000;109(4):575–587. doi: 10.1037//0021-843x.109.4.575. [DOI] [PubMed] [Google Scholar]
- Lieb K, Merklin G, Rieth C, Schuttler R, Hess R. Preattentive information processing in schizophrenia. Schizophr Res. 1994;14(1):47–56. doi: 10.1016/0920-9964(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Lubow RE. Construct validity of the animal latent inhibition model of selective attention deficits in schizophrenia. Schizophr Bull. 2005;31(1):139–153. doi: 10.1093/schbul/sbi005. Epub 2005 Feb 2016. [DOI] [PubMed] [Google Scholar]
- Lubow RE, Kaplan O, Abramovich P, Rudnick A, Laor N. Visual search in schizophrenia: latent inhibition and novel pop-out effects. Schizophr Res. 2000;45(1–2):145–156. doi: 10.1016/s0920-9964(99)00188-7. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Fuller RL, Braun EL, Robinson B, Summerfelt A, Gold JM. The speed of visual attention in schizophrenia: Electrophysiological and behavioral evidence. Schizophr Res. 2006;85(1–3):174–195. doi: 10.1016/j.schres.2006.03.040. Epub 2006 May 2019. [DOI] [PubMed] [Google Scholar]
- Milner B. Some effects of frontal lobectomy in man: The frontal granular cortex and behavior. McGraw-Hill; New York: 1964. [Google Scholar]
- Mirsky AF, Ingraham LJ, Kugelmass S. Neuropsychological assessment of attention and its pathology in the Israeli cohort. Schizophr Bull. 1995;21(2):193–204. doi: 10.1093/schbul/21.2.193. [DOI] [PubMed] [Google Scholar]
- Mirsky AF, Lochhead SJ, Jones BP, Kugelmass S. On familial factors in the attentional deficit in schizophrenia: A review and report of two new subject samples. Journal of Psychiatric Research. 1992;26(4):383–403. doi: 10.1016/0022-3956(92)90042-m. [DOI] [PubMed] [Google Scholar]
- Mori S, Tanaka G, Ayaka Y, Michitsuji S, et al. Preattentive and focal attentional processes in schizophrenia: A visual search study. Schizophrenia Research. 1996;22(1):69–76. doi: 10.1016/0920-9964(96)00049-7. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Faux SF, McCarley RW, Sands SF. Neuroleptics improve sustained attention in schizophrenia: A study using signal detection theory. Neuropsychopharmacology. 1991;4(2):145–149. [PubMed] [Google Scholar]
- Place EJS, Gilmore GC. Perceptual organization in schizophrenia. Journal of Abnormal Psychology. 1980;89(3):409–418. doi: 10.1037//0021-843x.89.3.409. [DOI] [PubMed] [Google Scholar]
- Posner MI, Snyder CR, Davidson BJ. Attention and the detection of signals. J Exp Psychol. 1980;109(2):160–174. [PubMed] [Google Scholar]
- Silverstein SM, Kovacs I, Corry R, Valone C. Perceptual organization, the disorganization syndrome, and context processing in chronic schizophrenia. Schizophr Res. 2000;43(1):11–20. doi: 10.1016/s0920-9964(99)00180-2. [DOI] [PubMed] [Google Scholar]
- Stroop J. Studies of interference in series verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Treisman A, Sato S. Conjunction search revisited. J Exp Psychol Hum Percept Perform. 1990;16(3):459–478. doi: 10.1037//0096-1523.16.3.459. [DOI] [PubMed] [Google Scholar]
- Van Selst M, Jolicoeur P. A solution to the effect of sample size and skew on outlier elimination. Quarterly Journal of Experimental Psychology. 1994;47A:631–650. [Google Scholar]
- Weickert TW, Goldberg TE, Marenco S, Bigelow LB, Egan MF, Weinberger DR. Comparison of cognitive performances during a placebo period and an atypical antipsychotic treatment period in schizophrenia: critical examination of confounds. Neuropsychopharmacology. 2003;28(8):1491–1500. doi: 10.1038/sj.npp.1300216. Epub 2003 Jun 1411. [DOI] [PubMed] [Google Scholar]
- Wells DS, Leventhal D. Perceptual grouping in schizophrenia: Replication of Place and Gilmore. Journal of Abnormal Psychology. 1984;93(2):231–234. doi: 10.1037//0021-843x.93.2.231. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. The Wide Range Achievement Test. Wide Range, Inc; Wilmington, Delaware: 1993. [Google Scholar]
- Wolfe JM. Visual search in continuous, naturalistic stimuli. Vision Res. 1994;34(9):1187–1195. doi: 10.1016/0042-6989(94)90300-x. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. What can 1 million trials tell us about visual search? Psychological Science. 1998;9(1):33–39. [Google Scholar]
- Wolfe JM, Cave KR, Franzel SL. Guided search: an alternative to the feature integration model for visual search. J Exp Psychol Hum Percept Perform. 1989;15(3):419–433. doi: 10.1037//0096-1523.15.3.419. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Electrophysiological measurement of rapid shifts of attention during visual search. Nature. 1999;400:867–869. doi: 10.1038/23698. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Serial deployment of attention during visual search. J Exp Psychol Hum Percept Perform. 2003;29(1):121–138. doi: 10.1037//0096-1523.29.1.121. [DOI] [PubMed] [Google Scholar]
- Zubin J. Problem of attention in schizophrenia. In: Kietzman M, Sutton S, Zubin J, editors. Experimental Approaches to Psychopathology. New York: Academic Press; 1975. pp. 139–166. [Google Scholar]