Abstract
The intestinal nematode parasite, Nippostrongylus brasiliensis, triggers potent type 2 immunity. Using OVA peptide as a model Ag, we have examined the adjuvant effects of this parasite on the in vivo development of Ag-specific Th2 cells from naive DO11.10 T cells. Our findings show that Th2 cells can develop from transferred naive OVA-specific DO11.10 T cells in recipient IL-4–/– mice inoculated with N. brasiliensis plus OVA. However, autocrine IL-4 is required for in situ Th2 cell differentiation since transferred IL-4Rα-deficient DO11.10 T cells showed greatly reduced Th2 cell development in inoculated IL-4–/– recipient mice. Surprisingly, we also found that IL-2 blockade promoted B7-dependent T cell cycling, but inhibited the development of OVA-specific Th2 cells. Furthermore, the effects of IL-2 occurred independently of CD25+ T regulatory cells. These studies establish a previously unrecognized requirement for autocrine IL-4 and IL-2 in Th2 responses elicited by nematode parasites.
Infectious agents can induce the development of effector Th cells that secrete specific sets of cytokines important in mediating the development of a host-protective response. Two polarized responses include the Th1 response, which is associated with elevations in IFN-γ and is protective against many intracellular pathogens, and the Th2 response, which is associated with elevations in IL-4 and other Th2 cytokines and provides protection against intestinal nematode parasites. Although considerable progress has been made toward understanding the mechanisms that lead to the development of IFN-γ-producing Th1 cells, the steps leading to the development of IL-4-producing Th2 cells during infectious disease are less clear. In particular, the role of IL-4 and IL-2 in initiating and stabilizing in vivo Ag-specific Th2 cell differentiation remains uncertain (1–4).
Previous studies have indicated an important role for IL-4 in the development of IL-4-producing Th2 cells (5–9). Culture of naive Ag-specific transgenic T cells with Ag-pulsed APCs results in optimal Th2 cell development if exogenous IL-4 is added. However, in the absence of exogenous IL-4, autocrine IL-4 can induce the development of a modest IL-4 response, particularly if IFN-γ and IL-12 are also neutralized (10). Further studies showed that these low levels of IL-4 can support the development of Th2 clones following multiple rounds of priming (11). These in vitro studies generally indicate that autocrine IL-4 can support modest in vitro Th2 cell differentiation in the absence of exogenous IL-4.
Several in vivo studies have also indicated a primary role for IL-4 in Th2 cell development (6, 12–14). However, nematode parasites have been shown to evoke a pronounced IL-4 response in vivo in STAT6–/– mice, as measured by elevations in serum IL-4 (15), suggesting that these infectious agents may stimulate a potent Th2 response in the absence of IL-4 signaling. As well as evoking pronounced Th2 cell differentiation, recent studies have also indicated that nematode parasites can trigger a pronounced IL-4 response by non-T cells, including eosinophils (16, 17) and basophils (18), suggesting an important alternative source of IL-4 in the absence of IL-4 signaling. In other immunization regimens, involving repeated challenges with Ag, autocrine IL-4 alone was required for an effective Th2 cell response leading to increased serum IgE levels (19). In IL-4Rα–/– mice infected with the helminth Schistosoma mansoni, IL-4-producing T cells can develop although at greatly reduced numbers compared with S. mansoni-infected wild-type (WT) mice. The degree to which T cell-derived IL-4 contributes to this strongly polarized Th2 response in WT mice remains uncertain (20). Other proposed sources for IL-4 during parasite infection include memory T cells (21), B cells (22), γδ T cells (23), eosinophils and basophils (15), and non-conventional T cells (24).
A number of in vitro studies have indicated that IL-2 also plays an important role in the activation of T cells and in their subsequent proliferation and expansion (25–27). IL-2 secretion is stimulated primarily by anti-CD28 costimulation in vitro, which preferentially stimulates the development of Th2 cells (28). Very recent studies have suggested that, in addition to its proliferation-inducing activity, IL-2 may also play an important role in Th2 cell differentiation by stabilizing the accessibility of the Il4 gene (29), although whether IL-2 is important in the in vivo development of Th2 cells during the potent type 2 responses that develop during helminth infection remains uncertain.
There is also the possibility that specific TCR-parasite Ag interactions may favor Th2 cell differentiation during helminth infection, contributing to the rapid burst of IL-4-producing T cells, as observed in the immune response to Leishmania major (30–32). Under these circumstances, the Th2 primary response might be more dependent on the initial activation of particular parasite-specific T cells clones than non-T cell or autocrine T cell sources of IL-4. It is also possible that bystander T cell activation or Th2 collateral priming, where previously activated Th2 cells drive naive T cells of a different Ag specificity to differentiate into Th2 cells (33), may play an important role in the development of the highly polarized Th2 responses that occur during infectious disease.
In this article, we have investigated the roles of IL-4 and IL-2 in mediating the adjuvant properties of Nippostrongylus brasiliensis that promote the in vivo differentiation of Ag-specific IL-4-producing T cells from naive T cells. We have focused on the DO11.10 T cell response to a non-parasite Ag, OVA, to obviate potential nonstereotypic effects of parasite Ag-specific T cell clones or cross-reactive memory cells, which might skew the response independently of adjuvant effects. Our findings in this system demonstrated that neither non-T cell nor bystander T cell IL-4 were required for the rapid development of IL-4-producing Th2 cells. Furthermore, autocrine IL-4 produced by these Ag-specific T cells was sufficient for the effective development of IL-4-producing Th2 cells but was not required for Ag-specific T cell expansion during the primary response. Unexpectedly, IL-2 blockade actually enhanced Ag-specific T cell expansion and inhibited Ag-specific Th2 cell development, independently of CD25+ T regulatory cells. These studies thus indicate that autocrine IL-4 is sufficient to drive the development of Ag-specific Th2 cells, which is IL-2 dependent during the primary in vivo immune response.
Materials and Methods
Mice
Breeding pairs of BALB/c IL-4–/– mice were purchased from The Jackson Laboratory. DO11.10 TCR-transgenic mice on an inbred BALB/c background were obtained from Dr. A. Sharpe (Harvard Medical School, Boston, MA). BALB/c mice genetically deficient for IL4Rα (IL-4Rα–/–) were obtained from The Jackson Laboratory and crossed to DO11.10 mice. F2 generations were genotyped and homozygous IL-4Rα–/–mice that expressed DO11.10 TCR were used in experiments. Female BALB/c WT mice were obtained from the Small Animal Division, National Cancer Institute (Fredrick, MD). All of the mice were maintained in a specific pathogen-free, virus Ab-free facility during the experiments. The studies reported here conformed to the principle for laboratory animal research outlined by the Animal Welfare Act and the Department of Health, Education and Welfare (National Institutes of Health) guidelines for the experimental use of animals.
Adoptive transfers
Peripheral lymph nodes and spleen were harvested from WT DO11.10 TCR-transgenic mice or IL-4Rα–/– DO11.10 mice that were age- and sex-matched to the adoptive transfer recipients. Single-cell suspensions were prepared by pressing tissue through a nylon strainer (BD Biosciences). The DO11.10 T cells were incubated with anti-CD4 beads and were purified by passing through an LS+ column (Miltenyi Biotec). In some experiments, the purified CD4+ T cells (with purity of ∼99% as determined by FACS) were resuspended at 5 × 107 cell/ml in PBS containing 0.1% BSA. A final concentration of 10 μM fluorescent dye CFSE (Molecular Probes) was added, and the cells incubated for 10 min at 37°C. The labeled cells were washed twice in cell culture medium containing 10% FCS (Invitrogen Life Technologies) before transfer. OVA-specific CD4+ T cells (5 × 106) were transferred to recipient mice by i.v. injection.
Parasite infection, OVA immunization, and blocking Ab treatment
HPLC-purified OVA323–339 with the sequence ISQAVHAAHAEINEAGR-COOH was synthesized by the Biomedical Instrumentation Center at the Uniformed Services University (Bethesda, MD). Third-stage N. brasiliensis larvae and 30 μg OVA peptide were injected intracutaneously in the ear of DO11.10 T cell transfer recipient mice. Some mice got N. brasiliensis alone or OVA alone as controls. In select experiments, groups of mice were also administered i.v. either 2 mg of anti-IL-2 Ab (S4B6) or 500 μg of anti-CD25 Ab (PC61) at doses previously shown to be effective at blocking either IL-2 (34) or depleting CD25+ T cells in vivo (35, 36). Control isotypes Abs were included in all experiments.
Cell sorting and cytokine gene expression by RT-PCR
Draining cervical lymph nodes (CLN) of recipient mice were removed after infection/immunization at the time indicated. For cell sorting of OVA-specific T cells, CLN cells were stained with PE-conjugated KJ1-26 mAb, and then labeled with anti-PE beads (Miltenyi Biotec). Labeled cells were passed through MS+ columns (Miltenyi Biotec) according to the protocol provided by the manufacturer. The KJ1-26+ population was collected and assessed for purity using FACS analysis. The KJ1-26+ population was 85–90% pure in all sorts. For RT-PCR, total RNA was extracted from purified cell populations with the RNA Isolation kit (Stratagene), specially developed for isolating small RNA quantities from tissue as previously described. Total RNA was then reverse transcribed as previously described (37). Real-time PCR kits (Applied Biosystems), specific for individual cytokines or rRNA, were used to quantitate differences in gene expression, and all data were normalized to constitutive rRNA values. The Applied Biosystems 7700 sequence detector was used for amplification of target mRNA, and quantification of differences between treatment groups was calculated according to the manufacturer's instructions.
ELISPOT
Two different ELISPOT assays were used. The first was used to monitor ex vivo cytokine secretion by lymph node cells (38). Briefly, single-cell lymph node suspensions were prepared in RPMI 1640 containing 10% heat-inactivated FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine (all from Invitrogen Life Technologies). Cells (0.5 × 106) were seeded into each well of an anti-IL-4 (clone BVD4-1D11.2)-coated Immulon IV 96-well microtiter plate (Microtiter). After short-term culture (5–12 h), the plate was washed several times with PBS followed by washes with PBS-Tween 20. Secondary biotinylated anti-IL-4 Ab was diluted in PBS, 0.05% Tween, and 5% FCS, added at 100 μl/well, and incubated overnight at 4°C. Plates were then washed and a 1/2000 dilution of streptavidin-alkaline phosphatase (Jackson ImmunoResearch Laboratories) was added. Plates were developed and results were counted as described. The second ELISPOT assay was modified to include the capability to quantitate IL-4-producing cells following in vitro restimulation with OVA peptide (39). Lymph node cells were cultured with 10 μg/ml OVA peptide for 3 days on anti-IL-4-coated plates, before being washed away with PBS and PBS-Tween 20. Secondary anti-IL-4 Ab was next added and subsequent steps were identical to those described for the first ELISPOT assay.
Flow cytometry
Lymph node cells were harvested and 1 × 106 cells were blocked with Fc Block (BD Pharmingen) and then incubated with anti-CD4-allophycocyanin-Cy7 (BD PharMingen), anti-CD44-allophycocyanin, and anti-CD62L-PE or anti-CD69-PE (BD Pharmingen). To assess B cell activation, CLN cells were stained with anti-B220-CyChrome and anti-MHCII-PE or anti-B7.2-PE (BD Pharmingen). After washes, cell were fixed with 1% paraformaldehyde (Fisher) and analyzed by flow cytometry using a BD LSRII (BD Biosciences). For CFSE-labeled cells, anti-CD4-allophycocyanin-Cy7 and KJ1-26-PE (Caltag Laboratories) were used to distinguish the DO11.10 T cells. Cell cycle progression was monitored by measuring sequential reductions in CFSE fluorescence of KJ1-2+CD4+ cells and the proliferation index was calculated using ModFit (Verity Software House) software.
Ex vivo intracellular cytokine measurement
For intracellular cytokine detection, 5 × 106 cells from cervical draining lymph nodes were incubated for 6 h with 10 μg/ml OVA peptide. GolgiStop (BD Pharmingen) was added to the culture for the last 4 h. Lymphocytes were harvested and incubated with Fc Block (2.4G2; BD Pharmingen) plus 10% rat serum (Sigma-Aldrich) for 20 min at room temperature. Cell surface markers were stained by anti-CD4-allophycocyanin-Cy7 (BD Pharmingen) and KJ1-26-PE mAb (Caltag Laboratories). Cells were fixed in 4% paraformaldehyde (Fisher) and permeabilized in 0.5% saponin (Sigma-Aldrich) before staining with allophycocyanin-conjugated rat anti-mouse IL-4 or anti-IFN-γ mAb (BD Pharmingen). More than 300,000 lymphocyte-gated events were collected, to obtain >2,000 KJ1-26+CD4+-gated events.
Results
Kinetics of Ag-specific CD4+ T cell cycling and IL-4 up-regulation during in vivo Th2 response
To provide a baseline for examining the role of IL-4 in the development and expansion of IL-4-producing T cells from naive T cells in vivo, CFSE-stained DO11.10 T cells were transferred to recipient BALB/c mice, which were then inoculated with the combination of 300 N. brasiliensis larvae plus OVA (30 μg). At days 2, 4, and 7 after inoculation, draining cervical lymph nodes were removed from individual mice (five mice per treatment group), pooled, and DO11.10 T cell expansion was examined by analyzing CFSE fluorescence. As shown in Fig. 1, by day 2 after inoculation, DO11.10 T cells had already undergone some proliferation with a significant number of cells having progressed to three to four divisions, and, by day 7, >40% of the cells had cycled more than nine divisions. DO11.10 T cells were also purified using magnetic bead cell sorting and assessed for elevations in IL-2, IL-4, and IFN-γ gene expression at each of these time points. At day 2 after inoculation, increases in IL-4 gene expression (∼10-fold), IL-2 gene expression (∼10-fold), and a smaller increase in IFN-γ gene expression (∼3-fold) were detected compared with cytokine levels in transferred DO11.10 T cells from untreated recipient mice. By day 4 after inoculation, IL-4 was markedly elevated (>400-fold), IL-2 was also increased (∼40-fold), and a smaller increase in IFN-γ (∼10-fold) was detected. Finally, by day 7, the Ag-specific DO11.10 T cell response was quite polarized, with IL-4 remaining at high levels and IFN-γ dropping to almost undetectable levels. IL-2 decreased but remained ∼20-fold elevated.
FIGURE 1.
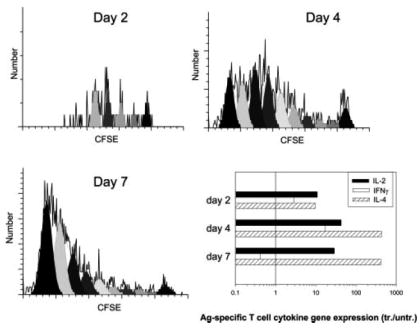
Ag-specific T cell cycling and IL-4 expression occurs in the draining lymph node as early as day 2 after inoculation. CD4+ T cells were purified from DO11.10 OVA-specific TCR-transgenic mice and labeled with CFSE before transfer to BALB/c WT recipient mice. Two days later, recipient mice were inoculated intracutaneously in the ear with a combination of OVA peptide plus N. brasiliensis. At days 2, 4, and 7 after inoculation, the draining CLN was removed from individual mice (five mice per treatment group), and pooled single-cell suspensions were stained with anti-CD4-allophycocyanin-Cy7 and KJ1-26-PE to distinguish DO11.10 T cells. Sequential reductions in CFSE fluorescence of KJ1-26-gated CD4+ cells were then used to measure cell cycle progression. CLN DO11.10 T cells were also purified using magnetic beads. IL-2, IL-4, and IFN-γ gene expression by sorted DO11.10 T cells were assessed by quantitative TaqMan real-time RT-PCR. DO11.10 T cell IL-2, IL-4, and IFN-γ mRNA levels were expressed as fold change compared with DO11.10 T cells sorted from untreated control mice. This experiment was repeated twice with similar results.
These studies indicate that Ag-specific T cell cytokine production and cell cycling occur in the lymph node as early as day 2 after inoculation. Initially, elevations in both IL-4 and IFN-γ are detected, but by day 7 the response is strongly polarized toward production of IL-4. Inoculation of mice with OVA peptide alone caused some proliferation but no appreciable increases in cytokines (data not shown), as previously described (39). It should be noted that previous studies have shown that transferred DO11.10 T cells on a WT, SCID, or RAG2–/– background exhibit the same degree of effector T cell development and function in recipient mice in vivo (39, 40), indicating that potential endogenous TCR signaling in DO11.10 WT mice does not influence the naive OVA-specific T cell response in vivo.
Non-T cell IL-4 and bystander T cell IL-4 production are not required for Ag-specific T cell expansion in vivo
Previous studies have shown the development of pronounced innate type 2 responses associated with IL-4 production by non-T cells during N. brasiliensis infection (17, 18). Other studies have suggested that IL-4 production by T cells can drive Th2 cell differentiation from naive cells of a different Ag specificity (33). To directly examine whether naive Ag-specific T cells require an innate type 2 response, collateral priming, or bystander T cells as a source of IL-4 for their differentiation into Th2 cells, 5 × 106 DO11.10 OVA-specific T cells from DO11.10 WT mice were transferred to WT or IL-4–/–recipients. Two days after adoptive transfer, recipient mice (five per treatment group) were inoculated intracutaneously in the ear with N. brasiliensis plus OVA. Seven days after inoculation, mice were killed and cervical ear lymph nodes were collected for analysis. The lymph node cell suspensions were stained for KJ-126 (anti-DO11.10 TCR Ab), CD69, CD44, and CD62L. Gated DO11.10 T cells showed pronounced increases of CD69 and CD44 and decreased CD62L in both recipient WT and IL-4KO recipient mice, consistent with an activated T cell phenotype (data not shown). To examine whether DO11.10 T cell cycle progression was also intact in immunized IL-4-deficient recipient mice, 5 × 106 sorted CD4 T cells were labeled with CFSE, as described in Materials and Methods, and transferred to BALB/c WT and IL-4KO recipients. Two days later, mice were inoculated intracutaneously in the ear with N. brasiliensis plus OVA and 7 days after inoculation, mice were killed and lymph node cell suspensions from cervical ear lymph nodes were stained for KJ1-26 and assessed for CFSE staining. As shown in Fig. 2a, extensive and comparable cell cycling was observed in both WT and IL-4KO recipient mice given N. brasiliensis plus OVA compared with corresponding untreated controls. These studies demonstrate that the strong host IL-4 response is not required to drive transferred DO11.10 T cell activation and proliferation, indicating that it occurs in the absence of a non-T cell or bystander T cell source of IL-4 in vivo.
FIGURE 2.
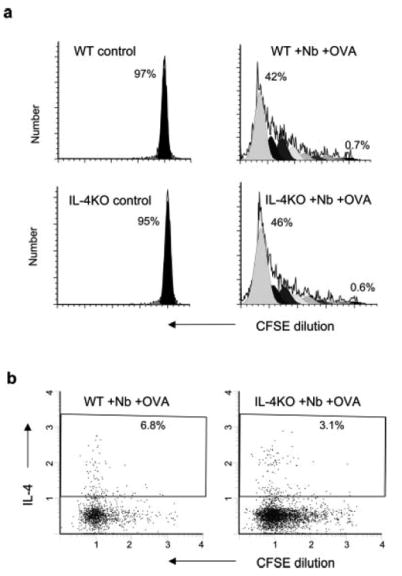
In vivo Ag-specific T cell expansion and cytoplasmic IL-4 levels are pronounced in the absence of non-T cell or bystander T cell IL-4. Five million purified DO11.10 CD4+ T cells were labeled with CFSE and transferred to recipient BALB/c IL-4–/– mice or WT mice. Two days later, recipient mice were immunized intracutaneously in the ear with N. brasiliensis (Nb) and OVA peptide (five animals per treatment group). At day 7 after immunization, the draining CLN cells were collected. a, Analysis of cell cycling was performed as described in Fig. 1. b, For ex vivo cytoplasmic cytokine staining, CLN cells were cultured with 10 μg/ml OVA peptide for 6 h with GolgiStop added for the last 4 h. Intracellular staining of IL-4 was performed as described in Materials and Methods. Data shown are for gated CD4+KJ1-26+ OVA-specific T cells. This experiment was repeated three times with similar results.
Increases in DO11.10 T cell IL-4 are sustained in the absence of a host IL-4 response
To examine whether naive Ag-specific T cells could also differentiate to produce IL-4 in the absence of a host IL-4 response, cytoplasmic IL-4 staining was performed following a 6-h restimulation with OVA Ag in vitro. As shown in Fig. 2b, DO11.10 T cells transferred into either WT or IL-4-deficient mice subsequently inoculated with N. brasiliensis plus OVA showed pronounced increases in IL-4 protein expression, although there was a consistent 50% decrease in the number of IL-4-producing DO11.10 T cells in IL-4-deficient mice. To further confirm that transferred DO11.10 T cells had effectively differentiated into IL-4-secreting cells in IL-4-deficient mice, an OVA-specific ELISPOT assay was used (39). As shown in Fig. 3a, pronounced elevations in IL-4 protein production by transferred WT DO11.10 T cells were detected by ELISPOT in IL-4-deficient recipients inoculated with N. brasiliensis plus OVA. Using magnetic bead cell sorting, we have further found marked and comparable elevations in IL-4 gene expression from purified DO11.10 T cells from WT and IL-4–/– recipient mice at day 7 after inoculation with N. brasiliensis plus OVA (Fig. 3b). Elevations in transferred DO11.10 T cell IL-4 and IFN-γ were also similar between inoculated WT and IL-4KO recipient mice on days 2 and 4 after inoculation (data not shown). To examine whether DO11.10 T cells were effectively producing IL-4 in vivo in microenvironments that would stimulate B cell activation, B cell MHC class II (MHCII) and CD86 expression was assessed. Previous studies have shown that increases in B cell MHCII expression are primarily IL-4 dependent in vivo (41–43). We found that increases in B cell surface MHCII expression and increases in the frequency of CD86+ B cells were primarily blocked in the draining cervical lymph node of N. brasiliensis-inoculated IL-4-deficient mice compared with inoculated WT mice (data not shown). As shown in Fig. 3c, increased B cell MHCII expression and increases in the frequency of CD86+ B cells were observed in IL-4-deficient mice, comparable to WT mice, following DO11.10 transfer and immunization with N. brasiliensis plus OVA. Taken together, these studies indicate that N. brasiliensis drives the development of functional Th2 cells in the absence of IL-4 derived from an innate type 2 response, B cells, or bystander T cells.
FIGURE 3.
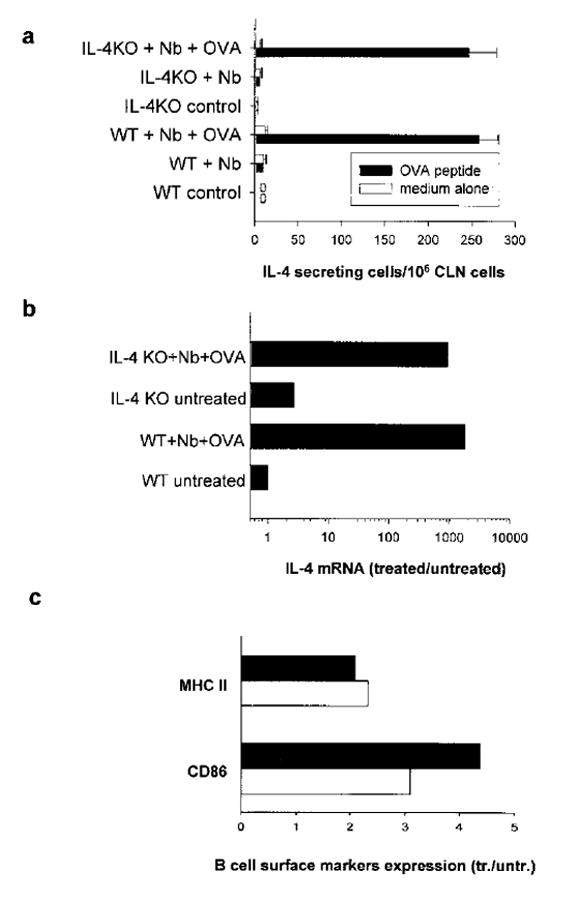
Transferred Ag-specific T cells effectively differentiated into IL-4-secreting cells in IL-4–/– mice. The DO11.10 T cell transfer and inoculation of recipient mice was performed as described in Fig. 2. a, CLN cells were cultured for 3 days with or without OVA restimulation to assess OVA-specific IL-4 secretion by ELISPOT assay. b, Transferred OVA-specific DO11.10 T cells were purified from CLN cells by incubating with KJ1-26-PE and anti-PE beads as described in Materials and Methods. IL-4 mRNA expression in sorted DO11.10 T cell populations from pooled treatment groups (five animals per group) was determined by quantitative TaqMan real-time PCR. c, CLN cells were pooled (five mice per treatment group) and stained with anti-B220 and anti-MHC II or anti-CD86 Abs. Expression of MHCII (mean fluorescence intensity) and CD86 (percentage of positive B cells) on gated B220+ cells were assessed by FACS analysis. Treatment groups included: IL-4–/– recipient mice (■) or WT recipient mice (□) inoculated with N. brasiliensis (Nb) + OVA, expressed relative to untreated recipient IL-4–/– mice or untreated recipient WT mice, respectively. This experiment was repeated twice with similar results.
Autocrine IL-4 is sufficient for the development of IL-4 producing Ag-specific T cells from naive T cells in vivo
To examine whether autocrine IL-4 produced by Ag-specific T cells drives their differentiation into IL-4-producing cells, DO11.10 and IL-4Rα–/– BALB/c mice were bred to produce homozygous IL-4Rα-deficient DO11.10 TCR OVA-transgenic mice. In brief, 5 × 106 purified DO11.10IL4Rα–/– OVA-specific T cells, stained with CFSE, were transferred to IL-4–/– recipients and compared with control DO11.10 WT T cells transferred to WT recipients. After 2 days, both groups of recipient mice (five per treatment group) were inoculated with 30 μg of OVA and 300 N. brasiliensis L3. As shown in Fig. 4a, IL-4Rα–/– DO11.10 T cell cycling was pronounced in recipient IL-4–/– mice as well as recipient WT mice. In contrast, IL-4Rα–/– DO11.10 T cell cytoplasmic IL-4 protein was markedly reduced (>10×) in IL-4–/– mice compared with controls (Fig. 4b). Similar results were obtained with IL-4 mRNA analyses of sorted DO11.10 T cells (data not shown). This was also reflected in markedly reduced elevations in B cell MHCII and CD86 expression (Fig. 4c). IL-4 secretion was also assessed using the OVA-specific ELISPOT assay. As shown in Fig. 5, IL-4 and IL-13 secretion were markedly reduced in IL-4–/– mice that had received IL-4Rα–/– DO11.10 T cells compared with IL-4–/– mice receiving WT DO11.10 T cells. The only difference between using WT and IL-4Rα–/– donor T cells in IL-4–/– recipient mice is that the transferred WT DO11.10 T cells were receiving signals from autocrine IL-4 in the IL-4–/– recipient, while these specific signals were blocked when using the transferred IL-4Rα–/– DO11.10 T cells. Similar results were obtained when this experiment was repeated. It should be noted that in an additional experiment similar reductions in IL-4 were obtained when IL-4Rα–/– DO11.10 T cells were transferred to WT recipients (data not shown). The use of WT recipients further extended our findings by excluding the possible influence on a Th2 response when IL-4 genes were constitutively knocked out in the recipients. It also indicates that the effects of any IL-4-mediated but T cell IL-4Rα-independent signaling is minimal in this system. Taken together, these studies indicate that T cell autocrine IL-4 is sufficient to support the rapid development of IL-4-producing Ag-specific T cells during nematode parasite infection. However, it should be noted that our results do not exclude the possibility that paracrine IL-4 can promote Th2 cell differentiation in the absence of autocrine IL-4.
FIGURE 4.
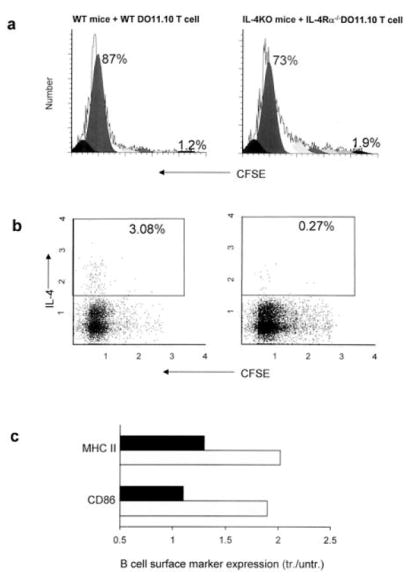
Cell cycling of transferred IL-4Rα–/–DO11.10 T cells in IL-4–/– recipients was pronounced, while IL-4 production was greatly reduced. Briefly, 5 × 106 DO11.10 IL-4Rα–/– OVA-specific T cells were stained with CFSE and then transferred to IL-4–/– recipients, while DO11.10 WT T cells were transferred to WT recipients (five animals per treatment group). Recipient mice were immunized as described in Fig. 2 and CLN cells were collected at day 7. a, Cell cycle progression of OVA-specific DO11.10 T cells was analyzed. Ex vivo DO11.10 T cell intracellular IL-4 staining (b) and B cell MHCII and B7.2 expression (c) were assessed as described in Fig. 3. Treatment groups included IL-4Rα–/–DO11.10 T cells transferred to IL-4–/– mice (■) or WT DO11.10 T cells transferred to WT mice (
 ) and inoculated with N. brasiliensis + OVA and expressed relative to untreated recipient IL-4–/– mice or untreated recipient WT mice, respectively. This experiment was repeated twice with similar results.
) and inoculated with N. brasiliensis + OVA and expressed relative to untreated recipient IL-4–/– mice or untreated recipient WT mice, respectively. This experiment was repeated twice with similar results.
FIGURE 5.
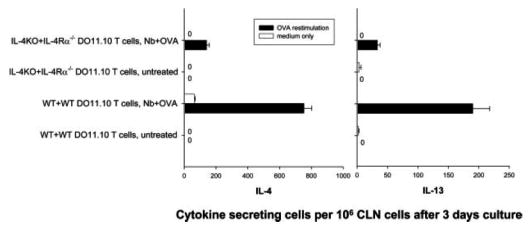
Th2 cytokine production from IL- 4Rα–/– DO11.10 T cells was markedly reduced in IL-4–/– recipient mice. Cell transfer and animal immunization were performed as described in Fig. 3. At day 7 after immunization, the draining CLN cells were collected. CLN cells were cultured for 3 day in vitro, with or without 10 μ/ml OVA peptide. IL-4 and IL-13 production were analyzed by ELISPOT. This experiment was repeated twice with similar results.
IL-2 blockade enhances expansion of Ag-specific T cell pool and inhibits Th2 cell differentiation
Our findings that IL-4 (either from non-T cells or T cells) was not required for in vivo cell cycling and expansion of DO11.10 T cells following immunization with OVA peptide and N. brasiliensis raised the possibility that IL-2 may instead drive Ag-specific T cell cycling during this response. DO11.10 T cells did show elevated IL-2 at early time points after inoculation (see Fig. 1). OVA-specific T cells, stained with CFSE, were transferred to BALB/c recipient mice. After 2 days, recipient mice (five per treatment group) were inoculated with 30 μg of OVA and 300 N. brasiliensis L3 and administered either anti-IL-2 (S4B6) or control Ab GL117. As shown in Fig. 6a, inoculated mice administered anti-IL-2 Ab showed pronounced DO11.10 T cell cycling, which was consistently higher than inoculated mice administered the control isotype. Seventy-four percent of DO11.10 T cells from mice administered N. brasiliensis plus OVA plus anti-IL-2 divided at least eight times, while 57% of inoculated mice given the control isotype showed this degree of proliferation. Analysis of the proliferation index showed more than a 2-fold increase in DO11.10 T cells from inoculated mice administered the anti-IL-2 Ab (Fig. 6b). These findings indicate that IL-2 down-regulates the number of divisions by Ag-specific T cells during the course of the Th2 response. Finally, to directly examine whether DO11.10 T cell expansion had resulted from the increased cell cycling, the total number of DO11.10 T cells was examined in each draining lymph node. A >2-fold increase was detected in inoculated mice administered anti-IL-2 Ab compared with the control (Fig. 6b). Ex vivo DO11.10 T cell cytoplasmic IL-4 levels were assessed and pronounced decreases in IL-4 levels (>10×) were obtained in anti-IL-2 Ab-treated mice inoculated with N. brasiliensis and OVA compared with the control group (Fig. 6c). These results indicate that IL-2 is not required for T cell expansion but is required for the differentiation of IL-4-producing T cells in vivo. These experiments, including DO11.10 T cell cycling, expansion, and IL-4 production were repeated twice with similar results.
FIGURE 6.
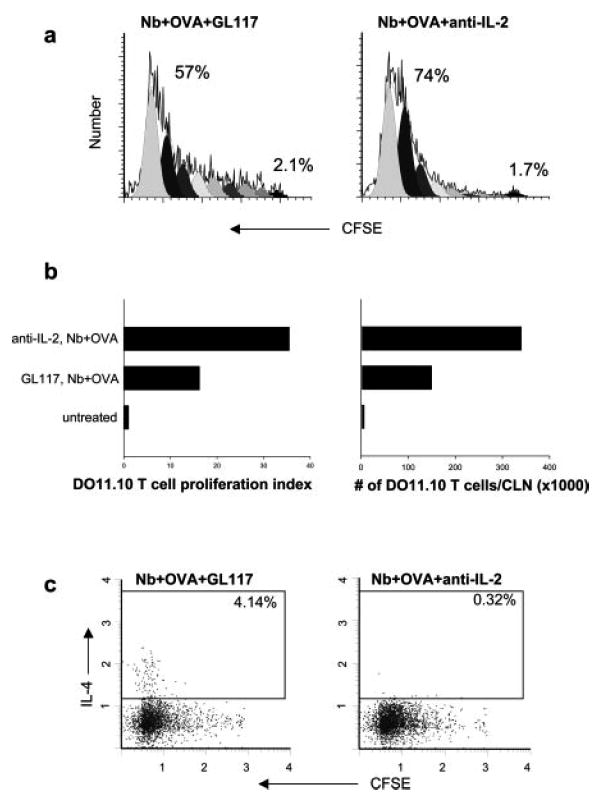
IL-2 inhibits Ag-specific T cell cycle progression while promoting the development of IL-4-producing T cells. Five million purified WT DO11.10 CD4+ T cells were labeled with CFSE and transferred to WT BALB/c mice. Two days later, recipient mice were administered 2 mg of anti-IL-2 (S4B6) or control Ab GL117 and immunized intracutaneously in the ear with N. brasiliensis (Nb) and OVA peptide (five animals per treatment group). At day 7 after immunization, the draining CLN cells were collected. a, Analysis of DO11.10 T cell cycling was performed as described in Fig. 1. b, The number of DO11.10 T cells in each CLN was calculated based on multiplying the percentage of KJ1-26+CD4+ cells measured by flow cytometry by the number of live cells in each CLN. c, Ex vivo cytoplasmic IL-4 staining was performed as described in Fig. 2. Data shown are with gated CD4+KJ1-26+ OVA-specific T cells. This experiment was repeated twice with similar results.
Previous studies have suggested that IL-2 signaling can differentially affect peripheral CD25+ T regulatory cells, in some systems stimulating or recruiting this population and in other cases inhibiting it or having minimal effect (44). The marked increase in T cell proliferation following IL-2 blockade suggested the possibility that CD25+ T regulatory cells, perhaps derived from the host response, may control Ag-specific T cell cycle progression and perhaps influence the development of Th2 cells. To examine this, OVA-specific T cells, stained with CFSE, were transferred to BALB/c recipient mice, which were then administered either anti-CD25 Ab, anti-IL-2 Ab, or control Ab i.v. After 2 days, recipient mice (five per treatment group) were inoculated with 30 μg of OVA and 300 N. brasiliensis L3. Previous studies have shown that populations of CD25+ T regulatory cells are effectively depleted by anti-CD25 Ab treatment (36). Depletion of CD25+ T cells was confirmed by FACS analysis at day 7 after inoculation (data not shown). As shown in Fig. 7, anti-CD25 Ab treatment had little effect on DO11.10 T cell cycling and did not result in an inhibition of IL-4 expression, instead increased IL-4 expression was detected. These results indicate that both increased proliferation and decreased IL-4 production resulting from IL-2 blockade were not mediated by CD25+ T regulatory cells and further that the CD25+ T cells were not a primary source of IL-4.
FIGURE 7.
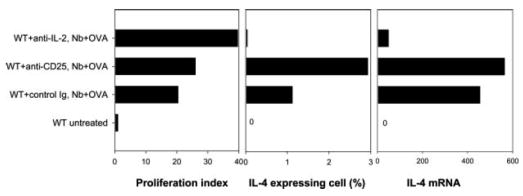
Comparison of effects of blocking anti-IL-2 and anti-CD25 on Ag-specific Th2 cell development elicited by N. brasiliensis (Nb). Five million purified WT DO11.10 CD4+ T cells were labeled with CFSE and transferred to WT BALB/c mice. Recipient mice were administered with either anti-IL-2, anti-CD25, or isotype control Ab and immunized in the ear with N. brasiliensis and OVA peptide (five animals per treatment group). At day 7 after immunization, the draining CLN cells were collected. Analysis of DO11.10 T cell cycling and ex vivo cytoplasmic IL-4 staining were performed as described in Fig. 2. OVA-specific DO11.10 T cells were also purified from the draining lymph node cells. IL-4 gene expression by the purified DO11.10 T cells was assessed as described in Fig. 3.
Discussion
We have examined the role of IL-4 and IL-2 in Ag-specific T cell differentiation to polarized IL-4-producing T cells during infectious disease. Our results show that IL-4 derived from the innate type 2 response, B cells, or bystander T cells are not required for N. brasiliensis to drive nonparasite Ag-specific Th2 cell development. However, in the context of this potent Th2-inducing agent, autocrine IL-4 alone can effectively mediate the rapid development of IL-4-producing T cells, although T cell expansion is largely IL-4 independent. Finally, our studies show that IL-2 is also not required for the in vivo expansion of Ag-specific T cells, but is required for their IL-4 expression through CD25+ T regulatory cell-independent mechanisms.
The development of a potent host Th2 response in the cervical lymph node paralleled the differentiation to Th2 cells of the transferred DO11.10 T cells in the draining lymph node following inoculation with OVA plus N. brasiliensis (39). We hypothesized that this strong background host type 2 cytokine response provided a source of IL-4, either from non-T cells or bystander T cells or through collateral priming, which then acted to drive the differentiation of DO11.10 CD4 T cells into Th2 cells. However, our findings indicate that sources of IL-4 other than Ag-specific T cells responding to the immunogen are not required for Th2 cell differentiation or migration in the lymph node microenvironment, in the context of this nematode parasite infection. Apparently, the potent adjuvant effect of N. brasiliensis that triggers the initial development of nonparasite Ag-specific Th2 cells is, at least to a large extent, IL-4 independent. We have recently shown that the Th2 response to N. brasiliensis is TLR-4 independent (45), excluding this pathogen-associated molecular pattern (PAMP) as playing a major role in this response. However, it remains a possibility that other as yet unidentified PAMPs are expressed by nematode parasites that can favor a Th2 response at particular doses of parasite Ag. It should also be considered that the host may recognize a general characteristic of the parasite, such as its large size or rough surface. Such structural features may stimulate an endogenous danger signal, which triggers the development of a Th2 response in the absence of Th1 response-inducing PAMPs.
Our results, examining Th2 cell differentiation in the context of the lymph node microenvironment, show both similarities and differences from previous studies of in vitro Ag-specific Th2 cell differentiation. In previously reported in vitro studies, exogenous IL-4 was required for optimal Ag-specific Th2 cell differentiation, but in its absence a small amount of IL-4 was produced that was sufficient to drive limited Th2 cell development, but only if IL-12 and IFN-γ were also blocked (10). A number of other studies have also indicated that autocrine IL-4 may be sufficient to at least promote some degree of Th2 cell differentiation in vitro (11, 46–48). Our studies extend these in vitro studies, demonstrating that in vivo autocrine IL-4 produced by Ag-specific T cells is sufficient to drive the rapid expansion of proliferating IL-4-producing Th2 cells during the primary response to an intestinal nematode parasite. Interestingly, recent studies of in vivo Th2 cell induction in response to schistosome-soluble egg Ag have shown a requirement for IL-4 that is independent of dendritic cell IL-4 (49). Our findings raise the possibility that Ag-specific T cell autocrine IL-4 may also be the essential cell source of IL-4 in this system.
The results reported in this study may at first appear to contradict earlier studies showing equivalent serum IL-4 elevations in N. brasiliensis-inoculated STAT6–/– and WT mice (15). However, these previous results examined the cumulative host type 2 response to this parasite, while our experimental system allowed specific examination of the development of conventional Ag-specific Th2 cells in the context of N. brasiliensis inoculation. It is possible that during the N. brasiliensis host response, cross-reactive memory cells or naive T cell clones with particular characteristics, including specific affinities for Ags expressed by N. brasiliensis, can develop into rapidly expanding Th2 cell populations in the absence of IL-4 signaling. Alternatively, accessory cell populations in the microenvironment of the lung or the intestine may provide signals that promote STAT6-independent Th2 cell differentiation and expansion. It is also possible that non-T cells are a source of IL-4 in STAT6–/– mice.
Our findings further demonstrate that in the absence of T cell autocrine IL-4, although Th2 cell differentiation was largely blocked, T cell cycling remained primarily intact. It has been suggested that IL-4 expression is linked to increased cell division number, with cell cycle-dependent epigenetic remodeling of the IL-4 locus leading to IL4 production (1, 50, 51). Our findings do show that IL-4 is predominantly produced by transferred WT DO11.10 T cells that have undergone greater than seven or eight divisions by 7 days after inoculation. As early as 2 days after inoculation, IL-4 expression is observed in those Ag-specific T cells that have undergone three to four cell divisions. However, clear separation between cell cycling and IL-4 production occurred when autocrine IL-4 was inhibited, suggesting that IL-4 signaling provides the essential transcription factors that are required along with cell cycling to effectively drive the development of Th2 cells in the lymph node. These results are consistent with previous in vitro findings that cell cycling alone is not sufficient to trigger Th2 cell differentiation (52, 53). It also indicates that IL-4 is not an essential factor in the growth and expansion of Ag-specific T cells during the response to N. brasiliensis. It may be that cognate and costimulatory molecule interactions are sufficient to drive the IL-4-independent cell cycle progression in the context of N. brasiliensis infection or that another cytokine is involved.
Given previous studies indicating that IL-2 is important in initial activation and expansion of T cells, IL-2 seemed a logical candidate for driving Ag-specific T cell expansion. A major function of CD28 in in vitro T cell priming is the stimulation of IL-2 production (28) and previous studies have shown that B7 costimulation is required for N. brasiliensis-driven DO11.10 T cell expansion (39). Our findings showed that blocking IL-2 did not down-regulate Ag-specific cell cycling in vivo, consistent with recent studies suggesting that CD28-mediated T cell stimulation can also occur through IL-2-independent pathways (54). Our observation that IL-2 actually inhibits Ag-specific T cell expansion in vivo is contrary to the notion that IL-2 is primarily a T cell growth factor, but is consistent with previous studies indicating that IL-2 can also down-regulate effector T cell responses (44, 55). Several studies have suggested that IL-2 signaling is required for the peripheral expansion, survival, and function of T regulatory cells (44, 56, 57), raising the possibility that the increased T cell expansion after IL-2 blockade may result from the potentially decreased T regulatory population. Our results show it is unlikely that the effects of anti-IL-2 Ab administration is due to inhibition of T regulatory cells, since anti-CD25 Ab treatment did not inhibit IL-4 expression and minimally enhanced proliferation. Our finding that the presence of IL-2 is critical for the effective development of IL-4-producing Th2 cells is in agreement with recent findings that IL-2 is essential for Th2 cell differentiation (29). In this study, IL-2 was shown to stabilize accessibility of the Il4 gene through the STAT5 signaling pathway (29). Other transcription factors, especially GATA-3, T-bet, and c-maf, have been linked to differential polarization of Th1 or Th2 cell subsets. With our system, further studies of these critical factors may lead to the understanding of mechanisms through which IL-2 and autocrine IL-4 drive the development of IL-4-producing T cells in vivo.
In summary, our results indicate that during the primary response the development of Ag-specific Th2 cells elicited by N brasiliensis requires IL-2 and autocrine IL-4 is sufficient to support their development. Furthermore, in the presence of autocrine T cell IL-4, other sources of IL-4 including bystander T cells, eosinophils, basophils, and B cells are not required.
Acknowledgments
We thank Karen M. Wolcott of the Biomedical Instrumentation Center for excellent help in flow cytometric analysis and Drs. Dragana Jankovic (National Institute of Allergy and Infectious Diseases, National Institutes of Health) and Nancy Noben-Trauth (Department of Immunology, George Washington University Medical Center) for careful reading of this manuscript and for helpful suggestions.
Abbreviations used in this paper
- WT
wild type
- CLN
cervical lymph node
- MH-CII
MHC class II
- PAMP
pathogen-associated molecular pattern
Footnotes
This work was supported by National Institutes of Health Grants AI31678 and AI55848 and by U.S. Department of Agriculture Current Research Information System 1265-32000-064-00D.
The opinions or assertions contained within are the private views of the authors and should not be construed as official or necessarily reflecting the views of the Uniformed Services University of the Health Sciences or the Department of Defense or the Department of Agriculture.
References
- 1.Coffman RL, Reiner SL. Instruction, selection, or tampering with the odds? Science. 1999;284:1283–1285. doi: 10.1126/science.284.5418.1283. [DOI] [PubMed] [Google Scholar]
- 2.Jankovic D, Sher A, Yap G. Th1/Th2 effector choice in parasitic infection: decision making by committee. Curr Opin Immunol. 2001;13:403. doi: 10.1016/s0952-7915(00)00234-x. [DOI] [PubMed] [Google Scholar]
- 3.Ekkens MJ, Liu Z, Liu Q, Foster A, Whitmire J, Pesce J, Sharpe AH, Urban JF, Gause WC. Memory Th2 effector cells can develop in the absence of B7-1/B7-2, CD28 interactions, and effector Th cells after priming with an intestinal nematode parasite. J Immunol. 2002;168:6344. doi: 10.4049/jimmunol.168.12.6344. [DOI] [PubMed] [Google Scholar]
- 4.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 5.Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796. [PubMed] [Google Scholar]
- 6.Le Gros G, Ben Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990;172:921. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 8.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 9.d'Ostiani CF, Del Sero G, Bacci A, Montagnoli C, Spreca A, Mencacci A, Ricciardi-Castagnoli P, Romani L. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans: implications for initiation of T helper cell immunity in vitro and in vivo. J Exp Med. 2000;191:1661. doi: 10.1084/jem.191.10.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noben-Trauth N, Hu-Li J, Paul WE. Conventional, naive CD4+ T cells provide an initial source of IL-4 during Th2 differentiation. J Immunol. 2000;165:3620. doi: 10.4049/jimmunol.165.7.3620. [DOI] [PubMed] [Google Scholar]
- 11.Noben-Trauth N, Hu-Li J, Paul WE. IL-4 secreted from individual naive CD4+ T cells acts in an autocrine manner to induce Th2 differentiation. Eur J Immunol. 2002;32:1428. doi: 10.1002/1521-4141(200205)32:5<1428::AID-IMMU1428>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Chatelain R, Varkila R, Coffman RL. IL-4 induces a Th2 response in Leishmania major-infected mice. J Immunol. 1992;148:1182. [PubMed] [Google Scholar]
- 13.Romani L, Mencacci A, Grohmann U, Mocci S, Mosci P, Puccetti P, Bistoni F. Neutralizing antibody to interleukin 4 induces systemic protection and T helper type 1-associated immunity in murine candidiasis. J Exp Med. 1992;176:19. doi: 10.1084/jem.176.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schopf LR, Mansfield JM. Characterization of a relatively rare class B, type 2 trypanosome variant surface glycoprotein gene. J Parasitol. 1998;84:284. [PubMed] [Google Scholar]
- 15.Finkelman FD, Morris SC, Orekhova T, Mori M, Donaldson D, Reiner SL, Reilly NL, Schopf L, Urban JF., Jr Stat6 regulation of in vivo IL-4 responses. J Immunol. 2000;164:2303. doi: 10.4049/jimmunol.164.5.2303. [DOI] [PubMed] [Google Scholar]
- 16.Shinkai K, Mohrs M, Locksley RM. Helper T cells regulate type-2 innate immunity in vivo. Nature. 2002;420:825. doi: 10.1038/nature01202. [DOI] [PubMed] [Google Scholar]
- 17.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 18.Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan ES, Urban JF, Jr, Dvorak AM, Finkelman FD, LeGros G, Paul WE. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitz J, Thiel A, Kuhn R, Rajewsky K, Muller W, Assenmacher M, Radbruch A. Induction of interleukin 4 (IL-4) expression in T helper (Th) cells is not dependent on IL-4 from non-Th cells. J Exp Med. 1994;179:1349. doi: 10.1084/jem.179.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jankovic D, Kullberg MC, Noben-Trauth N, Caspar P, Paul WE, Sher A. Single cell analysis reveals that IL-4 receptor/Stat6 signaling is not required for the in vivo or in vitro development of CD4+ lymphocytes with a Th2 cytokine profile. J Immunol. 2000;164:3047. doi: 10.4049/jimmunol.164.6.3047. [DOI] [PubMed] [Google Scholar]
- 21.Gollob KJ, Coffman RL. A minority subpopulation of CD4+ T cells directs the development of naive CD4+ T cells into IL-4-secreting cells. J Immunol. 1994;152:5180. [PubMed] [Google Scholar]
- 22.Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1:475. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 23.Ferrick DA, Schrenzel MD, Mulvania T, Hsieh B, Ferlin WG, Lepper H. Differential production of interferon-γ and interleukin-4 in response to Th1- and Th2-stimulating pathogens by γ/δ T cells in vivo. Nature. 1995;373:255. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 24.Noben-Trauth N, Shultz LD, Brombacher F, Urban JF, Jr, Gu H, Paul WE. An interleukin 4 (IL-4)-independent pathway for CD4+ T cell IL-4 production is revealed in IL-4 receptor-deficient mice. Proc Natl Acad Sci USA. 1997;94:10838. doi: 10.1073/pnas.94.20.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240:1169. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 26.Jain J, Loh C, Rao A. Transcriptional regulation of the IL-2 gene. Curr Opin Immunol. 1995;7:333. doi: 10.1016/0952-7915(95)80107-3. [DOI] [PubMed] [Google Scholar]
- 27.Minami Y, Kono T, Miyazaki T, Taniguchi T. The IL-2 receptor complex: its structure, function, and target genes. Annu Rev Immunol. 1993;11:245. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- 28.Seder RA, Germain RN, Linsley PS, Paul WE. CD28-mediated costimulation of interleukin 2 (IL-2) production plays a critical role in T cell priming for IL-4 and interferon γ production. J Exp Med. 1994;179:299. doi: 10.1084/jem.179.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cote-Sierra J, Foucras G, Guo L, Chiodetti L, Young HA, Hu-Li J, Zhu J, Paul WE. Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci USA. 2004;101:3880. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly BL, Stetson DB, Locksley RM. Leishmania major LACK antigen is required for efficient vertebrate parasitization. J Exp Med. 2003;198:1689. doi: 10.1084/jem.20031162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Launois P, Gumy A, Himmelrich H, Locksley RM, Rocken M, Louis JA. Rapid IL-4 production by Leishmania homolog of mammalian RACK1-reactive CD4+ T cells in resistant mice treated once with anti-IL-12 or -IFN-γ antibodies at the onset of infection with Leishmania major instructs Th2 cell development, resulting in nonhealing lesions. J Immunol. 2002;168:4628. doi: 10.4049/jimmunol.168.9.4628. [DOI] [PubMed] [Google Scholar]
- 32.Julia V, Rassoulzadegan M, Glaichenhaus N. Resistance to Leishmania major induced by tolerance to a single antigen. Science. 1996;274:421. doi: 10.1126/science.274.5286.421. [DOI] [PubMed] [Google Scholar]
- 33.Eisenbarth SC, Zhadkevich A, Ranney P, Herrick CA, Bottomly K. IL-4-dependent Th2 collateral priming to inhaled antigens independent of Toll-like receptor 4 and myeloid differentiation factor 88. J Immunol. 2004;172:4527. doi: 10.4049/jimmunol.172.7.4527. [DOI] [PubMed] [Google Scholar]
- 34.Via CS, Finkelman FD. Critical role of interleukin-2 in the development of acute graft-versus-host disease. Int Immunol. 1993;5:565. doi: 10.1093/intimm/5.6.565. [DOI] [PubMed] [Google Scholar]
- 35.Lohr J, Knoechel B, Jiang S, Sharpe AH, Abbas AK. The inhibitory function of B7 costimulators in T cell responses to foreign and self-antigens. Nat Immunol. 2003;4:664. doi: 10.1038/ni939. [DOI] [PubMed] [Google Scholar]
- 36.Mendez S, Reckling SK, Piccirillo CA, Sacks D, Belkaid Y. Role for CD4+ CD25+ regulatory T cells in reactivation of persistent leishmaniasis and control of concomitant immunity. J Exp Med. 2004;200:201. doi: 10.1084/jem.20040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svetic A, Finkelman FD, Jian YC, Dieffenbach CW, Scott DE, McCarthy KF, Steinberg AD, Gause WC. Cytokine gene expression after in vivo primary immunization with goat antibody to mouse IgD antibody. J Immunol. 1991;147:2391. [PubMed] [Google Scholar]
- 38.Lu P, Zhou XD, Chen SJ, Moorman M, Schoneveld A, Morris S, Finkelman FD, Linsley P, Claassen E, Gause WC. Requirement of CTLA-4 counter receptors for IL-4 but not IL-10 elevations during a systemic in vivo immune response. J Immunol. 1995;154:1078. [PubMed] [Google Scholar]
- 39.Liu Z, Liu Q, Pesce J, Whitmire J, Ekkens MJ, Foster A, VanNoy J, Sharpe AH, Urban JF, Jr, Gause WC. Nippostrongylus brasiliensis can induce B7-independent antigen-specific development of IL-4-producing T cells from naive CD4 T cells in vivo. J Immunol. 2002;169:6959. doi: 10.4049/jimmunol.169.12.6959. [DOI] [PubMed] [Google Scholar]
- 40.Merica R, Khoruts A, Pape KA, Reinhardt RL, Jenkins MK. Antigen-experienced CD4 T cells display a reduced capacity for clonal expansion in vivo that is imposed by factors present in the immune host. J Immunol. 2000;164:4551. doi: 10.4049/jimmunol.164.9.4551. [DOI] [PubMed] [Google Scholar]
- 41.Finkelman FD, Ohara J, Goroff DK, Smith J, Villacreses N, Mond JJ, Paul WE. Production of BSF-1 during an in vivo, T-dependent immune response. J Immunol. 1986;137:2878. [PubMed] [Google Scholar]
- 42.Noelle R, Krammer PH, Ohara J, Uhr JW, Vitetta ES. Increased expression of Ia antigens on resting B cells: an additional role for B-cell growth factor. Proc Natl Acad Sci USA. 1984;81:6149. doi: 10.1073/pnas.81.19.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu P, Urban JF, Zhou XD, Chen SJ, Morris SC, Finkelman FD, Gause WC. CD40-mediated costimulation contributes to lymphocyte proliferation, antibody production, eosinophilia, and mastocytosis during an in vivo type 2 response, but is not required for T cell IL-4 production. J Immunol. 1996;156:3327. [PubMed] [Google Scholar]
- 44.Nelson BH. IL-2, regulatory T cells, and tolerance. J Immunol. 2004;172:3983. doi: 10.4049/jimmunol.172.7.3983. [DOI] [PubMed] [Google Scholar]
- 45.Liu Z, Liu Q, Pesce J, Anthony RM, Lamb E, Whitmire J, Hamed H, Morimoto M, Urban JF, Jr, Gause WC. Requirements for the development of IL-4-producing T cells during intestinal nematode infections: what it takes to make a Th2 cell in vivo. Immunol Rev. 2004;201:57. doi: 10.1111/j.0105-2896.2004.00186.x. [DOI] [PubMed] [Google Scholar]
- 46.Croft M, Swain SL. Recently activated naive CD4 T cells can help resting B cells, and can produce sufficient autocrine IL-4 to drive differentiation to secretion of T helper 2-type cytokines. J Immunol. 1995;154:4269. [PubMed] [Google Scholar]
- 47.Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-α β-transgenic model. J Exp Med. 1995;182:1579. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med. 1995;182:1591. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacDonald AS, Pearce EJ. Cutting edge: polarized Th cell response induction by transferred antigen-pulsed dendritic cells is dependent on IL-4 or IL-12 production by recipient cells. J Immunol. 2002;168:3127. doi: 10.4049/jimmunol.168.7.3127. [DOI] [PubMed] [Google Scholar]
- 50.Bird JJ, Brown DR, Mullen AC, Moskowitz NH, Mahowald MA, Sider JR, Gajewski TF, Wang CR, Reiner SL. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- 51.Gett AV, Hodgkin PD. Cell division regulates the T cell cytokine repertoire, revealing a mechanism underlying immune class regulation. Proc Natl Acad Sci USA. 1998;95:9488. doi: 10.1073/pnas.95.16.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richter A, Lohning M, Radbruch A. Instruction for cytokine expression in T helper lymphocytes in relation to proliferation and cell cycle progression. J Exp Med. 1999;190:1439. doi: 10.1084/jem.190.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ben Sasson SZ, Gerstel R, Hu-Li J, Paul WE. Cell division is not a “clock” measuring acquisition of competence to produce IFN-γ or IL-4. J Immunol. 2001;166:112. doi: 10.4049/jimmunol.166.1.112. [DOI] [PubMed] [Google Scholar]
- 54.Marinari B, Costanzo A, Marzano V, Piccolella E, Tuosto L. CD28 delivers a unique signal leading to the selective recruitment of RelA and p52 NF-κB subunits on IL-8 and Bcl-xL gene promoters. Proc Natl Acad Sci USA. 2004;101:6098. doi: 10.1073/pnas.0308688101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature T lymphocyte apoptosis—immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 56.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 57.de la Rosa M, Rutz S, Dorninger H, Scheffold A. Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2480. doi: 10.1002/eji.200425274. [DOI] [PubMed] [Google Scholar]


