Abstract
Secreted protein acidic and rich in cysteine (SPARC) is highly expressed in human gliomas and promotes glioma invasion. We have shown by cDNA array analysis that SPARC upregulates membrane type 1-matrix metalloproteinase (MT1-MMP) and matrix metalloproteinase-2 (MMP-2) transcripts. To confirm these findings at the protein level and determine whether SPARC expression correlates with increased MMP activity, we used Western blot to assess the levels of MT1-MMP, and gelatin zymography to assess MMP-2 levels and activity. We also examined the expression, secretion, and cleavage of galectin-3, a target of MT1-MMP and MMP-2. Our data confirm that SPARC upregulates MT1-MMP levels and MMP-2 activity. There was also an increase in secreted galectin-3, as well as an increase in the proteolytically processed form of galectin-3. Previous studies have demonstrated that MT1-MMP, MMP-2 and galectin-3 are increased in gliomas. Our results suggest that their upregulation and activation may be a consequence of increased SPARC expression. These data provide a provisional mechanism whereby SPARC contributes to brain tumor invasion.
Keywords: SPARC, MMP-2, MT1-MMP, Galectin-3, glioma, invasion
Introduction
SPARC (also known as osteonectin and BM-40) is a 32-kDa secreted glycoprotein that has complex biological activities. As a matricellular protein, SPARC regulates cell-matrix interactions, but does not contribute significantly to the extracellular matrix (ECM) structure. SPARC is highly expressed in tissues that are undergoing morphogenesis, remodeling, and repair. The expression of SPARC modulates numerous cellular processes including, but not limited to, cell proliferation, adhesion, and spreading [4].
High levels of SPARC have been correlated with increased expression of specific MMPs, which degrade components of the ECM and may promote cell migration, such as during tumor cell invasion. Indeed, SPARC has been associated with invasion in several different types of cancer [3,4]. SPARC has been shown to increase the expression and/or activation of MMP-1 (collagenase), MMP-3 (stromelysin), and MMP-9 in cultured rabbit synovial fibroblasts [32], MMP-2 in breast cancer cell lines [10], and with MMP-2, MMP-3, and MMP-9 in glioma [27].
Galectin-3 is a 31-kDa member of the family of β-galactoside binding lectins that modulates normal development [21], and biological processes involved in cancer, including tumor invasion and metastasis [6]. It is secreted into the ECM where it mediates cell-cell and cell-matrix interactions. Galectin-3 has a C-terminal carbohydrate binding domain (CBD) and an N-terminal domain important for galectin-3 dimerization [29]. The N-terminal domain is made up of Gly-X-Tyr repeats, which makes the protein susceptible to proteolysis by MMP-2, MMP-9 [22,31], and the MT1-MMP soluble fragment [31]. Thus, cleavage of galectin-3 is considered to be an indicator of MMP-2, MMP-9, and MT1-MMP activity [31]. The processing of galectin-3 results in the generation of 22-kDa and/or 27-kDa degradation products that include the CBD and maintain carbohydrate binding activity [20,29,31]. Thus, cleavage of galectin-3 may be an additional function of the MMPs to regulate galectin-3 activity [20].
We have demonstrated that SPARC is highly expressed in human brain tumors [24,26]. Using the non-invasive U87MG glioma cell line that is low in endogenous SPARC, we have shown that SPARC induces tumor invasion in vitro [12] and in vivo [28]. To identify genes involved in SPARC-induced invasion, we performed a cDNA array analysis [13]. These data indicated that SPARC upregulation promoted increased MMP-2 and MT1-MMP transcript abundance, 2.2 and 2.3 fold, respectively. To confirm these findings at the protein level and to determine whether SPARC contributes to increased MMP activity, we used immunoblot and gelatin zymography to assess the levels of latent and active MT1-MMP and MMP-2. We also examined galectin-3, a target of these proteinases.
Materials and methods
Derivation of the U87MG-SPARC-transfected clones was previously described [12]. The SPARC-transfected (A2b2 and A2bi) and control vector-transfected (B1b2 and C2a2) clones were maintained in DMEM plus 10% FBS, geneticin (400 μg/ml), and puromycin (1 μg/ml) and grown in Opti-MEM as indicated.
Gelatin zymography was used to determine the effect of SPARC expression on MMP-2 levels and species. SPARC- and control-transfected clones were plated (2 × 105 cells) for 8 h, washed twice with PBS, and grown in serum-free media for 48-72 h. Conditioned media were collected and centrifuged. Aliquots (30 μl) of the conditioned media were subjected to 0.1% gelatin SDS-PAGE. The gels were incubated in renaturing buffer (2.5% Triton X-100) for 30 min, rinsed in water, and incubated at 37°C for 16 h in zymogram developing buffer (200 mM NaCl, 5 mM CaCl2, 0.02% Brij-35, 50 mM Tris-HCl, pH 7.5). Gels were stained with 0.5% Coomassie Brilliant Blue R-250 and de-stained in water containing 10% acetic acid and 30% methanol. A twin gel was stained with Coomassie Blue to show equal loading.
RT-PCR was performed to confirm the changes in MT1-MMP at the transcript level observed by cDNA array analysis. Cells (2 × 105)) were plated for 3 days, and RNA was isolated using the Tri-reagent kit (Molecular Research Center, Inc., Cincinnati, OH) according to the manufacturer's protocol. Intact RNA was verified by 1.1% formaldehyde gel electrophoresis, quantitated, and first strand cDNA synthesis and RT-PCR were performed as previously reported [13] using primers for MT1-MMP (forward 5'-ATAAACCCAAAAACCCCACC-3' and reverse 5'-ACACCCAATGCTTGTCTCC-3') and for GAPDH (forward 5'-CGTCTTCACCACCATGGAGA-3' and reverse 5'-CAGGGGTCTTACTCCTTGGA-3'). GAPDH was coamplified as a normalizing control.
For MT1-MMP analysis, cells (2 × 105) were plated for 18 h. Lysates were extracted using single detergent lysis buffer as described [29] and concentrations were determined using the BCA Protein Assay Kit (Pierce, Rockford, IL). Equal concentrations were resolved by 10% SDS-PAGE [29]. Proteins were transferred to nitrocellulose membranes and immunoblot analysis was performed with MT1-MMP antibody # AB-815 (Chemicon International, Temecula, CA) or the anti-MT1-MMP cytoplasmic tail antibody (Triple Point Biologics, Forest Grove, OR) with detection using Super Signal West Femto Enhanced Chemiluminescence reagents (Pierce). Membranes were stripped and probed with anti-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and ECL Western Blotting Detection Reagents (Amersham, Piscataway, NJ).
For galectin-3 studies, cells (4 × 105) were plated for 24 h, washed twice with Opti-MEM, and incubated in Opti-MEM for 2 days. Media were collected, treated with protease inhibitor cocktail (Calbiochem, San Diego, CA) to prevent further cleavage of galectin-3, and concentrated approximately 25-fold using Centricon Centrifugal Filters (Millipore, Bedford, MA). Cell lysates were extracted using single detergent lysis buffer as described previously [25]. Media and lysate samples were mixed with reducing Laemmli SDS sample buffer, boiled, and resolved by 10% SDS-PAGE, followed by transfer to Immobilon-P membranes (Millipore). Membranes were probed using the HL-31 polyclonal galectin-3 antibody (from Dr. Avraham Raz) that recognizes the full-length galectin-3 and its cleaved products. Protein was detected, and membranes were stripped and probed for actin detection as above. Densitometry analyses of the relative MMP-2, MT1-MMP, or galectin-3 levels in the SPARC-transfectants and control cells were performed using actin as a loading control as previously reported [13].
Recombinant human galectin-3 (from Dr. Avraham Raz) was incubated at 37°C with a recombinant catalytic domain of human MT1-MMP (Calbiochem) in a 1:10 molar ratio of MT1-MMP to galectin-3 in 50 mM Tris/HCl pH 7.5 buffer supplemented with 150 mM NaCl, 5 mM CaCl2 and 0.02% Brij 35. At various times, an aliquot of the reaction containing ∼40 ng of galectin-3 was collected and mixed with SDS-sample buffer. Samples were then resolved by reducing 12% SDS-PAGE followed by immunoblot analysis using the HL-31 polyclonal galectin-3 antibody as above. To determine the cleavage site of galectin-3 by MT1-MMP, galectin-3 was incubated for 1 h at 37°C with the catalytic domain of MT1-MMP as described above. Three μg of galectin-3 were then subjected to 12% reducing SDS-PAGE, transferred to a PVDF membrane, and stained with Coomassie Blue R-250. The protein band corresponding to the ∼22-kDa degradation product was excised and sent to ProSeq (Boxford, MA) for N-terminal sequencing.
Results
In this report, we utilized zymography and immunoblot analyses of SPARC- and control-transfected cells to determine whether increases in transcript observed using cDNA array analysis correlated with increases in protein expression and/or activity. Immunoblot analysis (Fig. 1A) confirmed the increased expression of SPARC in the cell lysates and conditioned media of the SPARC-expressing clones relative to the vector-transfected clones. Zymography (Fig.1B) indicated that increased SPARC expression was associated with increased MMP-2 activity in both the lysates and conditioned media. A twin Coomassie Blue-stained gel of the lysates indicated equal loading (data not shown).
Figure 1.
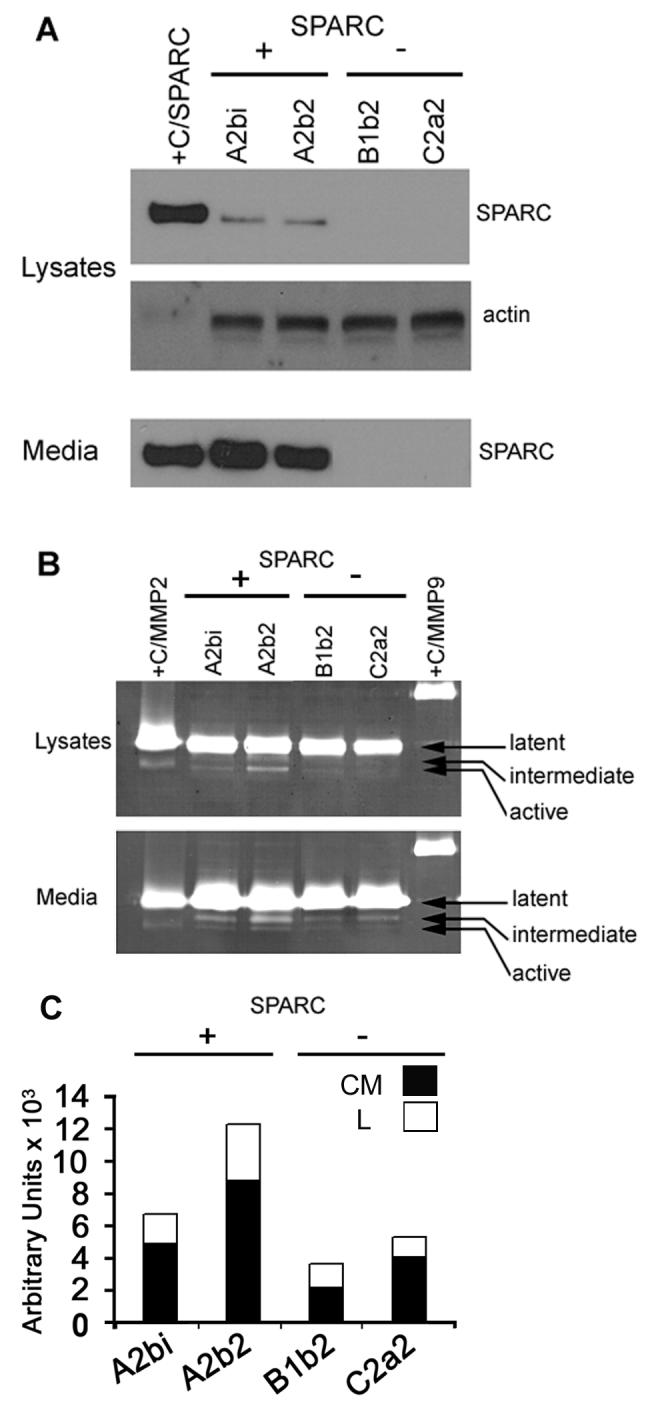
Increased SPARC correlates with increased MMP-2 expression and activity. A. Immunoblot analysis demonstrating increased SPARC in lysates and conditioned media of SPARC- (A2bi, A2b2) versus control- (B1b2, C2a4) transfected cells. +C/SPARC: SPARC positive control. Actin: loading control. B. Zymography demonstrating increased MMP-2 expression and activation in lysates and conditioned media of SPARC- versus control-transfected cells. (+C/MMP2) and (+C/MMP9): MMP-2 and MMP-9 positive controls. The latent, intermediate, and active forms are indicated. C. Densitometric analyses demonstrating increased active MMP-2 in the lysates (L) and intermediate MMP-2 in the conditioned medium (CM) of the SPARC- versus control-transfected cells. (The active MMP-2 signals in CM were too weak to quantitate). Representative results from n = 3 experiments.
We next examined the expression of MT1-MMP, which along with TIMP-2, is the physiological activator of MMP-2. Using RT-PCR, increased levels of MT1-MMP transcript were observed in SPARC- versus control-transfected cells (Fig. 2A). Using immunoblot analysis, MT1-MMP protein levels were also higher in the SPARC-transfected clones compared to controls. The protein was detected using antibodies that detect the cytoplasmic tail and the hinge region (Fig. 2A). Densitometry indicated that the SPARC-transfected cells had > 2-fold increase in MT1-MMP transcript (Fig. 2B) and protein (Fig. 2C) compared to control-transfected cells. No changes were observed in TIMP-2 levels (data not shown).
Figure 2.

SPARC increases expression of MT1-MMP. A. RT-PCR analysis demonstrating an increase in MT1-MMP transcript in SPARC- (A2b2, A2bi) versus control- (C2a2, B1b2) transfected cells. GAPDH serves as the internal control. Immunoblot analysis demonstrating increased MT1-MMP in SPARC- versus control-transfected cells using the AB-815 antibody to the hinge region or the antibody to the cytoplasmic tail. Actin: loading control (only one actin blot is illustrated). Note: The lanes for the protein standard were moved closer from another region of the same blots. B and C. Densitometric analyses demonstrating increased MT1-MMP transcript (B) and protein (densitometry for the cytoplasmic tail antibody immunoblot is shown [C]) in the SPARC- versus control-transfected cells. Representative results from n = 3 experiments.
We previously reported that galectin-3 is cleaved by MMP-2 and MMP-9 at the Ala62-Tyr63 peptide bond generating a ∼22-kDa fragment [20]. Using conditioned media containing a recombinant soluble ectodomain of MT1-MMP we have also reported an increase in galectin-3 processing, which indicated MT1-MMP activity [31]. However, the kinetics and cleavage site of MT1-MMP-dependent galectin-3 degradation were not reported. Here we examined the kinetics of galectin-3 degradation by MT1-MMP in a purified system and determined the N-terminal sequence of the cleaved product. To this end, recombinant galectin-3 was incubated with a recombinant catalytic domain of human MT1-MMP and aliquots were collected at various times for immunoblot analysis. MT1-MMP cleaved galectin-3 (31-kDa) to a ∼22-kDa product in a time-dependent manner (Fig. 3). The ∼22-kDa degradation product was readily detected after 10-min incubation, and after 2 h most of the galectin-3 was converted to the degradation product. N-terminal sequencing of the 22-kDa fragment revealed an N-terminus starting with Tyr63 consistent with a cleavage at the Ala62-Tyr63 peptide bond. These results establish galectin-3 as an MT1-MMP substrate via a cleavage site that is also targeted by MMP-2 and MMP-9 [20].
Figure 3.

MT1-MMP cleaves galectin-3. Recombinant human galectin-3 was incubated with a recombinant catalytic domain of human MT1-MMP in a 1:10 molar ratio of MT1-MMP to galectin-3. Samples were taken at the indicated time points and resolved by reducing 12% SDS-PAGE followed by immunoblot analysis using anti-galectin-3 antibody recognizing full length and cleaved galectin-3.
Since galectin-3 is a target of MMP-2 and MT1-MMP, we examined protein levels of the 31-kDa and the proteolytically processed forms. Immunoblot (Fig. 4A) and densitometric (Fig. 4C) analyses of equally loaded lysates showed similar levels of galectin-3 in the SPARC- versus control-transfected cells, and only the 31-kDa galectin-3 was present (Fig. 4A). In contrast, immunoblot (Fig. 4B) and densitometric (Fig. 4D) analyses of conditioned media showed increased secretion of full-length galectin-3 and the 27-kDa and 22-kDa forms in the SPARC- versus control-transfected cells. The increased secretion of the full-length galectin-3 did not completely account for the increase in galectin-3 processing, as there was a greater percentage of the cleaved forms in the SPARC-transfected cells than in control cells (Fig. 4D), thus indicating an increase in protease activity with increased SPARC expression.
Figure 4.

SPARC increases galectin-3 secretion and cleavage. A and C. Immunoblot and densitometric analysis demonstrating no difference in galectin-3 levels in lysates of SPARC- (A2bi, A2b2) versus control- (B1b2, C2a2) transfected cells. Actin (A) is the loading control used for normalization in (D). B and D. Immunoblot and densitometric analysis showing the increase of full-length and both cleaved forms of galectin-3 in conditioned media from SPARC versus control-transfected cells. The relative levels of the 31-kDa (black), the 27-kDa (grey), and the 22-kDa (white) proteins are illustrated (D). The bars in total indicate relative levels of total secreted galectin-3 by each cell line. Representative results from n = 3 experiments.
Discussion
We and others have demonstrated that increased SPARC expression correlates with glioma invasion in vitro [12] and in vivo [27,28]. In vivo, we observed that increased SPARC expression promoted tumor invasion of individual cells along blood vessels, through the corpus collosum, and into adjacent brain tissue [28]. cDNA array analysis identified MT1-MMP and MMP-2 as possible candidates that might promote this invasion [13]. In this study, we determined that increased SPARC expression induced an increase in MT1-MMP transcript and protein, an upregulation and activation of MMP-2, and an increase in the secretion and cleavage of galectin-3, which is a target of both MT1-MMP and MMP-2. Although the exact means whereby SPARC increases the expression of these proteins is unknown, these data provide a possible mechanism whereby SPARC promotes glioma invasion by increasing the degradation of the surrounding ECM via MMP activation and/or altering tumor cell adhesion and motility via galectin-3 secretion and/or cleavage.
The role of MMPs in glioma invasion is well documented [2,8,23]. Differential roles have been suggested whereby MMP-9 contributes primarily to invasion along established blood vessels, but MT1-MMP and MMP-2 may regulate both invasion and angiogenesis [9]. Indeed, activation of pro-MMP-2 requires MT1-MMP [11]. In gliomas, MT1-MMP expression correlates with tumor grade [18,19] and invasiveness [15]. Furthermore, transfection of U251 cells with MT1-MMP displayed prominent activation of MMP-2 and increased invasive growth in vitro [17], and promoted invasion of C6 cells in central nervous system white matter [1].
Increased MMP-2, along with increased MMP-9 and MMP-3, has also been observed in a genetically defined model of SPARC-induced glioma invasion [27]. Invasion was attributed to MMP-3. However, the effects of MMP-2 on invasion may have been clouded by the expression of the other MMPs. That study did not evaluate MT1-MMP expression.
A benefit to the U87MG cell line used here is the lack of MMP-9 expression, thereby eliminating possible confounding effects, especially with respect to cleavage of galectin-3 [20]. Our data suggest that SPARC-induced upregulation and activation of MT1-MMP and MMP-2 may contribute to SPARC-induced invasion.
Although reports on the expression of galectin-3 in gliomas have been conflicting, galectin-3 is expressed in a number of glioma cells lines [16]. We previously reported [5] that galectin-3 expression increases during glioma progression, whereas other studies have found a global decrease in expression [7,14]. This discrepancy may be due to the specific regions of tumors analyzed, as higher galectin-3 expression is associated with the invasive regions in vivo. Indeed, glioblastoma cells demonstrated greater motility on galectin-3 in vitro, suggesting that increased galectin-3 promotes invasion [7]. In addition, galectin-3 expression is not restricted to the tumor cells. Therefore, galectin-3 expression from other normal cell sources must also be considered, as heterogeneous expression within the tumors results from various cell types including microglia and endothelial cells [30].
The increasing expression of MMP-2 and MMP-9 associated with glioma progression could result in more cleavage of galectin-3. Since the cleaved fragment is present and stable in vivo and can compete for cell surface or ECM binding, it has been speculated that cleavage would result in changes in cell adhesion and motility [22]. Our results show that there is an increase in galectin-3 secretion by the more invasive SPARC-expressing cells, and much of the secreted protein is cleaved. Galectin-3 cleavage in these cells is likely mediated by MMP-2 and/or MT1-MMP. Indeed, here we confirmed that MT1-MMP can readily accomplish the degradation of galectin-3 to the ∼22-kDa product by hydrolyzing the same peptide bond cleaved by gelatinases.
In summary, these studies suggest a novel mechanism whereby SPARC promotes glioma invasion, and this function of SPARC warrants further investigation.
Acknowledgements
We are grateful for the continued support of the Barbara Jane Levy family (SAR) and for the NIH/NCI grants R37 CA046120-19 (AR) and CA61986-11 (RF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Belien AT, Paganetti PA, Schwab ME. Membrane-type 1 matrix metalloprotease (MT1-MMP) enables invasive migration of glioma cells in central nervous system white matter. J Cell Biol. 1999;144:373–384. doi: 10.1083/jcb.144.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellail AC, Hunter SB, Brat DJ, Tan C, Van Meir EG. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem. Cell Biol. 2004;36:1046–1069. doi: 10.1016/j.biocel.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Bos TJ, Cohn SL, Kleinman HK, Murphy-Ullrich JE, Podhajcer OL, Rich JN. International Hermelin brain tumor symposium on matricellular proteins in normal and cancer cell-matrix interactions. Matrix Biol. 2004;23:63–69. doi: 10.1016/j.matbio.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Bradshaw AD, Sage EH. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest. 2001;107:1049–1054. doi: 10.1172/JCI12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bresalier RS, Yan PS, Byrd JC, Lotan R, Raz A. Expression of the endogenous galactose-binding protein galectin-3 correlates with the malignant potential of tumors in the central nervous system. Cancer. 1997;80:776–787. [PubMed] [Google Scholar]
- 6.Calfice S, Castronovo V, van den Brule F. Galectin-3 and cancer. Int J Oncol. 2004;25:983–992. [PubMed] [Google Scholar]
- 7.Camby I, Belot N, Rorive S, Lefranc F, Maurage CA, Lahm H, Kaltner H, Hadari Y, Rouchoux MM, Brotchi J, Zick Y, Salmon I, Gabius HJ, Kiss R. Galectins are differentially expressed in supratentorial pilocytic astrocytomas, astrocytomas, anaplastic astrocytomas and glioblastomas, and significantly modulate tumor astrocyte migration. Brain Pathol. 2001;11:12–26. doi: 10.1111/j.1750-3639.2001.tb00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demuth T, Berens ME. Molecular mechanisms of glioma cell migration and invasion. J Neurooncol. 2004;70:217–228. doi: 10.1007/s11060-004-2751-6. [DOI] [PubMed] [Google Scholar]
- 9.Forsyth PA, Wong H, Laing TD, Rewcastle NB, Morris DG, Muzik H, Leco KJ, Johnston RN, Brasher PM, Sutherland G, Edwards DR. Gelatinase-A MMP-2), gelatinase B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. Br J Cancer. 1999;79:1828–1835. doi: 10.1038/sj.bjc.6990291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilles C, Bassuk JA, Pulyaeva H, Sage EH, Foidart JM, Thompson EW. SPARC/osteonectin induces matrix metalloproteinase 2 activation in human breast cancer cell lines. Cancer Res. 1998;58:5529–5536. [PubMed] [Google Scholar]
- 11.Gingras D, Page M, Annabi B, Beliveau R. Rapid activation of matrix metalloproteinase-2 by glioma cells occurs through a posttranslational MT1-MMP-dependent mechanism. Biochim Biophys Acta. 2000;1497:341–350. doi: 10.1016/s0167-4889(00)00071-9. [DOI] [PubMed] [Google Scholar]
- 12.Golembieski WA, Ge S, Nelson K, Mikkelsen T, Rempel SA. Increased SPARC expression promotes U87 glioblastoma invasion in vitro. Int J Dev Neurosci. 1999;17:463–472. doi: 10.1016/s0736-5748(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 13.Golembieski WA, Rempel SA. cDNA array analysis of SPARC-modulated changes in glioma gene expression. J Neurooncol. 2002;60:213–226. doi: 10.1023/a:1021167211131. [DOI] [PubMed] [Google Scholar]
- 14.Gordower L, Decaestecker C, Kacem Y, Lemmers A, Gusman J, Burchert M, Danguy A, Gabius H, Salmon I, Kiss R, Camby I. Galectin-3 and galectin-3-binding site expression in human adult astrocytic tumours and related angiogenesis. Neuropathol Appl Neurobiol. 1999;25:319–330. doi: 10.1046/j.1365-2990.1999.00192.x. [DOI] [PubMed] [Google Scholar]
- 15.Guo P, Imanishi Y, Cackowski FC, Jarzynka MJ, Tao HQ, Nishikawa R, Hirose T, Hu B, Cheng SY. Up-regulation of angiopoietin-2, matrix metalloprotease-2, membrane type 1 metalloprotease, and laminin 5 gamma 2 correlates with the invasiveness of human glioma. Am J Pathol. 2005;166:877–890. doi: 10.1016/s0002-9440(10)62308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lahm H, Andre S, Hoeflich A, Fischer JR, Sordat B, Kaltner H, Wolf E, Gabius HJ. Comprehensive galectin fingerprinting in a panel of 61 human tumor cell lines by RT-PCR and its implications for diagnostic and therapeutic procedures. J Cancer Res Clin Oncol. 2001;127:375–386. doi: 10.1007/s004320000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakada M, Kita D, Futami K, Yamashita J, Fujimoto N, Sato H, Okada Y. Roles of membrane type 1 matrix metalloproteinase and tissue inhibitor of metalloproteinases 2 in invasion and dissemination of human malignant glioma. J Neurosurg. 2001;94:464–473. doi: 10.3171/jns.2001.94.3.0464. [DOI] [PubMed] [Google Scholar]
- 18.Nakada M, Nakamura H, Ikeda E, Fujimotor N, Yamashita J, Sato H, Seiki M, Okada Y. Expression and tissue localization of membrane-type 1, 2, and 3 matrix metalloproteinases in human astrocytic tumors. Am J Pathol. 1999;154:417–428. doi: 10.1016/S0002-9440(10)65288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nuttall RK, Pennington CJ, Taplin J, Wheal A, Yong VW, Forsyth PA, Edwards DR. Elevated membrane-type matrix metalloproteinases in gliomas revealed by profiling proteases and inhibitors in human cancer cells. Mol Cancer Res. 2003;1:333–345. [PubMed] [Google Scholar]
- 20.Ochieng J, Fridman R, Nangia-Makker P, Kleiner DE, Liotta LA, Stetler-Stevenson WG, Raz A. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry. 1994;33:14109–1414. doi: 10.1021/bi00251a020. [DOI] [PubMed] [Google Scholar]
- 21.Ochieng J, Furtak V, Lukyanov P. Extracellular functions of galectin-3. Glycoconj J. 2004;19:527–535. doi: 10.1023/B:GLYC.0000014082.99675.2f. [DOI] [PubMed] [Google Scholar]
- 22.Ochieng J, Green B, Evans S, James O, Warfield P. Modulation of the biological functions of galectin-3 by matrix metalloproteinases. Biochim Biophys Acta. 1998;1379:97–106. doi: 10.1016/s0304-4165(97)00086-x. [DOI] [PubMed] [Google Scholar]
- 23.Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer. 2003;3:489–501. doi: 10.1038/nrc1121. [DOI] [PubMed] [Google Scholar]
- 24.Rempel SA, Ge S, Gutierrez JA. SPARC: a potential diagnostic marker of invasive meningiomas. Clin Cancer Res. 1999;5:237–241. [PubMed] [Google Scholar]
- 25.Rempel SA, Golembieski WA, Fisher JL, Maile M, Nakeff A. SPARC modulates cell growth, attachment and migration of U87 glioma cells on brain extracellular matrix proteins. J Neurooncol. 2001;53:149–160. doi: 10.1023/a:1012201300188. [DOI] [PubMed] [Google Scholar]
- 26.Rempel SA, Golembieski WA, Ge S, Lemke N, Elisevich K, Mikkelsen T, Gutierrez JA. SPARC: a signal of astrocytic neoplastic transformation and reactive response in human primary and xenograft gliomas. J Neuropathol Exp Neurol. 1998;57:1112–1121. doi: 10.1097/00005072-199812000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Rich JN, Shi Q, Hjelmeland M, Cummings TJ, Kuan CT, Bigner DD, Counter CM, Wang XF. Bone-related genes expressed in advanced malignancies induce invasion and metastasis in a genetically defined human cancer model. J Biol Chem. 2003;278:15951–15957. doi: 10.1074/jbc.M211498200. [DOI] [PubMed] [Google Scholar]
- 28.Schultz C, Lemke N, Ge S, Golembieski WA, Rempel SA. Secreted protein acidic and rich in cysteine promotes glioma invasion and delays tumor growth in vivo. Cancer Res. 2002;62:6270–6277. [PubMed] [Google Scholar]
- 29.Shekhar MP, Nangia-Makker P, Tait L, Miller F, Raz A. Alterations in galectin-3 expression and distribution correlate with breast cancer progression: functional analysis of galectin-3 in breast epithelial-endothelial interactions. Am J Pathol. 2004;165:1931–1941. doi: 10.1016/S0002-9440(10)63245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strik HM, Deininger MH, Frank B, Schluesener HJ, Meyermann R R. Galectin-3: cellular distribution and correlation with WHO-grade in human gliomas. Neurooncol. 2001;53:13–20. doi: 10.1023/a:1011874800612. [DOI] [PubMed] [Google Scholar]
- 31.Toth M, Osenkowski P, Hesek D, Brown S, Merouch S, Sakr W, Mobashery S, Fridman R. Cleavage at the stem region releases an active ectodomain of the membrane type 1 matrix metalloproteinase. Biochem J. 2005;387:497–506. doi: 10.1042/BJ20041324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tremble PM, Lane TF, Sage EH, Werb Z. SPARC, a secreted protein associated with morphogenesis and tissue remodeling, induces expression of metalloproteinases in fibroblasts through a novel extracellular matrix-dependent pathway. J Cell Biol. 1993;121:1433–1144. doi: 10.1083/jcb.121.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]


