Abstract
There are few prospective studies assessing risk factors for onset of temporomandibular (TMD) pain disorders in any age group. The aim of this prospective cohort study was to identify risk factors for onset of clinically significant TMD pain (i.e., pain meeting research diagnostic criteria for myofascial pain and/or arthralgia) during early adolescence. Subjects were 1,996 boys and girls, initially 11 years old, randomly selected from a large nonprofit health care system. Subjects completed a baseline telephone interview and were followed up with mailed questionnaires every 3 months for 3 years. At baseline and all follow ups, subjects were asked to report the presence of facial pain in the past 3 months. Subjects reporting a first onset of facial pain received a standardized clinical examination. In multivariate analyses, baseline predictors of clinically significant pain included female gender [Odds Ratio (OR) = 2.0, 95% Confidence Interval (CI) = 1.2–3.3] and negative somatic and psychological symptoms including somatization (OR = 1.8, CI = 1.1–2.8), number of other pain complaints (OR = 3.2, CI = 1.7–6.1) and life dissatisfaction (OR = 4.1, CI = 1.9–9.0). Many of the risk factors for onset of clinically significant TMD pain in adolescents are similar to risk factors for onset of TMD and other pain problems in adults, as well as risk factors for onset of other pain conditions in adolescents. These findings suggest that the development of TMD pain in adolescence may reflect an underlying vulnerability to musculoskeletal pain that is not unique to the orofacial region.
Keywords: temporomandibular disorders, adolescents, gender, chronic pain, somatization, incidence
INTRODUCTION
The etiologies of pain-related temporomandibular joint and muscle disorders (TMD’s) are poorly understood. Although several studies have assessed risk factors for these disorders, most have used case-control designs (Drangsholt and LeResche, 1999). This is not the most definitive design for assessing pain-related risk factors, as observed case-control differences might have arisen after pain onset, rather than predating the pain. For example, a case-control study cannot readily determine whether depressive symptoms reflect a risk factor for developing pain or a response to enduring pain.
A few studies have assessed risk factors for TMD pain using prospective designs. Kitai et al. (1997) studied onset of temporomandibular pain on function in 361 adolescent girls over a 5-year period. None of the several occlusal variables measured at baseline predicted pain onset. A prospective cohort study of 803 adults (Von Korff et al., 1993) found that baseline presence of multiple pain conditions elsewhere in the body predicted onset of TMD pain in the next 3 years. Severe depression (odds ratio (OR) =1.6) and female gender (OR=1.5) also predicted onset, but were not statistically significant (p>0.10). A recent 3-year prospective study (Diatchenko et al., 2005) found that, in the 170 young women analyzed, specific polymorphisms of the Catechol-O-Methyl-Transferase gene predicted onset of TMD pain meeting Research Diagnostic Criteria for TMD (RDC/TMD criteria) (Dworkin and LeResche, 1992). The same polymorphisms were associated with a summary score of sensitivity to a battery of experimental pain stimuli. However, the influence of other possible risk factors for onset of TMD was not reported.
In addition to these studies of pain onset, some prospective studies have examined factors predicting continuation or worsening of TMD pain. Depression has been found to predict poor treatment outcome (Grossi et al., 2001) and progression from acute to chronic jaw-related pain (Wright et al., 2004). Pain in other body sites at baseline was found to be associated with increased risk of onset of dysfunctional TMD pain in women (John et al., 2003), and with persistence of myofascial TMD pain (Rammelsberg et al., 2003). Thus, the literature suggests that presence of pain elsewhere in the body, female gender and perhaps pre-existing depressive symptoms are associated with onset and maintenance of temporomandibular pain in adults.
Many adults with TMD pain report that their condition began during adolescence (Von Korff et al., 1988). If gender, presence of other pain conditions and psychological symptoms are related to TMD onset, these factors may begin to play a role during the adolescent period. It is also possible that these factors are not strongly associated with pain onset, but are associated with the tendency for pain, if present, to persist and become more severe. The aim of the present prospective cohort study was to identify risk factors for onset of clinically significant TMD pain, that is, pain associated with an RDC/TMD diagnosis of myofascial pain or arthralgia, between the ages of 11 and 14 years. We also assessed risk factors for onset of facial pain that did not meet these strict diagnostic criteria.
METHODS
Study Sample
Subjects in this longitudinal study were boys and girls, initially 11 years old, selected from the enrollees of Group Health Cooperative, a large nonprofit integrated health care system in Washington State. Initial telephone interviews took place from May 2000 through April 2001. During this period, individuals were sampled from the Group Health enrollment database on a monthly basis. Each monthly sample consisted of all enrollees (except those sampled in previous months) who lived in the Puget Sound region and were 11 years 0 months through 11 years 10 months of age. This selection criterion was chosen to allow sufficient time for the child to be interviewed before his or her 12th birthday. If multiple children meeting the age criteria resided in the same household, one child was chosen at random to participate. Children who were not sufficiently proficient in English to be able to understand the interview questions, or whose parents were not sufficiently proficient in English to be able to provide informed consent were considered ineligible.
Data Collection Procedures
An advance letter was sent to the parents of the selected children; the envelope contained a separate letter to the child and a fact sheet explaining the study procedures. Parents and children were informed that if they did not wish to be contacted about the study, they could telephone the study office to refuse participation. Households not refusing initial contact were telephoned by a female survey interviewer. The interviewer spoke with the child’s parent or legal guardian and explained the study procedures in detail. In order for the child to participate in the study, the parent or legal guardian was required to provide informed consent and the child was required to provide informed assent.
Data on history and presence of facial pain, back pain, headache, and stomach pain in the past 3 months, as well as information on demographics and suspected risk factors were collected from the child through a telephone survey at baseline. Subjects received a $5 gift certificate to a local video store for completing the baseline interview. In addition, consenting parents and legal guardians participated in a brief telephone interview concerning their own pain experience, educational level, relationship to the child and marital status.
Three months after completing the baseline interview, the child received a brief mailed questionnaire with a prepaid return envelope. The questionnaire inquired into the presence in the past 3 months of each of the four pain conditions. A limited number of mutable risk factors were also assessed in the mailed questionnaire. Identical questionnaires were sent every three months for the next three years. Subjects who failed to respond to a questionnaire were given a reminder telephone call and sent a new questionnaire if necessary. Subjects were paid $5 for completion of each mailed questionnaire. Subjects who actively refused further participation, who could not be located through the health plan records or the post office (forwarding addresses) or who failed to respond to two questionnaires in a row were not sent further questionnaires. Finally, an attempt was made to contact the entire sample, (including those lost to mail follow up and passive drop outs, but excluding active refusals) for a final telephone follow up 36 months after the initial telephone survey. The follow up interview was similar to that completed at baseline.
Subjects who reported a first onset of facial pain on any follow up questionnaire or at the final 36 month interview, were followed up with an in-home interview and examination. Subjects were considered to have a first onset of facial pain if they had not reported a history of facial pain at baseline or at any prior follow up. The examination and interview were conducted by trained, dental hygienist interviewer/examiners. All procedures were approved by the Institutional Review Boards of Group Health Cooperative and the University of Washington.
Measures
Baseline interview
The child’s age and sex were obtained from Group Health’s enrollment database and confirmed with both the parent and the child. The parent was asked to report his or her own educational level and to report whether he/she had had a problem with any of 6 pain conditions (back pain, headache, facial pain, stomach pain, chest pain or joint pain) at any time. Those parents with a history of a pain condition were asked whether the pain problem had occurred in the past 6 months. Data on the child’s race was gathered directly from the child.
Children were asked if they had ever had a problem with each of four pain conditions: facial pain (“pain in any of the following places: the muscles of the face, the joint in front of the ear or inside the ear, other than an ear infection”), back pain, headache and stomach pain. They were asked to report only pain that had lasted a whole day or more, or that had occurred several times in a year. Subjects were specifically instructed not to report “little aches and pains that didn’t last very long, like a short headache or sore muscles after exercising.” Subjects who reported having experienced a given pain condition were reminded of the severity criteria and asked to report whether the pain condition had occurred in the past 3 months. Date anchors were provided to help the subjects recall the 3-month period (e.g., if an interview was conducted in early December, the subject was asked if the pain had occurred “in the past 3 months, that is, since the beginning of the school year.”) The pain questions for both the parent and the child were similar to those used in our prior epidemiologic research (Von Korff et al., 1988; Von Korff et al., 1993).
In addition to questions on pain conditions, the baseline interview included standardized questions on school performance (McAfee et al., 2005), school satisfaction (Bachman et al., 1986) general health status (Von Korff et al., 1988), sedentary activities such as television, video, phone and computer use (Bachman et al., 1986), physical activity level (Koo and Rohan, 1999) history of smoking (McAfee et al., 2005) and life satisfaction (Bachman et al., 1986), as well as questions on history of orthodontic treatment developed for this study. Subjects reported height and weight and these measurements were converted to body mass index (BMI) percentiles for age and gender using the 2000 CDC Growth Chart Standards (Centers for Disease Control, 2006). These standards are based on U.S. national data collected from 1963–1980. The CDC has not updated these norms in order to avoid normalization of the current epidemic of obesity; currently over 17% of adolescents in the U.S. are above the 95th percentile. Subjects at or above the 95th percentile were classified as obese; those at or above the 85th percentile but below the 95th were classified as overweight. The Rosenberg Self Esteem Scale (Rosenberg, 1965), designed specifically to assess self esteem among adolescents, was also administered. Depression and somatization were assessed using abbreviated scales derived from the SCL-90 (Derogotis and Cleary, 1977). The SCL-90 was originally designed for use with adults and older adolescents, but it has been used successfully in studies of children as young as 11 years of age. We used data from the adult population of the same health care system (Von Korff et al., 1988) to identify 6 depression items and 5 somatization items from the original SCL-90 scales that showed high correlations with the respective full scale (0.95 and 0.96 respectively) and adequate internal consistency (0.81 and 0.75 respectively). In this sample of 11-year olds, internal consistency was 0.73 for depression, and 0.68 for somatization. Average item scores (0–4) were calculated for each scale.
Follow up measures of facial pain
The definition of facial pain at follow up was the same as at baseline, i.e., “pain any of the following places: the muscles of the face, the joint in front of the ear or inside the ear, other than an ear infection” in the past 3 months.
TMD Pain Diagnoses Derived from Examination
Subjects reporting a first onset of facial pain in response to any of the follow up questionnaires or the final telephone interview were contacted by a registered dental hygienist and asked to participate in an interview and examination in the subject’s home. The four hygienists conducting these examinations were trained and calibrated on examination methods for the Research Diagnostic Criteria for TMD (RDC/TMD) examination (Dworkin and LeResche, 1992). After the examination data were collected, RDC/TMD diagnoses were computed by algorithm. Subjects meeting criteria for myofascial pain with or without limited opening (Axis 1, Group 1a or 1b disorders) and/or for arthralgia or arthritis (Axis 1, Group 3a or 3b) were considered to have an RDC/TMD pain diagnosis.
Outcome Variable
Subjects were classified into one of three categories: 1) no facial pain report, 2) facial pain report with confirmed RDC/TMD pain diagnosis (i.e., clinically significant pain), or 3) facial pain report without confirmed RDC/TMD pain diagnosis.
Data Analysis and Statistical Methods
The analysis was restricted to subjects without a prior history of facial pain at baseline survey. In addition, subjects had to have at least a Month 3 follow up questionnaire. Only data prior to the first missing follow up were used. Subjects excluded from the analyses due to lack of follow-up at 3 months were different from the subjects included in the analyses on a number of variables potentially associated with facial pain, including gender, race, general health, history of orthodontic treatment, other current pain conditions, school performance, self esteem, depression, somatization, history of smoking, sedentary activity, BMI, parent’s lifetime pain conditions, and parent’s education level. To account for these differences between subjects with and without follow-up at 3 months, weighted multinomial logistic regression was performed for all regression analyses (Robins et al., 1995).
Weighting Method
A multivariate logistic regression was used to obtain predicted probabilities of follow up at 3 months. Variables used to predict follow up at Month 3 were all of the variables listed above that differed significantly between those subjects who completed the Month 3 follow up and those who did not. The inverse of the predicted probabilities of follow up at 3 months were then used as weights in all multinomial regression analyses to obtain adjusted estimates for odds ratios. Following the method of Robins et al. (1995), a modified empirical variance estimator that adjusted for the estimation of the weights was used to estimate the standard errors, and to compute the statistical significance and confidence intervals.
Multinomial logistic regression was used to determine the association between the risk factors and the three categories of the outcome variable, pain status (i.e., no pain, pain meeting RDC/TMD criteria or pain report only). All regression analyses controlled for the number of months a subject was followed before the first missing follow up or up to the first pain report, whichever occurred first. Initially, bivariate analyses were used to assess the relationship of each baseline variable to the outcome variables. Baseline variables found to be significant predictors of outcome in the bivariate analyses were then entered into multivariate models.
There were only minor differences between the weighted and unweighted results. For example, all variables in the multivariate analysis that were statistically significant at a 0.05 significance level (Table 3) were the same for the weighted and unweighted analyses. The differences in the odd ratio (OR) estimates were usually less than 10%, and the largest difference (15%) was for the OR for a pain diagnosis (versus no pain) for the variable life satisfaction (neutral or dissatisfied vs. very satisfied), the weighted OR was 4.1 and the unweighted OR was 3.6. The data shown in Tables 2 and 3 are the weighted estimates.
Table 3.
Baseline Predictors of First Onsets of RDC/TMD Pain Diagnoses and TMD Pain Report Only (Multivariate Analyses) – Weighted for missing 3-month follow-up1
| Measure | P-value Overall | TMD Pain Diagnosis | Pain Report Only |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | ||
| Female vs. Male | .002 | 2.01 (1.2–3.3)*** | 1.47 (1.1–2.0)** |
| Race | .14 | ||
| Asian vs. white | .28 (.1–1.0) | .70 (.4–1.2) | |
| Black vs. white | .72 (.3–1.6) | .67 (.4–1.1) | |
| Other vs. white | .57 (.3–1.2) | .67 (.4–1.0) | |
| Somatization (0–4) | 0.043 | 1.80 (1.1–2.8)* | 1.18 (.8–1.6) |
| Self-esteem (0–6) | .61 | .98 (.8–1.2) | .93 (.8–1.1) |
| Satisfaction w/life in general (vs. Very Satisfied) | .004 | ||
| Satisfied | 1.68 (1.0–2.9) | .89 (.7–1.2) | |
| Neutral or dissatisfied | 4.12 (1.9–9.0)**** | .99 (.5–1.8) | |
| No. of other pains2 | .003 | ||
| 1 vs 0 | 0.87 (.5–1.6) | .93 (.7–1.3) | |
| 2–3 vs 0 | 3.22 (1.7–6.1)**** | 1.50 (.9–2.4) | |
| Ever smoked (yes vs. no) | .089 | .59 (.2–1.8) | .37 (.2–.9)* |
| Ever orthodontic treatment (yes vs. no) | .25 | 0.96 (.6–1.7) | 1.28 (.9–1.8) |
| BMI | .21 | ||
| Overweight vs. normal | .92 (.5–1.7) | .72 (.5–1.1) | |
| Obese vs. normal | 1.02 (.5–2.0) | .64 (.4–1.0) | |
| Parent’s # of pain conditions (lifetime) | .098 | ||
| 1–2 vs. 0 | 1.29 (.4–4.1) | .98 (.6–1.6) | |
| 3–6 vs. 0 | 2.38 (.8–7.4) | .91 (.6–1.5) | |
| Parent’s education (vs professional/grad school) | .017 | ||
| ≤ High school | .33 (.1–.8)* | .59 (.4–.9)* | |
| Some College | .57 (.3–1.1) | .88 (.6–1.3) | |
| College | 1.02 (.5–2.1) | .91 (.6–1.4) |
All results are adjusted for the number of months a subject was followed before the first missing follow-up or until the first pain report.
Pain in the past 3 months, yes vs. no
p <0.05,
p < 0.01,
p < 0.005,
p < 0.001
Table 2.
Baseline Predictors of First Onsets of RDC/TMD Pain Diagnoses and TMD Pain Report Only (Bivariate Analyses) – Weighted for missing 3-month follow-up1
| Measure | Overall P-value | TMD Pain Diagnosis | Pain Report Only |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | ||
| Female vs. Male | .001 | 1.89 (1.2–3.0)** | 1.54 (1.2–2.0)*** |
| Race | .060 | ||
| Asian vs. white | .36 (.1–1.1) | .74 (.4–1.3) | |
| Black vs. white | .86 (.4–1.8) | .60 (.4–.9)* | |
| Other vs. white | .80 (.4–1.5) | .60 (.4–.9)* | |
| Depression (0–4) | <.001 | 1.87 (1.4–2.5)**** | .92 (.8–1.2) |
| Somatization (0–4) | <.001 | 2.20 (1.5–3.2)**** | 1.09 (.8–1.5) |
| Self-esteem (0–6) | .058 | .81 (.7–.9)* | .97 (.9–1.1) |
| Satisfaction w/life in general (vs. Very Satisfied) | .001 | ||
| Satisfied | 1.67 (.9–2.9) | .90 (.7–1.2) | |
| Neutral or dissatisfied | 4.22 (2.0–8.7)**** | .94 (.5–1.6) | |
| No. of other pains2 | <.001 | ||
| 1 vs 0 | 1.11 (.6–2.0) | .98 (.7–1.4) | |
| 2–3 vs 0 | 4.64 (2.6–8.1)**** | 1.43 (.9–2.2) | |
| Back pain2 | <.001 | 3.90 (2.2–6.8)**** | 1.41 (.9–2.2) |
| Headache2 | <.001 | 2.65 (1.6–4.4)**** | 1.25 (.8–1.8) |
| Stomach pain2 | .027 | 1.89 (1.2–3.1)* | .98 (.7–1.4) |
| Ever smoked (yes vs. no) | .034 | .81 (.3–2.2) | .32 (.1–.8)** |
| Ever orthodontic treatment (yes vs. no) | .031 | 1.14 (.7–1.9) | 1.49 (1.1–2.0)** |
| BMI | .031 | ||
| Overweight vs. normal | .94 (.5–1.7) | .66 (.4–.9)* | |
| Obese vs. normal | .96 (.5–1.9) | .54 (.3–.8)** | |
| Parent’s # of pain conditions (lifetime) | .029 | ||
| 1–2 vs. 0 | 1.70 (.6–4.7) | 1.07 (.6–1.8) | |
| 3–6 vs. 0 | 3.04 (1.1–8.2)* | .95 (.6–1.5) | |
| Parent’s education (vs professional/grad school) | .040 | ||
| ≤ High school | .51 (.2–1.2) | .54 (.3–.9)** | |
| Some College | .83 (.4–1.6) | .84 (.6–1.2) | |
| College | 1.10 (.6–2.2) | .89 (.6–1.3) |
All results are adjusted for the number of months a subject was followed before the first missing follow-up or until the first pain report.
Pain in the past 3 months, yes vs. no
p <0.05,
p < 0.01,
p < 0.005,
p < 0.001
RESULTS
Initially, 1,996 subjects completed the baseline interview, a response rate of 49%. Baseline interview data for these subjects were previously reported as part of a cross-sectional survey of 11–17 year olds (LeResche et al., 2005). As described in that publication, the response rate did not differ by gender or age and the sample was racially similar to the underlying population of the health care system. It is also demographically similar to the population of the greater Seattle, Washington metropolitan area. Among the 1,996 initial respondents, 1,674 reported no history of TMD pain at baseline and thus were eligible for the risk factor analyses. The analyses for this study were confined to those subjects who completed at least the Month 3 follow up questionnaire, a total of 1,310 subjects. Figure 1 displays the outcomes for all subjects initially approached for the study.
Fig 1.
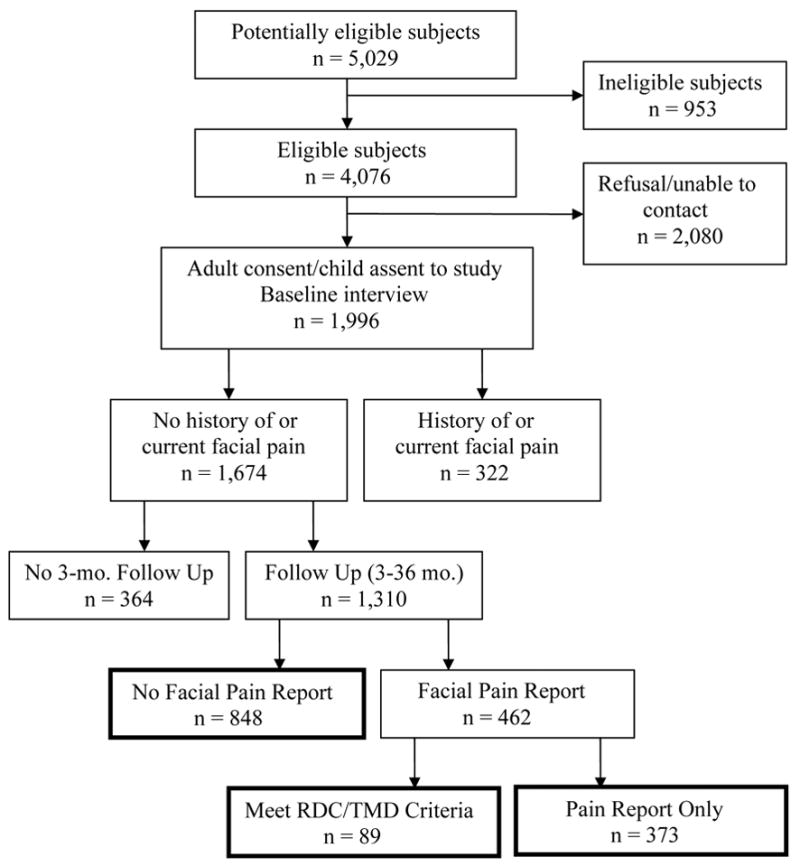
Outcome of subjects approached for the study. Boxes for the three outcome categories compared in the tables are identified with bold lines.
Among the 1,310 subjects in the sample, 848 (64.7%) reported no facial pain prior to the first missing follow up; 89 subjects (6.8% of the cohort) had an onset of clinically significant TMD pain, i.e., reported facial pain and were assigned an RDC/TMD pain diagnosis. Of these, 36 subjects met criteria for myofascial pain only, 40 for arthralgia only and 13 for both disorders. The remaining 373 subjects in the cohort (28.5%) reported a first onset of facial pain but were found on examination not to meet criteria for an RDC/TMD pain diagnosis.
The characteristics of the three outcome groups are shown in Table 1. Overall, slightly more girls than boys participated, although there were somewhat more boys than girls in the no pain group. In all three groups, most subjects reported their race as white. The modal level of parental education was some college or vocational school, but a substantial proportion of the parents had completed college or graduate education. Over three-quarters of the parents in all groups were married or living as married.
Table 1.
Sample Characteristics by Pain Status
| Pain Status | ||||||
|---|---|---|---|---|---|---|
| No Pain (n = 848) | Pain Diagnosis (n = 89) | Pain Report Only (n = 373) | ||||
| N | % | N | % | N | % | |
| Gender | 429 | 50.6 | 37 | 41.6 | 171 | 45.8 |
| Male | ||||||
| Female | 419 | 49.4 | 52 | 58.4 | 202 | 54.2 |
| Race | 78 | 9.2 | 3 | 3.4 | 26 | 7.0 |
| Asian | ||||||
| Black | 67 | 7.9 | 10 | 11.2 | 32 | 8.6 |
| Other | 130 | 15.3 | 14 | 15.7 | 44 | 11.8 |
| White | 573 | 67.6 | 62 | 69.7 | 271 | 72.7 |
| Parent’s education | 147 | 17.3 | 11 | 12.4 | 49 | 13.1 |
| <=High School | ||||||
| Some college | 310 | 36.6 | 34 | 38.2 | 153 | 41.0 |
| College | 222 | 26.2 | 27 | 30.3 | 98 | 26.3 |
| Prof/grad | 169 | 19.9 | 17 | 19.1 | 73 | 19.6 |
| Parent marital status | ||||||
| Married/living as married | 658 | 77.6 | 71 | 79.8 | 295 | 79.1 |
| Never married | 36 | 4.2 | 5 | 5.6 | 15 | 4.0 |
| Separated | 26 | 3.1 | 2 | 2.2 | 10 | 2.7 |
| Divorced | 95 | 11.2 | 10 | 11.2 | 45 | 12.1 |
| Widowed | 13 | 1.5 | 1 | 1.1 | 5 | 1.3 |
| Unknown | 20 | 2.4 | 0 | 0 | 3 | 0.8 |
| Satisfaction with life | ||||||
| Very satisfied | 358 | 42.2 | 22 | 24.7 | 154 | 41.3 |
| Satisfied | 439 | 51.8 | 50 | 56.2 | 193 | 51.7 |
| Neutral/dissatisfied | 51 | 6.0 | 17 | 19.1 | 26 | 7.0 |
| Number of other pains1 | ||||||
| 0 | 616 | 72.6 | 43 | 48.3 | 240 | 64.3 |
| 1 | 166 | 19.6 | 17 | 19.1 | 84 | 22.5 |
| 2–3 | 66 | 7.8 | 29 | 32.6 | 49 | 13.1 |
| Back pain1 | 62 | 7.3 | 26 | 29.2 | 47 | 12.6 |
| Headache1 | 92 | 10.8 | 27 | 30.3 | 63 | 16.9 |
| Stomach pain1 | 159 | 18.8 | 30 | 33.7 | 80 | 21.4 |
| Ever smoked | 26 | 3.1 | 5 | 5.6 | 7 | 1.9 |
| Orthodontic treatment | 263 | 31.0 | 24 | 27.0 | 122 | 32.7 |
| BMI | ||||||
| normal (<85%) | 580 | 68.4 | 60 | 67.4 | 283 | 75.9 |
| overweight (≥85% – <95%) | 146 | 17.2 | 16 | 18.0 | 53 | 14.2 |
| obese (≥95%) | 122 | 14.4 | 13 | 14.6 | 37 | 9.9 |
| Parent’s # of pain conditions (lifetime) | ||||||
| 0 | 78 | 9.2 | 4 | 4.5 | 38 | 10.2 |
| 1–2 | 401 | 47.3 | 26 | 29.2 | 161 | 43.2 |
| 3–6 | 369 | 43.5 | 59 | 66.3 | 174 | 46.6 |
Past 3 months
Table 2 shows the results of the bivariate multinomial logistic regression analyses for the individual baseline variables that were indicative of an association with the onset of clinically significant TMD pain meeting RDC/TMD diagnostic criteria and/or a facial pain report only (overall p-value <0.10). Female gender was the only variable that predicted both the onset of an RDC/TMD pain diagnosis and the onset of facial pain report alone. In the bivariate analyses, the odds of experiencing an onset of a pain condition meeting RDC/TMD criteria (i.e., clinically significant facial pain) were significantly increased among children with higher levels of depression and somatic symptoms at baseline. Children with low self esteem at baseline, as well as those who reported themselves to be neutral or dissatisfied with life in general were also at greater risk for facial pain meeting RDC/TMD diagnostic criteria. The probability of developing clinically significant facial pain was increased for children who reported experiencing any one of the other pain conditions at baseline, and the probability increased with the number of other pain conditions reported, such that, for children with 2–3 pain conditions, the odds of experiencing an onset of clinically significant pain were over 4 times those of children with none of the other pain conditions at baseline. Finally, children whose parents had a lifetime history of 3 or more pain conditions were at significantly increased risk for onset of a facial pain meeting RDC/TMD criteria.
In contrast, risk factors for reporting an onset of facial pain only (without an RDC/TMD diagnosis) included race, smoking status, orthodontic treatment, and body mass index (BMI). Children who reported their race as white were significantly more likely to experience an onset of facial pain not meeting diagnostic criteria than those who reported that their race was black or “other.” Never having smoked and having undergone orthodontic treatment were each predictors of an onset of facial pain (without an RDC/TMD diagnosis) during the follow up period. Children who were obese or overweight were less likely to experience facial pain than those of normal weight and those whose parents had a high school education or less were also significantly less likely to report facial pain, compared with children whose parents had a professional or graduate education.
Baseline variables showing no statistically significant relationship with future facial pain or RDC/TMD pain diagnoses included self rated general health status, amount of time spent in sedentary activities, physical activity level, self rated school performance, school satisfaction and parental marital status. The overall p-values for these variables ranged from 0.21–0.94.
Variables were selected for inclusion in the multivariate model if they were indicative of a bivariate relationship with either facial pain alone or with the presence of an RDC/TMD pain diagnosis (overall p-value < 0.10). Because each of the individual pain conditions was highly correlated with total number of pain conditions, only the total number of pain conditions was included in the multivariate model. Depression and somatization scores were also highly intercorrelated (r = 0.62). Somatization was included in the final multivariate model because it showed the higher level of statistical significance for predicting clinically significant pain. In a model including depression in place of somatization, there was still some indication that higher levels of depression or were predictive of an RDC/TMD pain diagnosis, but the relationship between depression and clinically significant pain did not achieve statistical significance.
In the final multivariate model (Table 3), female gender continued to predict both onset of clinically significant TMD pain meeting RDC/TMD criteria as well as facial pain report only; low parental education was also significantly associated with lower risk of experiencing both clinically significant pain and pain report without an RDC/TMD diagnosis. Other baseline variables associated with significantly increased risk of experiencing an onset of TMD pain meeting RDC/TMD criteria during the follow up period included high somatization, low satisfaction with life in general and number of other pains at baseline.
In the multivariate model, children with a prior history of smoking remained at lower risk for facial pain report not meeting RDC/TMD criteria. However, the associations of pain onset with race, orthodontic treatment and BMI found in the bivariate analyses were no longer statistically significant.
DISCUSSION
This 3-year prospective cohort study found that, among subjects reporting no history of facial pain at age 11, a number of factors predicted the onset of a pain condition meeting RDC/TMD criteria by age 14. These factors included female gender and negative somatic and psychological symptoms – somatization, number of existing pain conditions and report of being neutral about or dissatisfied with life at age 11. These factors are similar to some of the risk factors predicting onset of a range of pain conditions in adults. Our findings suggest that the relationship of these factors to pain onset can occur quite early in the lifespan, and may reflect a predisposition to develop pain that has its basis in genetic factors, early life experiences or the interaction of these factors.
Among the young adolescents in this study, 28.5% reported a first onset of facial pain on one of the follow up questionnaires, but had no RDC/TMD diagnosis on examination. Only 6.8% both reported pain and met RDC/TMD criteria when examined – an annualized rate of 2.3%. Thus, the use of written questionnaires at these very short (3-month) follow-up intervals resulted in a large number of pain reports that did not meet diagnostic criteria for clinically significant pain.
It may be that, despite instructions not to report “little aches and pain that didn’t last very long,” subjects reported pain problems that were fleeting or minor. It is also possible that, simply because of the frequency of the questionnaires, subjects had an opportunity to report pain that was of substantial intensity and even of several days’ duration, but was no longer present when the subject was examined an average of 7 weeks after answering the questionnaire. Such pain might be forgotten with longer follow-up intervals, e.g., the 1-year follow-up typical in prospective studies. Reports of pain that did not meet diagnostic criteria for a TMD pain disorder could also represent the early stages of development of a more significant pain condition. However, this hypothesis cannot be tested without further follow up of the cohort.
Given that a substantial number of subjects reported facial pain that did not meet RDC/TMD criteria upon examination, it is not surprising that, by and large, different predictive factors were found for pain report alone and for clinically significant pain (i.e., pain report plus the presence of an RDC/TMD diagnosis of myofascial pain and/or arthralgia). In the multivariate analyses, two factors were associated with both pain report and clinically significant pain. First, girls were significantly more likely than boys both to report facial pain that did not meet RDC/TMD criteria for a pain diagnosis, and to report facial pain that did meet these criteria. This gender difference parallels the prevalence patterns seen in adults (Drangsholt and LeResche, 1999). Secondly, low parental education was associated with both a lower rate of facial pain report and a lower rate of pain meeting diagnostic criteria. The reason for this difference by parental education/socioeconomic status (SES) is not clear. The literature on the relationship of SES to pain report in children is mixed. A number of studies have found a relationship between low socioeconomic status and higher rates of various kinds of pain in children and/or adolescents (e.g., Sillanpää et al., 1991; Hotopf et al., 1998; Anttila et al., 2002; Grøholt et al., 2003); however, there are also studies that found no relationship between pain and socioeconomic status (e.g., Kristjansdottir, 1996; Mikkelsson et al., 1997). It may be that report of facial pain, as opposed to other pain conditions, is less acceptable in families of lower SES. The only other factor in the multivariate analyses predicting onset of facial pain alone was never having smoked. This result was unexpected, but given that less than 3% of subjects reported ever having smoked, this relationship should be examined in samples with a larger number of smokers.
In contrast, the factors predicting clinically significant TMD pain represent a cluster of negative somatic and psychological symptoms, i.e., somatization, number of other pains complaints and life dissatisfaction at baseline. It should be noted that the somatization scale used in this study did not include items related to pain. Thus both pain-related and non-pain related somatic symptoms at baseline predicted first onset of TMD pain. It is interesting that somatization was associated with TMD pain meeting RDC/TMD criteria, but not with facial pain alone. This finding is contrary to the theory that somatization reflects a tendency to self-report inconsequential symptoms of questionable clinical significance, and is more consistent with the idea that somatization is a marker of heightened sensitization to diverse stimuli.
In adults, negative affect, somatic symptoms and other pain complaints have been found to be associated with the onset and maintenance of TMD pain (Von Korff et al., 1993; Grossi et al., 2001; John et al., 2003; Wright et al., 2004) as well as other pain problems (Von Korff et al., 1993; McBeth et al., 2001). Similar factors have been found to predict onsets of back pain (Jones et al., 2003a) and chronic widespread pain (Jones et al., 2003b) in population-based studies of adolescents. It has been hypothesized that early experience of somatic symptoms (including pain in other body sites) may result in a hypervigilance to bodily symptoms (Rollman et al., 2004) that can sensitize an individual to develop additional pain complaints. If so, our results suggest that such hypervigilance may already be present in early adolescence. It is also possible that the presence of other pain complaints and somatic symptoms indicate an underlying vulnerability to pain that has a genetic basis (Diatchenko et al., 2005; Mogil and Devor, 2004). Of course, these explanations need not be mutually exclusive.
It is notable that in the multivariate model, even after controlling for pain complaints and somatization, dissatisfaction with life in general emerged as a strong independent predictor of the onset of clinically significant TMD pain. To our knowledge, life dissatisfaction has not been measured in other pain studies. It may be that life dissatisfaction in adolescents indicates a negative affectivity (Watson and Clark, 1984) that may manifest itself as depression in adults.
Our study has a number of limitations and several strengths. Because we were interested in tracking the dynamic nature of pain during puberty, we employed very frequent follow up (every 3 months). Few other pain onset studies use such short follow-up intervals. With this high frequency of follow up, rates of onset of facial pain were much higher than we initially predicted based on studies using a 1-year follow up period, and fewer of the facial pain reports resulted in a TMD diagnosis than was the case in a similar study of adults (LeResche, 1995). These observations point to the importance of diagnostic confirmation of self report of pain onsets, particularly in studies with frequent follow ups, to ascertain new occurrences of the pain condition of interest. Since few longitudinal studies have employed frequent follow ups in assessing onsets of pain conditions, this is an area that deserves increased methodological research.
The prospect of such frequent follow up probably also contributed to the fact that a number of subjects with no history of facial pain at baseline were lost to the study early on – even before the first follow up – and thus we could not utilize their data for these analyses. These early drop outs differed in many ways from the subjects retained in the cohort, but we were able to adjust for these factors in the weighted analyses presented in this paper.
Although we excluded subjects who reported a history of TMD pain at baseline (a requirement of cohort studies) we found a relatively high occurrence of new cases in the first 9 months of the follow-up period. This pattern raises the possibility that some of the new onset cases we detected had experienced facial pain before, but had forgotten about it. To the extent that this is true, the incidence rate might be an overestimate of the actual incidence. This limitation is, however, inherent in all cohort studies that rely on self report for some aspect of the case definition.
Despite its limitations, this is one of the few large, population-based prospective studies of the onset of any kind of pain in adolescents. One of the unique advantages of this study was the use of a standardized clinical examination to determine if the pain problem met specified diagnostic criteria. Other studies examining risk factors for pain onset have lacked such diagnostic assessments, either because they are not available for the pain conditions studied or it was not feasible to use them in field studies. We were able to confirm that many risk factors for onset of clinically significant TMD pain in adolescents are similar to the risk factors for onset of TMD and other pain problems in adults, and are also similar to risk factors for onset of other pain conditions in adolescents. These findings suggest that individuals who develop TMD pain in adolescence may have an underlying vulnerability to experience pain that is not unique to the orofacial region.
Acknowledgments
We thank Kimberly Huggins, Katie Saunders and Dr. Laura Richardson for comments on earlier versions of this paper. We also wish to thank the dental hygienists who collected the examination data -- Perdis Jacobsen, Barbara Omalev, Barbara Appleby and Nancy Alleman. This study was supported by Grant No. DE08773 from the National Institutes Health (National Institute of Dental and Craniofacial Research), USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anttila P, Metsähonkala L, Aromaa M, Sourander A, Salminen J, Helenius H, Alanen P, Sillanpää M. Determinants of tension-type headache in children. Cephalalgia. 2002;22:401–408. doi: 10.1046/j.1468-2982.2002.00381.x. [DOI] [PubMed] [Google Scholar]
- Bachman JG, Johnston LD, O’Malley PM. Monitoring the future: questionnaire responses from the nation’s high school seniors. Institute of Social Research, University of Michigan; Ann Arbor: 1986. [Google Scholar]
- Centers for Disease Control Growth Charts: United States. http://www.cdc.gov/growthcharts/
- Derogatis LR, Cleary PA. Confirmation of the dimensional structure of the SCL-90: A study in construct validation. J Clin Psychol. 1977;33:981–989. [Google Scholar]
- Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- Drangsholt M, LeResche L. Temporomandibular disorder pain. Chapter 15 . In: Crombie IK, Croft PR, Linton SJ, LeResche L, Von Korff M, editors. Epidemiology of Pain. IASP Press; Seattle: 1999. pp. 203–233. [Google Scholar]
- Dworkin SF, LeResche L. Research Diagnostic Criteria for Temporomandibular Disorders: Review, criteria, examinations and specifications, critique. J Craniomandib Disord Facial Oral Pain. 1992;6:301–355. [PubMed] [Google Scholar]
- Grøholt E-K, Stigum H, Nordhagen R, Köhler L. Recurrent pain in children, socio-economic factors and accumulation in families. Eur J Epidemiol. 2003;18:965–975. doi: 10.1023/a:1025889912964. [DOI] [PubMed] [Google Scholar]
- Grossi ML, Goldberg MB, Locker D, Tenenbaum HC. Reduced neuropsychologic measures as predictors of treatment outcome in patients with temporomandibular disorders. J Orofac Pain. 2001;15:329–339. [PubMed] [Google Scholar]
- Hotopf M, Carr S, Mayou R, Wadsworth M, Wessely S. Why do children have chronic abdominal pain, and what happens to them when they grow up? Population based cohort study. BMJ. 1998:1196–1200. doi: 10.1136/bmj.316.7139.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John MT, Miglioretti DL, LeResche L, Von Korff M, Critchlow CW. Widespread pain as a risk factor for dysfunctional temporomandibular disorder pain. Pain. 2003;102:257–263. doi: 10.1016/S0304-3959(02)00404-9. [DOI] [PubMed] [Google Scholar]
- Jones GT, Watson KD, Silman AJ, Symmons DP, Macfarlane GJ. Predictors of low back pain in British schoolchildren: a population-based prospective cohort study. Pediatrics. 2003;111:822–828. doi: 10.1542/peds.111.4.822. 2003a. [DOI] [PubMed] [Google Scholar]
- Jones GT, Silman AJ, Macfarlane GJ. Predicting the onset of widespread body pain among children. Arthritis Rheum. 2003;48:2615–2621. doi: 10.1002/art.11221. 2003b. [DOI] [PubMed] [Google Scholar]
- Kitai N, Takada K, Yasuda Y, Verdonck A, Carels C. Pain and other cardinal TMJ dysfunction symptoms: A longitudinal survey of Japanese female adolescents. J Oral Rehabil. 1997;24:741–748. doi: 10.1046/j.1365-2842.1997.00567.x. [DOI] [PubMed] [Google Scholar]
- Koo MM, Rohan TE. Comparison of four habitual physical activity questionnaires in girls aged 7–15 yr. Med Sci Sports Exerc. 1999;31:421–427. doi: 10.1097/00005768-199903000-00011. [DOI] [PubMed] [Google Scholar]
- Kristjansdottir G. Prevalence of self-reported back pain in school children: A study of sociodemographic differences. European Journal of Pediatrics. 1996;155:984–986. doi: 10.1007/BF02282892. [DOI] [PubMed] [Google Scholar]
- LeResche L, Mancl LA, Drangsholt MT, Saunders K, Von Korff M. Relationship of pain and symptoms to pubertal development in adolescents. Pain. 2005;118:201–209. doi: 10.1016/j.pain.2005.08.011. [DOI] [PubMed] [Google Scholar]
- LeResche L. Research diagnostic criteria for TMD. In: Fricton JR, Dubner RB, editors. Orofacial Pain and Temporomandibular Disorders. Raven Press; New York: 1995. pp. 189–203. [Google Scholar]
- McAfee T, Ludman E, Grothaus L, Zbikowski SM, Bush T, Hollis J, Polen M, Curry SJ. Physician tobacco advice to preteens in a smoking-prevention randomized trial: steering clear. J Pediatr Psychol. 2005;30:371–376. doi: 10.1093/jpepsy/jsi031. [DOI] [PubMed] [Google Scholar]
- McBeth J, Macfarlane GJ, Benjamin S, Silman AJ. Features of somatization predict the onset of chronic widespread pain: results of a large population-based study. Arthritis Rheum. 2001;44:940–946. doi: 10.1002/1529-0131(200104)44:4<940::AID-ANR151>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Mikkelsson M, Sourander A, Piha J, Salminen JJ. Psychiatric symptoms in preadolescents with musculoskeletal pain and fibromyalgia. Pediatrics. 1997;100:220–227. doi: 10.1542/peds.100.2.220. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Devor M. Introduction to pain genetics. In: Mogil JS, editor. The Genetics of Pain. IASP Press; Seattle: 2004. pp. 1–17. [Google Scholar]
- Rammelsberg P, LeResche L, Dworkin S, Mancl L. Longitudinal outcome of temporomandibular disorders: a 5-year epidemiologic study of muscle disorders defined by research diagnostic criteria for temporomandibular disorders. J Orofac Pain. 2003;17:9–20. [PubMed] [Google Scholar]
- Robins JM, Rotnitzsky A, Zhao LP. Analysis of semiparametric regression models for repeated outcomes in the presence of missing data. J Am Stat Assoc. 1995;90:106–121. [Google Scholar]
- Rollman GB, Abdel-Shaheed J, Gillespie JM, Jones KS. Does past pain influence current pain: biological and psychosocial models of sex differences. Eur J Pain. 2004;8:427–433. doi: 10.1016/j.ejpain.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. Society and the Adolescent Self-Image. Princeton, NJ: Princeton University Press; 1965. [Google Scholar]
- Sillanpää M, Piekkala P, Kero P. Prevalence of headache at preschool age in an unselected child population. Cephalalgia. 1991;11:239–242. doi: 10.1046/j.1468-2982.1991.1105239.x. [DOI] [PubMed] [Google Scholar]
- Von Korff M, Dworkin SF, LeResche L, Kruger A. An epidemiologic comparison of pain complaints. Pain. 1988;32:173–183. doi: 10.1016/0304-3959(88)90066-8. [DOI] [PubMed] [Google Scholar]
- Von Korff M, LeResche L, Dworkin SF. First onset of common pain symptoms: A prospective study of depression as a risk factor. Pain. 1993;55:251–258. doi: 10.1016/0304-3959(93)90154-H. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. Negative affectivity: the disposition to experience aversive emotional states. Psychol Bull. 1984;96:465–490. [PubMed] [Google Scholar]
- Wright AR, Gatchel RJ, Wildenstein L, Riggs R, Buschang P, Ellis E., 3rd Biopsychosocial differences between high-risk and low-risk patients with acute TMD-related pain. J Am Dent Assoc. 2004;135:474–483. doi: 10.14219/jada.archive.2004.0213. [DOI] [PubMed] [Google Scholar]


