Abstract
Exposure of T cells to their specific antigen normally results in proliferation, but in the presence of high and repeatedly administered doses of antigen, T cells may undergo apoptosis. Here we demonstrate that i.v. administration of as little as 100 μg of recombinant P2 protein twice daily completely prevents experimental autoimmune neuritis induced by adoptive transfer of neuritogenic P2-specific T cells or by immunization with the neuritogenic P2–peptide-spanning amino acids 53–78. Antigen treatment started after disease onset markedly ameliorated experimental autoimmune neuritis. The mechanism of action may be through programmed T cell death; a profound increase of the rate of apoptosis was seen in inflammatory foci of peripheral nerves and in the spleen. There was no cytokine switch by our Th1 cells after exposure to their specific antigen, but increased secretion of interferon γ and tumor necrosis factor α was demonstrated. High antigen dose therapy using recombinant, pathogen-free protein may prove useful for the treatment of autoimmune inflammatory disorders of the nervous system.
Keywords: neuritis, recombinant myelin proteins
Experimental autoimmune neuritis (EAN) is an inflammatory demyelinating disease of the peripheral nervous system and serves as an animal model for human demyelinating neuropathies (1). EAN can be actively induced by the inoculation of Lewis rats with peripheral nerve myelin (2) or its main immunogenic compound, the P2 protein (3). Of interest, both bovine P2 and human recombinant P2 (which differs from bovine P2 by nine amino acid residues) are neuritogens in the Lewis rat (4). Another approach takes advantage of the adoptive transfer of activated, P2-specific, neuritogenic T lymphocytes (AT-EAN) (5). Clinical course, histopathology (6), and neurophysiology (7) of AT-EAN have been well characterized.
Recently, antigen-specific therapy with guinea pig myelin basic protein has been used to treat experimental autoimmune encephalomyelitis, an inflammatory disease of the central nervous system (8). Here we investigated whether recombinant myelin protein P2 could be used therapeutically in two EAN model diseases. This would circumvent the potential problems in prevention or therapy of comparable human disease with natural compounds, like the transmission of bovine spongiform encephalopathy to humans (9), in future human studies.
MATERIALS AND METHODS
Animals.
Female Lewis rats from Charles River Breeding Laboratories were 6–8 weeks old and had body weights of 125–160 g. Animals were housed in plastic cages in a room with natural lighting and were given commercial food pellets and water ad libitum. All experiments were conducted according to Bavarian state regulations for animal experimentation and were approved by the responsible authorities.
Expression and Purification of Recombinant Human Myelin Proteins.
Full length human P2 and the extracellular domain of human P0 were produced in Escherichia coli [rhP2 in BL21(DE3)pLysS and rhP0 in BL21(DE3) cells; Novagen] and were purified by nickel-nitrilo-tri-acetic acid affinity and gel filtration chromatography as described (4).
T Cell Culture and Proliferation Assays.
Neuritogenic P2-specific T cell lines G5 and G7 were established as described from Lewis rats (5, 10). T cell activation was tested in vitro using RPMI 1640 medium supplemented with 1% normal rat serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mM glutamine. All cell culture reagents were obtained from GIBCO. For experimentation, all T cells were taken from the same stage of activation (fifth cycle of restimulation).
T cell proliferation assays were performed in 96-well flat bottom microtiter plates; 1.5 × 104 responder G5 or G7 T cells, 7.5 × 105 irradiated (3000 rad) thymocytes, and various concentrations of rhP2 or the neuritogenic P2–peptide (amino acids 53–78) in a total volume of 100 μl were seeded per well. After 48 h, cells were labeled with 0.2 μCi (1 Ci = 37 GBq)/well tritiated thymidine (Amersham/Buchler) for 16 h and harvested at the indicated time points. The cells were collected on glass fiber filter paper by a Betaplate 96-well harvester (Pharmacia LKB). The radioactivity associated with the dried filter was quantified with a 96-well Betaplate liquid scintillation counter (Pharmacia LKB). For measuring cytokine secretion, T cells were cultured at a density of 7 × 105/ml in 12-well plates in medium comprised as described above for T cell activation.
Induction of EAN.
For active EAN, Lewis rats were inoculated in the footpad with 50 μl of an emulsion of an equal volume of 100 μg synthetic P2–peptide (amino acids 53–78 of the bovine P2 protein) in saline and complete Freund’s adjuvant with Mycobacterium tuberculosis (Difco) at a final concentration of 0.5 mg/ml. The peptide was synthesized by solid-phase stepwise elongation on an Applied Biosystems model 430 synthesizer.
AT-EAN was induced by tail vein injection of 8 × 106 P2-specific, CD4+-activated T cells (G7). Animals were killed at the time points mentioned above by intracardiac perfusion with Ringer’s solution containing 2 × 104 units/liter heparin followed by 4% paraformaldehyde in 0.1 M phosphate buffer at pH 7.4 (10).
Scoring.
Animals were weighed and inspected for clinical signs of disease on a daily basis. Disease severity was assessed clinically using a 10-grade scale ranging from 0 to 10 (11, 12): 0 = normal; 1 = less lively, reduced tone of tail; 2 = limp tail, impaired righting; 3 = absent righting; 4 = gait ataxia; 5 = mild paraparesis of the hind limbs; 6 = moderate paraparesis; 7 = severe paraparesis or paraplegia; 8 = tetraparesis; 9 = moribund; and 10 = death.
Immunocytochemistry.
Eight-micrometer paraffin sections were mounted on poly-l-lysine-coated slides and were processed as described (10). Tissue sections were deparaffinized in xylene and 96% ethanol, treated with chloroform, and washed in 0.05 M Tris buffer. Cellular infiltrates were characterized in serial sections by incubation with the mAb B 115-1 (dilution 1:500; Holland Biotechnology, Leiden, The Netherlands) staining pan T cells and ED 1 identifying macrophages (dilution 1:1000; Serotec) for 1 h at room temperature. We then used the avidin–biotin complex detection system (Dako) and 3,3′-diaminobenzidine as peroxidase substrate. Finally, sections were counterstained with hematoxylin, dehydrated, and mounted in Eukitt (Kindler, Freiburg, Germany). Coded sections from the sciatic nerve were examined by blinded observers. The number of B 115-1- and ED 1-stained cells was evaluated quantitatively in longitudinal nerve sections in five fields of 0.8 mm2 each at ×200 primary magnification per marker and animal.
Detection of Apoptosis by in Situ Tailing (IST).
IST was performed on paraffin-embedded tissue. Tissue sections were incubated for 1 h with 50 μl of a reaction mixture containing 1 μl of digoxigenin-labeled nucleotides (digoxigenin/DNA labeling mixture; Boehringer Mannheim) and 12 units of terminal transferase (Promega) as described (13). The reaction was stopped by adding 0.5 M EDTA. Sections were then treated for 1 h with an alkaline phosphatase-labeled anti-digoxigenin antibody (Boehringer Mannheim) at a dilution of 1:600. Color reaction was visualized by alkaline phosphatase histochemistry using nitroblue tetrazolium/5-bromo-4-chloro-3-indoylphosphate p-toluidine salt (Boehringer Mannheim) as a chromogen. After IST, the same sections were stained for T cells using the B 115-1 antibody and the avidin–biotin complex detection system with alkaline phosphatase. Fast red salts served as a chromogen.
ELISA Assays.
P2 sandwich ELISA. Microtiter plates were coated with monoclonal P2 antibodies at concentrations of 10 and 50 μg/ml. Plates were blocked with 2.5% casein in 0.3 M NaOH (pH 7.2) overnight at 4°C and washed five times with PBS/0.1% Tween 20. Then, blood samples from rats 0, 1, 4, and 10 h after injection of 500 μg of rhP2 and rhP2 standards (from 1000 to 10 ng/ml) were added and incubated for 1 h at 37°C. Polyclonal P2 antibodies from rabbit diluted 1:1000 in PBS/0.1% Tween 20 were added and incubated for 1 h at 37°C. The plates were washed, and horseradish peroxidase-coupled goat anti-rabbit antibody (Dianova, Hamburg, Germany) diluted 1:5000 in PBS/0.1% Tween 20 was added and incubated for 1 h at room temperature on a rotatory shaker (250 rpm). Finally, antibody binding was visualized using 2 mM ABTS [2, 2′-azino-bis-(ethyl-benzthiazoline-6-sulfonic acid); Boehringer Mannheim] in 0.1 M citric acid, 0.1 M sodium phosphate (pH 4.3), and 2.5 mM hydrogen peroxide. OD was read at wavelengths 405 and 450 nm with an ELISA reader.
Cytokine ELISA.
All studies were done with the sandwich ELISA technique following the principles given above. Interleukin 4 (IL-4) was measured using the commercially available BioSource Cytoscreen Rat IL-4 kit (Medgenix, Ratingen, Germany) following the instructions given by the supplier. The enclosed IL-4 standard had a biological activity of 1.7 × 10−4 units/pg. For the other cytokines, we coated the plates using monoclonal hamster anti-mouse tumor necrosis factor α (TNF-α) at 3 μg/ml (Genzyme) or monoclonal anti-rat interferon γ (IFN-γ) at 6 μg/ml (Holland Biotechnology) as capture antibodies. For standard proteins, we used recombinant mouse TNF-α (biological activity: 4 × 10−2 units/pg; Genzyme) or recombinant rat IFN-γ (biological activity: 4 × 10−3 units/pg; Holland Biotechnology). For detection, we added a polyclonal rabbit anti-mouse TNF-α (Endogen, Cambridge, MA) or a polyclonal hamster anti-mouse IFN-γ (Genzyme). Antibody binding was visualized using horseradish peroxidase-coupled goat anti-rabbit or goat anti-hamster antibody (Dianova) at a dilution of 1:1000 and ABTS [2,2′-azino-bis-(ethyl-benzthiazoline-6-sulfonic acid)] as described above. OD was read at 405 nm.
Statistical Evaluation.
Statistical analysis was performed using ANOVA/MANOVA and the Mann–Whitney U rank sum test using the spss 4.0 computer program (SPSS, Chicago).
RESULTS
Prevention of AT-EAN.
First, we studied AT-EAN because its course is much more synchronous than disease induced by immunization. As controls, we used ovalbumin and an irrelevant recombinant myelin protein, the extracellular domain of rhP0 that is not recognized by our CD4+ T cell line (data not shown). rhP0 was generated in a similar bacterial expression system (4).
In our first experiment, Lewis rats were treated twice daily with 500 μg of rhP2 i.v. for 7 days starting on day 1 after adoptive transfer of activated P2-specific T cells. All animals remained completely free of disease until day 24. In contrast, Lewis rats receiving ovalbumin exhibited the typical disease course of EAN, reached the maximum of disease on day 6 after cell injection, resulting in paraplegia or tetraparesis, and began to remit thereafter. At the end of this study (day 24), the ovalbumin control group still had residual clinical signs of neuritis (data not shown).
In a more extensive analysis, groups of rats received either 500 μg of rhP2 twice daily, 500 μg of rhP2 plus 500 μg of ovalbumin once daily, 100 μg of rhP2 twice daily, or 100 μg of rhP2 plus 100 μg of ovalbumin once per day.
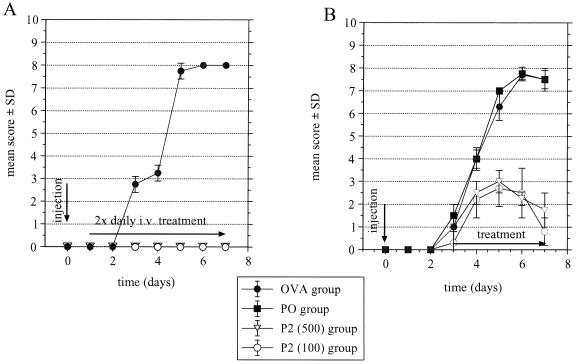
With 500 μg of rhP2 twice daily, results were identical to those of the first experiment (Fig. 1A). The group that received only 20% of that dose and only once per day rather than twice also did not develop AT-EAN nor did they exhibit weight loss. Blood samples were taken from rats 0, 1, 4, and 10 h after injection of 500 μg of rhP2. Using a sandwich ELISA with a sensitivity of 10 ng/ml, no measurable levels of circulating P2 were detected.
Figure 1.
(A) Treatment of AT-EAN with different dosages of rhP2. Lewis rats were injected with 8 × 106, activated, P2-specific T line cells. On days 1–7, one group received 500 μg of rhP2 i.v. twice daily or 100 μg of rhP2 once daily, and control animals were treated with 500 μg of ovalbumin i.v. twice daily. (B) Treatment of ongoing AT-EAN with rhP2. Rats were injected i.v. with 8 × 106 T cell blasts. Three days later, animals were divided into four groups and received, for 4 days, twice daily, either 500 μg of rhP2, 100 μg of rhP2, 500 μg of ovalbumin, or 100 μg of rhP0. Differences between the ovalbumin or rhP0 groups and treatment with 100 or 500 μg of rhP2 were significant at P < 0.05. OVA, ovalbumin.
In the sciatic nerve, severe inflammation was seen in ovalbumin-treated EAN rats (Fig. 2A) with strong infiltration of T cells and macrophages (Table 1). Sciatic nerves of rats treated with 500 μg of rhP2 twice daily (Fig. 2C) had essentially no infiltrates (Table 1). Only a few infiltrating T cells and macrophages (Table 1) were seen in the sciatic nerves of rats treated with 100 μg of rhP2 once daily (Fig. 2B) or in rats receiving rhP2 with ovalbumin (Table 1).
Figure 2.
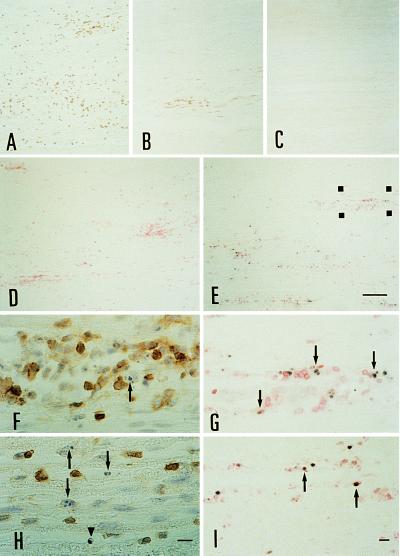
T cell infiltration in AT-EAN on day 7 in (A) the ovalbumin control group, (B) the 1× 100 μg of rhP2 group, and (C) the 2× 500 μg of rhP2 group. (D and E) Double-labeling of apoptotic T cells in the sciatic nerve. Nuclei with fragmented DNA are labeled black by IST followed by anti-T cell immunochemistry visualized with fast red salt. Microphotographs show representative sections from control rhP0 animals (D) and rhP2-treated recipients (E), which were treated once on days 6 and 7 and killed 6 h later. Note the high number of apoptotic nuclei occurring in the sciatic nerve of rhP2-treated animals. (Bar for A–E = 100 μm.) (G) The region marked in E at higher magnification. Apoptotic T cells are indicated by arrowheads. (I) Apoptotic fragments (black) are engulfed by macrophages labeled with ED 1 (red). (F and H) T cells displaying typical nuclear changes characteristic for apoptosis (arrows). Some cells still have a preserved membrane signal (arrowhead). F is a higher magnification of the region marked in E stained on a serial section. (Bars for F–I = 10 μm.)
Table 1.
Treatment of early and ongoing AT-EAN: quantitative analysis of inflammatory cells
| Treatment per day | Rats, n | Duration of treatment, days postimmunization | Mean score ± SD at disease peak | T cell infiltration at disease peak, T cells/mm2* | Macrophage infiltration at disease peak, macrophages/mm2* |
|---|---|---|---|---|---|
| Early disease | |||||
| 2× 500 μg of OVA | 2 | 1–7 | 7.75 ± 0.3 | 248.7 ± 21.5 | 113.2 ± 17.8 |
| 1× 100 μg of rhP2 + 1× OVA | 2 | 1–7 | 0 | 21.5 ± 5.8† | 13.1 ± 5.2† |
| 2× 100 μg of rhP2 | 2 | 1–7 | 0 | 13.7 ± 4.1† | 9.2 ± 3.2† |
| 1× 500 μg of rhP2 + 1× OVA | 2 | 1–7 | 0 | 13.5 ± 4.8† | 8.5 ± 2.8† |
| 2× 500 μg of rhP2 | 2 | 1–7 | 0 | 6.3 ± 2.7† | 3.2 ± 2.2† |
| Ongoing disease | |||||
| 2× 500 μg OVA | 3 | 3–7 | 7.7 ± 0.2 | 258.7 ± 38.1 | 100.4 ± 38.8 |
| 2× 500 μg of rhP0 | 3 | 3–7 | 7.75 ± 0.3 | 249.2 ± 26.7 | 95.7 ± 19.1 |
| 2× 100 μg of rhP2 | 3 | 3–7 | 3.0 ± 0.5‡ | 37.6 ± 12.2† | 16.1 ± 7.2† |
| 2× 500 μg of rhP2 | 3 | 3–7 | 2.7 ± 0.8‡ | 6.5 ± 2.8† | 2.2 ± 1.7† |
OVA, ovalbumin.
Mean ± SD.
P < 0.01 vs. ovalbumin;
P < 0.05 vs. ovalbumin.
As a side reaction, animals receiving 500 μg of rhP2 twice daily exhibited a 5% weight loss early after initiation of the therapy whereas body weight in the 100 μg of rhP2 twice daily-treated group was not affected. At early autopsy, we observed focal necrosis in the liver occurring as early as 24 h after P2 treatment. Moreover, serum plasma activity of alanine aminotransferase and aspartate aminotransferase as determined by a standard photometric method was elevated 3-fold compared with the rhP0 control animals. After day 2, animals in the high dose group did well and showed no further weight loss.
Treatment of Ongoing AT-EAN.
Antigen-specific therapy of ongoing AT-EAN was initiated at the beginning of overt disease on day 3. Rats treated twice daily with 500 μg or twice daily with 100 μg of rhP2 developed only mild disease, with the maximum of disease on day 5 after cell injection. As early as day 6, animals in both rhP2-treated groups began to remit (Fig. 1B).
In these experiments rhP0 was used as a nerve-specific control antigen. This is another neuritogenic protein without cross-reactivity to P2. In antigen proliferation assays, rhP0 was not recognized by our neuritogenic T cell lines (data not shown). We found that rhP0 did not mitigate disease severity (Fig. 1B).
In the sciatic nerve of the ovalbumin- and rhP0-treated rats, histological analysis revealed severe inflammation with infiltrating T cells and macrophages (Table 1). In contrast, we found only a few infiltrates in both rhP2 treatment groups. Although there was no difference in the clinical score between the rhP2-treated groups, histological analysis again showed that treatment with 500 μg of rhP2 resulted in less inflammation compared with the 100 μg of rhP2 group (Table 1).
Treatment of Active EAN Induced by Immunization with P2–Peptide.
Early treatment. RhP2 antigen therapy was initiated on day 4 after immunization with P2–peptide-spanning amino acids 53–78, i.e., those in the latent phase. Groups of three Lewis rats were treated with 100 μg of rhP2 twice daily i.v. Results are given in Table 2. The animals treated with rhP2 remained free of disease until they were killed on day 19. Therapy was discontinued on day 13. In contrast, Lewis rats receiving the control antigen rhP0 developed EAN, with the maximum of disease on day 16 (Table 2; experiment 1).
Table 2.
Treatment of early and ongoing actively induced EAN
| Treatment, 2× 100 μg/day | Rats, n | Duration of treatment, days post-immunization | Mean score ± SD at disease peak | Mean T cells/mm2 ± SD in the sciatic nerve |
|---|---|---|---|---|
| Early | ||||
| rhP2 (exp. 1) | 3 | 4–13 | 0 | 11.3 ± 3.7* |
| rhP0 (exp. 1) | 3 | 4–13 | 3.3 ± 0.5 | 347.3 ± 38.7 |
| rhP2 (exp. 2) | 3 | 4–13 | 0 | 10.6 ± 2.8* |
| rhP0 (exp. 2) | 3 | 4–13 | 5.7 ± 1.9 | 359.7 ± 41.7 |
| Ongoing | ||||
| rhP2 | 3 | 13–18 | 3.8 ± 2.9† | 95.7 ± 25.7* |
| rhP0 | 3 | 13–18 | 6.5 ± 0.4 | 360.2 ± 32.2 |
Exp., experiment.
P < 0.01 vs rhP0;
P < 0.05 vs. rhP0.
This experiment was repeated with similar results, except that the rhP0-treated control group was more severely affected (Table 2; experiment 2). According to our experience, frequent general anesthesia during the induction period of active EAN is a stress factor that critically influences the strength of disease. This may explain the variability of disease severity between the placebo-treated control groups in these two experiments.
Treatment of ongoing, active EAN.
Therapy was started on day 13 after immunization with P2–peptide-spanning amino acids 53–78 when overt signs of disease already were present. Three animals received 100 μg of rhP2 twice daily i.v., and a control group received an equal amount of rhP0. Between days 15 and 18, the rhP0 control group displayed moderate paraparesis or paraplegia whereas the rhP2-treated group reached the maximum of disease on days 14–15, resulting in gait ataxia only (Table 2).
Mechanisms of Therapeutic Action.
The possible mechanisms of action were examined in vitro and in situ.
Suppression of proliferation response at high antigen doses.
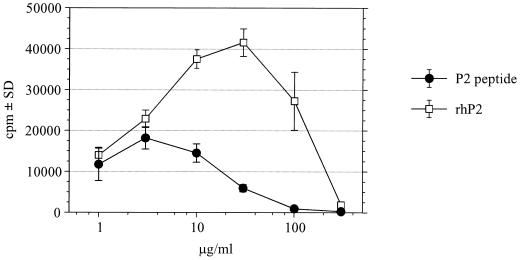
Fig. 3 shows that proliferation of the neuritogenic T cell line G5 was maximal at 3 μg/ml of the neuritogenic P2–peptide (amino acids 53–78) and at 30 μg/ml of the rhP2 protein and then steadily declined at greater concentrations. Similar results were obtained with the T cell line G7 (data not shown).
Figure 3.
High antigen dose suppression of rat T cell line G5. T cells were incubated with increasing doses of rhP2 or the neuritogenic P2 53–78 peptide as described. [3H]Thymidine incorporation was measured after 2 days during the last 16 h of restimulation and is expressed as mean cpm (± SD) of triplicate cultures.
Cytokine production after exposure to P2 protein in vitro.
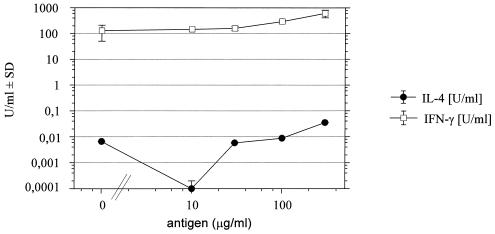
Fig. 4 shows a dose–response curve for the secretion of IFN-γ and IL-4 5 h after addition of P2 to T cell cultures. There was spontaneous production of IFN-γ, which is a typical feature of Th1 cells. Secretion of IL-4 was below 1 unit/ml even at the highest doses of antigen. With TNF-α, we noted some higher variability, and in most cases a plateau of secretion (≈40 units/ml) was reached at a rhP2 concentration of 30 μg/ml (data not shown).
Figure 4.
Dose-dependent secretion of IFN-γ and IL-4 after exposure of P2 T cell lines to rhP2 in vitro. Supernatants were taken 5 h after addition of P2. Values represent mean ± SD from two experiments. Note that the y axis has a logarithmic scale.
Apoptosis in spleen.
Following observations by others about homing of activated T cells (14) and T cell apoptosis in spleen (15), we studied whether T cell apoptosis occurs in the spleen on days 1 and 2 after injection of 8 × 106 neuritogenic, P2-specific T cells after i.v. treatment with 500 μg of rhP2 or the control antigen rhP0. There were very few apoptotic T cells in the spleen of rhP0 control rats (5.7 ± 3.7 apoptotic T cells/mm2), as identified by IST and immunocytochemistry. A 5-fold increase of apoptotic T cells occurred in the spleen of the rhP2-treated group (28.7 ± 4.7 apoptotic T cells/mm2; P < 0.01), as detected by double labeling techniques.
Apoptosis in the sciatic nerve in situ.
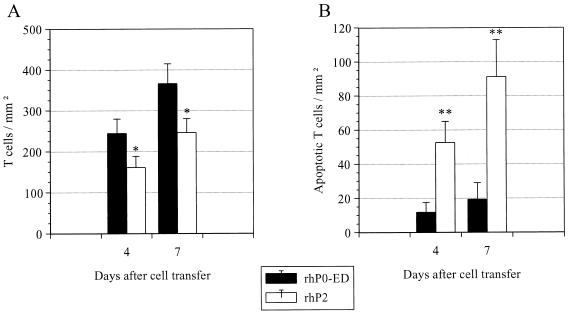
Using a similar approach as described (15), we studied T cell apoptosis in situ after treatment with rhP2 starting at early (day 3/4) or late (day 6/7) stages of AT-EAN (10). Animals received 500 μg of rhP2 or an equal amount of the control antigen rhP0 on day 3 or 6, followed by a second injection 12 h later, and then were killed another 6 h later. Histological analysis of the sciatic nerve of rhP2 recipients (Fig. 2 E and G) showed a reduction of inflammatory infiltrates by ≈30% compared with rhP0 controls (Figs. 2D and 5). By morphological criteria, T cells exhibited typical signs of apoptosis (Fig. 2 F and H).
Figure 5.
(A) Immunocytochemical analysis of T cell infiltration in rhP2-treated rats and rhP0 control animals in AT-EAN. On day 3 or 6 after i.v. injection with 8 × 106, activated, P2-specific T line cells, Lewis rats received 500 μg of rhP2 or rhP0, which was repeated after 12 h (day 4 or 7); 6 h later, animals were killed, and the sciatic nerve was removed. Values indicate mean density of T cells/mm2 ± SD of three animals per group (∗, P < 0.05). (B) T cell apoptosis in treatment groups vs. spontaneous T cell apoptosis occurring in control animals. Values indicate mean density of apoptotic cells/mm2 ± SD of three animals per group (∗∗, P < 0.01).
The percentage of apoptotic T cells was ≈7-fold higher in the rhP2-treated rats (32.6 ± 7.7% on day 4 and 37.0 ± 8.8% on day 7) than in rhP0-treated control EAN rats (4.8 ± 2.4% on day 4 and 5.3 ± 2.7% on day 7). Abundant apoptotic fragments were engulfed by macrophages (Fig. 2I). When we compared i.v. treatment with recombinant P2 (500 μg) and the neuritogenic peptide (250 μg), the recombinant protein induced apoptosis in situ at a much higher efficacy than the peptide (rhP2: 27.6 ± 6.1%; P2–peptide: 12.9 ± 3.8%; rhP0–control: 6.5 ± 2.6%).
DISCUSSION
Immune responses induced by a protein antigen can be suppressed specifically by high doses of the same antigen in adult animals, as was recently shown in mouse experimental autoimmune encephalomyelitis (8). It was proposed that T cell receptor reengagement at an appropriate stage of the cell cycle may be the underlying mechanism leading to T cell apoptosis. This results in termination of T cell-induced inflammation, similar to T cell apoptosis occurring during the natural disease course of experimental encephalomyelitis, where T cell apoptosis was first described (16, 17). We have investigated whether the human recombinant antigen P2 is effective in the experimental counterpart of experimental autoimmune encephalomyelitis in the peripheral nervous system, EAN. We show here that i.v. administration of high doses of rhP2 has a pronounced beneficial effect on AT-EAN and EAN actively induced with synthetic P2–peptide 53–78. In both EAN models, antigen treatment completely prevented disease when administered before overt clinical disease and still markedly ameliorated the disease course when given later. As little as 100 μg of rhP2 given once daily on days 1–7 after cell injection was sufficient to protect animals from clinical signs of AT-EAN. The specificity of our findings was supported by the observation that the irrelevant antigens ovalbumin and neuritogenic rhP0 obtained in a similar expression system were ineffective and failed to suppress EAN.
We then searched for a possible mechanism of action and found a high degree of T cell apoptosis in peripheral nerve within 18 h after initiation of the treatment. Moreover, there was a systemic pro-apoptotic effect, as indicated by augmented apoptosis of P2-specific T cells in the spleen after injection of rhP2. The control antigen P0 did not induce apoptosis nor did it depress disease activity. We know that T cell activation occurs in peripheral immune organs like spleen (15) before inflammation of the nerve, so the finding of increased apoptosis in spleen leads us to suggest that apoptosis may be the crucial mechanism to explain the efficacy of antigen therapy. Antigen exposure in vitro augmented secretion of IFN-γ and TNF-α in our Th1-type cell lines, speaking against cytokine switching toward a Th2 type as an underlying mechanism for T cell apoptosis.
Of interest, treatment with rhP2 was more effective than the neuritogenic peptide spanning amino acids 53–78 in augmenting apoptosis in situ. This finding indicates that antigen processing must occur in situ and is in accord with commonly accepted theories about the pathogenesis of autoimmune disorders of the nervous system (18). The lower efficacy of peptide treatment may be due to the fact that our T cell lines were not of clonal origin. They probably harbor other P2-reactive cells that do not recognize the neuritogenic epitope. Only a limited amount of mAb is available to investigate rat T cell receptor usage at the protein level, so further characterization is not possible at present.
With our highest antigen dose, we observed focal liver necrosis at early time points. McFarland et al. (19) demonstrated that high dose antigen therapy caused increased levels of TNF-α. They speculated about a possible contamination of their antigen preparation. In view of the susceptibility of liver cells to TNF-α-mediated cell death (20), focal liver necrosis may be mediated by TNF-α. However, this side effect did not seem to be relevant because animals exhibited no further signs of weight loss for up to 24 days of observation. Our antigen preparations were checked and were found to be free from pathogens.
In some diseases, multiple autoantigens may be involved during the natural disease course. This has been shown in experimental encephalomyelitis as an animal model for multiple sclerosis (21) and in nonobese, diabetic mice as an animal model for diabetes (22). In these conditions, i.v. therapy with multiple antigens may be needed for effective treatment of the disease. We tried to modulate EAN induced by immunization with peripheral bovine myelin, a mixture containing multiple autoantigens including P0, P2, and myelin basic protein. In this case, we failed to obtain a significant effect by sole i.v. administration of P2, but we observed a significant improvement by combining P2 and P0 (A.W. and R.G., unpublished observations). High antigen dose therapy using recombinant, pathogen-free protein may prove useful for the treatment of inflammatory autoimmune disorders of the nervous system.
Acknowledgments
We thank Dr. Th. Hünig for helpful suggestions and critical reading, Dr. Hans Lassmann for many productive discussions on the subject, and Dr. Nanette Schloot for helpful discussion on autoimmunity in the nonobese, diabetic mouse. Alexandra L. Bunz and Helga Brünner provided excellent technical assistance. This study was supported by grants from the Deutsche Forschungsgemeinschaft Go 459/3-1.
Footnotes
Abbreviations: EAN, experimental autoimmune neuritis; AT-EAN, adoptive transfer EAN; IST, in situ tailing; rhP2, recombinant human P2; rhP0, recombinant human P0; IL, interleukin; TNF, tumor necrosis factor; INF, interferon.
References
- 1.Hartung H P, Stoll G, Toyka K V. In: Peripheral Neuropathy. Dyck P J, Thomas P K, Griffin J W, Low P A, Poduslo J F, editors. Philadelphia: Saunders; 1993. p. 418. [Google Scholar]
- 2.Brostoff S W, Levit S D, Powers J M. Nature (London) 1977;268:752–753. doi: 10.1038/268752a0. [DOI] [PubMed] [Google Scholar]
- 3.Kadlubowski M, Hughes R A C. Nature (London) 1979;277:140–141. doi: 10.1038/277140a0. [DOI] [PubMed] [Google Scholar]
- 4.Weishaupt A, Giegerich G, Jung S, Gold R, Enders U, Pette M, Hayasaka K, Hartung H-P, Toyka K V. J Neuroimmunol. 1995;63:149–156. doi: 10.1016/0165-5728(95)00139-5. [DOI] [PubMed] [Google Scholar]
- 5.Linington C, Izumo S, Suzuki M, Uyemura K, Meyermann R, Wekerle H. J Immunol. 1984;133:1946–1950. [PubMed] [Google Scholar]
- 6.Izumo S, Linington C, Wekerle H, Meyermann R. Lab Invest. 1985;53:209–218. [PubMed] [Google Scholar]
- 7.Heininger K, Stoll G, Linington C, Toyka K V, Wekerle H. Ann Neurol. 1986;19:44–49. doi: 10.1002/ana.410190109. [DOI] [PubMed] [Google Scholar]
- 8.Critchfield J M, Racke M K, Zuniga-Pflücker J C, Cannella B, Raine C S, Goverman J, Lenardo M J. Science. 1994;263:1139–1143. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- 9.Wickham E A. Br Med J. 1996;312:988–989. doi: 10.1136/bmj.312.7037.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zettl U K, Gold R, Toyka K V, Hartung H-P. J Neuropathol Exp Neurol. 1995;54:540–547. doi: 10.1097/00005072-199507000-00008. [DOI] [PubMed] [Google Scholar]
- 11.King R H M, Craggs R I, Cross M L P, Thomas P K. Exp Neurol. 1985;87:9–19. doi: 10.1016/0014-4886(85)90129-3. [DOI] [PubMed] [Google Scholar]
- 12.Hartung H P, Schäfer B, Heininger K, Stoll G, Toyka K V. Brain. 1988;111:1039–1059. doi: 10.1093/brain/111.5.1039. [DOI] [PubMed] [Google Scholar]
- 13.Gold R, Schmied M, Giegerich G, Breitschopf H, Hartung H P, Toyka K V, Lassmann H. Lab Invest. 1994;71:219–225. [PubMed] [Google Scholar]
- 14.Klinkert W E F. J Immunol. 1987;139:1030–1036. [PubMed] [Google Scholar]
- 15.Zettl U K, Gold R, Toyka K V, Hartung H-P. Acta Neuropathol. 1996;91:360–367. doi: 10.1007/s004010050437. [DOI] [PubMed] [Google Scholar]
- 16.Pender M P, Nguyen K B, McCombe P A, Kerr J F. J Neurol Sci. 1991;104:81–87. doi: 10.1016/0022-510x(91)90219-w. [DOI] [PubMed] [Google Scholar]
- 17.Schmied M, Breitschopf H, Gold R, Zischler H, Rothe G, Wekerle H, Lassmann H. Am J Pathol. 1993;143:446–452. [PMC free article] [PubMed] [Google Scholar]
- 18.Wekerle H, Schwab M, Linington C, Meyermann R. Eur J Immunol. 1986;16:1551–1557. doi: 10.1002/eji.1830161214. [DOI] [PubMed] [Google Scholar]
- 19.McFarland H I, Critchfield J M, Racke M K, Mueller J P, Nye S H, Boehme S A, Lenardo M J. In: Immunobiology of Proteins and Peptides. Atassi M Z, Bixler G S Jr, editors. Vol. 8. New York: Plenum; 1995. pp. 157–166. [Google Scholar]
- 20.Leist M, Gantner F, Jilg S, Wendel A. J Immunol. 1995;154:1307–1316. [PubMed] [Google Scholar]
- 21.Hafler D A, Weiner H L. Immunol Rev. 1995;144:75–107. doi: 10.1111/j.1600-065x.1995.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 22.Haskins K, Wegmannn D. Diabetes. 1996;45:1299–1305. doi: 10.2337/diab.45.10.1299. [DOI] [PubMed] [Google Scholar]