Abstract
B cells play an important role in the allergic response by producing allergen-specific Igs as well as by serving as antigen-presenting cells. We studied the involvement of B cells in the development of responses in a murine model of allergic airway sensitization. Normal and B cell-deficient (μMt−/−) B10.BR mice were sensitized via the airways to ovalbumin; Ig production, cytokine elaboration from local lymph node cells, development of airway hyperresponsiveness, and histological changes in the airways were evaluated. Both strains of mice had increased production of T helper 2-like cytokines and developed an accumulation of eosinophils in the bronchial tissue after airway sensitization. However, only wild-type mice produced allergen-specific antibodies and exhibited altered airway function. B cell-deficient mice reconstituted with anti-ovalbumin IgE during the course of sensitization developed increases in airway responsiveness. These results indicated that neither B cells nor IgE were necessary for the induction of a T helper 2-type cytokine response or eosinophil infiltration of the airways after allergic sensitization but that IgE was required as a second signal for the development of airway hyperresponsiveness in this model of airway sensitization.
Keywords: IgE, mAb
The allergic response of atopic patients is characterized by the production of unusually high amounts of allergen-specific IgE against common environmental antigens. Indeed, in atopic diseases such as bronchial asthma, there is a close correlation between serum IgE levels and the prevalence (1) and severity (2) of the disease. IgE bound to high affinity IgE receptors on mast cells and basophils triggers the activation of these cells after cross-linking by antigen, thus initiating the allergic cascade (3). Activated mast cells release tryptase and histamine, central elements in anaphylactic and immediate-type allergic reactions. Other mediators released by mast cells, such as prostaglandins and leukotrienes, have proinflammatory properties that enhance and sustain allergic inflammation (4). Furthermore, mast cells have been shown to produce the T helper 2 (TH2)-type cytokines: interleukin 4 (IL-4), a major inducer of B cell isotype switching to IgE, and IL-5, the primary factor for eosinophil activation and differentiation (5). IgE receptors have also been demonstrated on eosinophils (6, 7), essential cells in the allergic asthmatic response (8).
In addition to IgE production, B cells have a second, important role in the development of the allergic response. B cells are important effectors of IgE-mediated enhancement of T cell function via the low affinity surface receptor for IgE (FcɛRII/CD23). In vitro, B cells can effectively focus and present low concentrations of antigens via CD23 to T cells (9). In vivo, CD23-deficient mice show normal antibody responses to 2,4-dinitrophenyl–ovalbumin (OVA) but impaired IgE-mediated enhancement of Ig (including IgE) production (10). Anti-CD23 antibody treatment of rats inhibits antigen-specific IgE production (11), and treatment of allergen-sensitized mice with anti-CD23 antibody before airway allergen challenge reduces pulmonary eosinophil infiltration (12).
These results suggest that B cells may play a role in the induction of an allergic response after airway sensitization in mice. In the model used, BALB/c mice were sensitized (in the absence of adjuvant) exclusively via the airways, mimicking a “natural” mode of allergic sensitization to aeroallergens. Airway sensitization in this way induces allergen-specific IgE (13) and T cell activation (14), which is associated with immediate cutaneous hypersensitivity (15) and increased airway responsiveness (16). The development of airway hyperreactivity (AHR) in this model is associated with, or dependent on, three distinct events: allergen challenge of the airways, IL-5-mediated eosinophil airway infiltration, and production of allergen-specific IgE. We demonstrated previously that the development of AHR in this model is inhibited by anti-IL-5 antibody treatment (17), pretreatment with nebulized interferon γ (IFN-γ) (18), transfer of IFN-γ-producing CD8+ T cells (19), or use of a low IgE responder mouse strain (SJL) (16). All of these interventions inhibit or reduce TH2-type cytokine production and/or eosinophil airway infiltration. As a corollary, nude mice required both IL-5 and antigen-specific IgE to reconstitute AHR. Here, we show that sensitization of B cell-deficient mice via the airways lead to significant eosinophil infiltration of lung tissue and increased local production of IL-5 but not AHR. Passive sensitization of B cell-deficient mice with anti-OVA IgE antibody during the course of airway sensitization completely restored the development of AHR, indicating that IgE was required as an essential second signal in addition to eosinophil infiltration for the development of increased airway responsiveness in this model.
MATERIALS AND METHODS
Animals.
Female B10.BR mice from 8 to 12 weeks of age were obtained from The Jackson Laboratories. B cell-deficient mice (μMt−/−) (20) were obtained from The Jackson Laboratories and were backcrossed for five generations to B10.BR wild-type mice. Spleens and lymph nodes from μMt−/− mice contained <3% B220+ cells, and cells from μMt+/+ mice were 70% B220+ in spleen and ≥30% B220+ in lymph nodes. Age- and sex-matched normal and B cell-deficient mice were used in all experiments. The mice were maintained on OVA-free diets. All experimental animals in this study were used under a protocol approved by the Institutional Animal Care and Use Committee of the National Jewish Medical and Research Center.
Airway Sensitization and Antibody Treatment.
Mice (three to four mice per group per experiment) were sensitized via the airways by nebulization of OVA (Sigma; 1% in PBS) on 10 consecutive days for 20 min daily as described (21). Control animals were exposed to nebulized PBS following the same protocol. Normal and B cell-deficient controls showed similar airway responsiveness and lung cell composition (data not shown). B cell-deficient mice in some experimental groups received 0.4 μg of i.v. anti-OVA IgE antibody (22) in 200 μl of PBS on days 4, 6, and 8 of the protocol. Age-matched control mice received similar amounts of anti-2,4,6,-trinitrophenyl IgE (PharMingen) following the same protocol. There were no differences between OVA-sensitized mice with or without anti-2,4,6-trinitrophenyl IgE treatment in any parameter tested (data not shown). Airway responsiveness was assessed on day 12, 2 days after the last nebulization, and the mice were killed to obtain tissues and cells for further assays.
Measurement of Anti-OVA Antibody and Total Ig Levels.
Anti-OVA Ig serum levels were measured by ELISA as described (15). The antibody titers of the samples were related to pooled standards that were generated in the laboratory. Total IgE levels were determined using the same method as described in ref. 15. Total Ig levels were calculated by comparison with known mouse IgE and IgG standards (PharMingen). The limit of detection was 100 pg/ml for IgE and 1 ng/ml for IgG.
Cell Preparation and Culture.
Peribronchial lymph nodes (PBLNs) were harvested, and mononuclear cells were purified by passing the tissue through stainless steel mesh followed by density gradient centrifugation (Organon Teknika–Cappel). T cells were isolated by nylon wool passage to a purity of ≥85% CD3+ cells. Cells were washed three times in PBS and resuspended in RPMI 1640 medium (GIBCO) containing 10% fetal calf serum (FCS), 100 units/ml penicillin, 100 μg/ml streptomycin, 5 mM glutamine, and 50 μM 2-mercaptoethanol. Antigen-presenting cells (APCs) were prepared from spleens of B10.BR mice by preparing mononuclear cells, by depleting mononuclear cells of T cells by incubation with antithymocyte antiserum followed by rabbit complement, and by irradiating with 3000 rad. T cells were plated in 96-well, round bottom plates at 200,000 cells/well and cultured in the presence or absence of OVA and mitogen at 37°C for 48 h. APCs were added at 400,000 cells/well. Cell-free supernatants were harvested and stored at −20°C.
Measurement of Cytokines.
Cytokine levels in the supernatants were measured by ELISA. In brief, ELISA plates were coated with purified anticytokine antibodies (all reagents from PharMingen) and blocked with 10% FCS/PBS. Samples and dilution rows of purified cytokines as standards were incubated at 4°C overnight. Biotinylated anticytokine antibodies followed by avidin–peroxidase and 3-ethylbenzthiazoline-6-sulfonic acid substrate were used for detection. The limit of detection for IL-4 and IL-5 was 4 pg/ml.
Proliferation Assay.
PBLN T cells were cultured in 96-well, round bottom plates at 200,000 cells/well in the presence or absence of OVA and mitogen for 5 days. APCs were added at a 2:1 ratio. Incorporation was measured 6 h after addition of 1 μCi (1 Ci = 37 GBq) of [3H]thymidine (ICN).
Monitoring AHR.
Airway responsiveness was determined in vitro as described (16). In brief, tracheal smooth muscle segments ≈0.5 cm in length were placed in Krebs–Henseleit baths suspended by triangular supports transducing the force of contractions. Electrical field stimulation was delivered with increasing frequencies until peak contractile responses were reached. ES50, the frequency leading to 50% of maximal contractions, was calculated from linear plots and was compared for the different treatment groups.
Isolation of Lung Cells.
Lung cells were isolated as described (22). In brief, lungs were perfused with warmed (37°C) calcium- and magnesium-free Hanks’ balanced salt solution (HBSS) containing 10% FCS, 0.6 mM EDTA, 100 units/ml penicillin, and 100 μg/ml streptomycin via the right ventricle at a rate of 4 ml/min for 4 min. Lungs were removed and cut into 300-μm pieces. Four milliliters of HBSS containing 175 units/ml collagenase (type IA; Sigma), 10% FCS, 100 units/ml penicillin, and 100 μg/ml streptomycin was added to the minced lungs and incubated for 60 min in an orbital shaker at 37°C. The digested lungs were sheared with a sterile 20-gauge needle and filtered through 45- and 15-μm filters. The filters were washed with HBSS/2% FCS (45 μm, 1 × 10 ml; 15 μm, 2 × 10 ml). Cells were resuspended in HBSS and counted using a hemocytometer, and cytospin slides were prepared. Slides were stained with leukostat (Fisher), and cell differentiation percentages were determined by counting at least 300 cells using light microscopy.
Immunohistochemistry.
After perfusion via the right ventricle, lungs were inflated through the tracheas and fixed with 2 ml of 10% formalin. Major basic protein (MBP) in lung sections was localized as described (17). Blocks of the left lung tissue were cut around the main bronchus and embedded in paraffin blocks, and 5-μm tissue sections were affixed to microscope slides, deparaffinized, and incubated in normal rabbit serum for 2 h at 37°C. The slides were then stained with either rabbit anti-mouse MBP [kindly provided by G. Gleich (Mayo Clinic, Rochester, MN) and J. Lee (Mayo Clinic, Scottsdale, AZ)] or with normal rabbit control serum and incubated overnight at 4°C. After washing and incubating in 1% chromotrope 2R (Harleco, Philadelphia) for 30 min, the slides were placed in fluorescein-labeled goat anti-rabbit IgG for 30 min at 37°C. The slides were examined in a blinded fashion using a Zeiss microscope equipped with a fluorescein filter system. Numbers of eosinophils in the submucosal tissue around central airways were evaluated using the iplab2 software (Signal Analytics, Vienna, VA) for Macintosh counting four different sections per animal.
Statistical Analysis.
ANOVA was used to determine the levels of difference between all groups. Pairs of groups were compared by Student’s t test. Comparisons for all pairs were performed by the Tukey–Kramer honestly significant difference test for airway responsiveness and histology data. P values for significance were set to 0.05. Values for all measurements are expressed as mean ± SD, except for values for ES50, which are presented as mean ± SEM.
RESULTS
Total and OVA-Specific IgE and IgG Serum Levels After Sensitization.
Normal B10.BR and B cell-deficient μMt−/− B10.BR mice were sensitized via the airways after 10 days of nebulization with OVA. Serum levels of OVA-specific and total Igs were measured 1 day after completion of the nebulization protocol. Sensitization with OVA via the airways resulted in significantly increased serum levels of anti-OVA IgE and IgG1 in normal (μMt+/+) B10.BR mice (Table 1). Sensitization did not significantly alter total IgE and IgG serum levels. In contrast, serum levels of total and OVA-specific Ig were below the limit of detection before and after airway sensitization in μMt−/− mice. This confirms the inability of the B cell-deficient mice to generate any antibody responses (20). B cell-deficient mice passively sensitized with anti-OVA IgE during sensitization demonstrated serum levels of antibody that were roughly one-third of the levels in actively sensitized normal animals (Table 1).
Table 1.
OVA-specific antibody and total Ig levels in the serum
| Strain | Sensitization | OVA-specific Ig, units/ml
|
Total IgE levels, ng/ml | |
|---|---|---|---|---|
| IgE | IgG1 | |||
| B10.BR | PBS × 10 | <10 | <10 | 19 ± 4 |
| μMt−/− | PBS × 10 | <10 | <10 | <10 |
| B10.BR | OVA × 10 | 1777 ± 354* | 1393 ± 242* | 24 ± 6 |
| μMt−/− | OVA × 10 | <10 | <10 | <10 |
| μMt−/− plus α-OVA IgE | OVA × 10 | 581 ± 73* | <10 | 12 ± 2 |
Serum titers for OVA-specific antibodies and total IgE were determined by ELISA in mice: normal control mice (B10.BR/PBS, n = 8); B cell-deficient control mice (μMt−/−/PBS, n = 8); normal OVA-sensitized mice (B10.BR/OVA, n = 12); B cell-deficient, OVA-sensitized mice (μMt−/−/OVA, n = 12); and B cell-deficient, OVA-sensitized mice receiving anti-OVA antibody treatment (μMt−/− plus α-OVA-IgE/OVA, n = 8). Presented are the means ± SD.
Significant (P < 0.05) differences compared with the nonsensitized group.
Proliferative Responses to OVA.
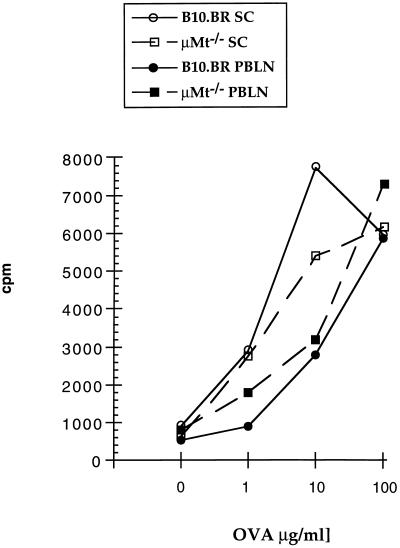
To assess antigen-specific proliferative responses of T cells after sensitization with OVA, PBLN cells were cultured for 3 days in the absence or presence of OVA or mitogen. Sensitization of normal B10.BR mice to OVA via the airways resulted in dose-dependent, OVA-specific responses in T cells prepared from PBLN as well as spleen (Fig. 1). Airway sensitization of μMt−/− mice resulted in OVA-specific T cell responses that were similar to those seen in the wild-type animals. This indicated that antigen-specific T cell responsiveness was induced to OVA to a similar extent in B cell-deficient and B cell-sufficient animals.
Figure 1.
Proliferative responses of splenic and peribronchial lymph node T cells. Mice were sensitized to OVA via the airways using the 10-day sensitization protocol. Two days after completion of sensitization, PBLN T cells (2 × 105 per well) were cultured in triplicate in the absence or presence of OVA together with APCs (4 × 105 per well) for 72 h. Thymidine uptake was measured after pulsing the cells with 1 μCi of [3H]thymidine for 6 h. Expressed are the mean cpm of one of three representative experiments. SE for all values was less than 5%.
Cytokine Production by Local Lymph Node Cells.
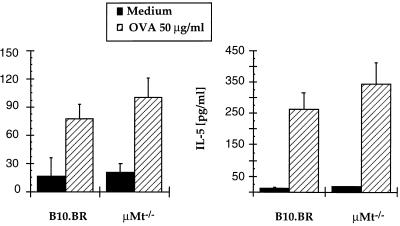
To evaluate the importance of B cells in the induction of TH2-type T cell responses after allergic sensitization, we measured the production of IL-4 and IL-5 by cultured PBLN T cells during 48 h with OVA. Sensitization via the airways significantly enhanced in vitro IL-4 and IL-5 production by PBLN T cells compared with nonsensitized animals. In nonsensitized control mice, production of IL-4 and IL-5 in PBLN T cells cultured with medium or OVA was less than 10 pg/ml (not shown). After airway sensitization, production of IL-4 and IL-5 specifically in response to OVA by PBLN T cells was increased (Fig. 2). In vitro production of IL-4 and IL-5 after airway sensitization was similar in μMt−/− mice and normal wild-type mice.
Figure 2.
IL-4 and IL-5 production by peribronchial lymph node T cells after OVA sensitization. Mice were sensitized to OVA via the airways for 10 days. Two days after completion of sensitization, PBLN T cells were cultured (2 × 105 cells per well) in the absence or presence of OVA (50 μg/ml) together with APCs (4 × 105 per well) for 24 h. Cytokine levels in supernatants were determined by ELISA. Expressed are the mean ± SD (pg/ml) from three independent experiments.
Airway Reactivity.
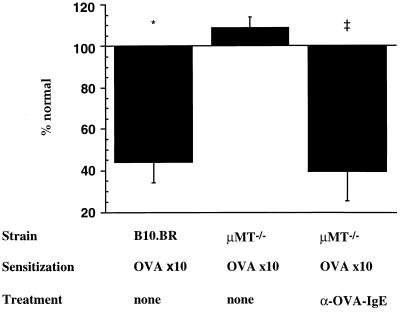
To monitor the development of AHR after airway sensitization to OVA, we measured the reactivity of tracheal smooth muscle segments to electrical field stimulation by measuring the electrical frequencies that caused 50% maximal contraction, the ES50 value in hertz. PBS-sensitized control animals had mean ± SEM ES50 levels of 4.0 ± 0.4 Hz. Sensitization of normal B10.BR mice via the airways on 10 consecutive days significantly increased airway reactivity, resulting in ES50 levels of 2.3 ± 0.4 Hz (P < 0.02 compared with PBS controls). In contrast, μMt−/− mice did not demonstrate any altered airway reactivity after the 10-day sensitization protocol, exhibiting ES50 levels of 4.3 ± 0.2 Hz similar to nonsensitized controls. However, passive sensitization of OVA-sensitized μMt−/− mice with anti-OVA IgE antibody on days 4, 6, and 8 of the 10-day protocol completely restored the development of AHR; B cell-deficient mice receiving OVA-specific IgE during the course of OVA exposure via the airways exhibited ES50 values of 2.4 ± 0.7 Hz (P < 0.03 compared with μMt−/− OVA-sensitized animals without IgE antibody). This ES50 value was similar to that of OVA-sensitized normal B10.BR mice (Fig. 3). These data confirm the importance of antigen-specific IgE in the development of increased airway responsiveness after allergic airway sensitization to OVA. The data also show that the only essential role of B cells in this process is production of IgE.
Figure 3.
Anti-OVA IgE, but not B cells, are required for the development of airway hyperresponsiveness. Normal B10.BR (n = 12) and B cell-deficient μMt−/− mice (n = 12) were sensitized to OVA via the airways for 10 days. A different group of μMt−/− mice received anti-OVA IgE antibody treatment on days 4, 6, and 8 of the 10-day protocol (n = 8). Control animals were exposed to nebulized PBS (n = 8). Two days after the last challenge, in vitro airway reactivity was measured by electrical field stimulation of tracheal smooth muscle segments. Compared are the frequencies leading to 50% maximal contraction, the ES50 ± SEM in hertz, from three independent experiments as a percentage of the control ES50 value (100%) in (PBS) nonsensitized animals (4 ± 0.4 Hz). ∗, P < 0.02 vs. PBS; ‡, P < 0.03 vs. OVA-sensitized μMt−/− mice without anti-OVA IgE treatment. μMt−/− mice receiving anti-2,4,6-trinitrophenyl IgE during 10-day OVA nebulization had similar ES50 levels as OVA-sensitized, nontreated μMt−/− mice.
Lung Cell Composition After Airway Sensitization.
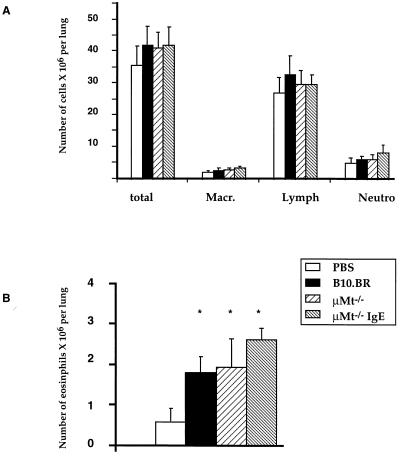
To evaluate the importance of B cells in the development of allergic inflammation after airway sensitization, total leukocyte and differential counts in isolated lung cells of individual mice were compared for the following groups: (PBS) nonsensitized mice, OVA-sensitized normal B10.BR mice, and OVA-sensitized μMt−/− mice with or without anti-OVA IgE treatment on days 4, 6, and 8 of the 10-day sensitization protocol. The absolute numbers of leukocytes, lymphocytes, and neutrophils were slightly, but not significantly, increased in OVA-sensitized animals compared with nonsensitized mice (Fig. 4). Numbers of eosinophils were significantly increased in OVA-sensitized B10.BR mice by ≈3-fold. Airway sensitization of μMt−/− mice resulted in similar numbers of eosinophils compared with nonsensitized mice. Treatment of μMt−/− mice with anti-OVA IgE further increased the numbers of eosinophils in the lungs slightly but not significantly compared with noninjected B cell-deficient mice (Fig. 4). These data indicate that eosinophil infiltration of lung tissue does not depend on the presence of B cells during sensitization and that passive sensitization of B cell-deficient mice with anti-OVA IgE may enhance eosinophil accumulation.
Figure 4.
Airway sensitization to OVA increases eosinophil infiltration of lung cells in normal and in B cell-deficient mice. Normal B10.BR (n = 12) and B cell-deficient μMt−/− mice (n = 12) were sensitized to OVA via the airways for 10 days. A different group of μMt−/− mice received anti-OVA IgE antibody treatment on days 4, 6, and 8 of the 10-day protocol (n = 8). Control animals were exposed to nebulized PBS (n = 8). Relative numbers of different types of leukocytes isolated by lung digestion were determined by light microscopy. Expressed are the means ± SD of the percentage of the different cell types. ∗, P < 0.01 vs. PBS.
Localization of Eosinophils by Immunohistochemistry.
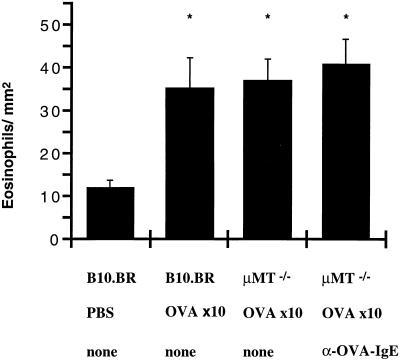
To localize eosinophils in the lung tissue of normal and B cell-deficient mice, immunohistochemistry with specific anti-MBP antibody was performed on formalin-fixed lung sections. The numbers of MBP-positive cells were measured in the peribronchial tissue using computer-assisted analysis and were compared for the different groups. In normal B10.BR as well as in μMt−/− mice with or without anti-OVA IgE antibody, sensitization of the airways significantly increased the number of peribronchial eosinophils (Fig. 5). These data indicated that airway sensitization induced peribronchial eosinophil infiltration regardless of the presence or absence of B cells.
Figure 5.
Localization of eosinophils in peribronchial tissue after airway sensitization with OVA. Control animals were exposed to nebulized PBS (n = 8). Normal B10.BR (n = 12) and B cell-deficient μMt−/− mice (n = 12) were sensitized to OVA via the airways for 10 days. A different group of μMt−/− mice received anti-OVA IgE antibody treatment on days 4, 6, and 8 of the 10-day protocol (n = 8). Sections of peribronchial tissue were stained with rabbit anti-mouse MBP antibodies. Four sections around similarly sized central airways were chosen randomly, and eosinophils in the connecting tissue were counted in a blinded fashion. Expressed are the mean ± SD numbers of eosinophils per square millimeter of lung tissue. ∗, P < 0.01 vs. PBS.
DISCUSSION
We investigated the influence of B cells on T cell function, airway inflammation, and development of AHR in a mouse model of allergic airway sensitization. We used B cell-deficient mice (μMt−/−) (20) and their wild-type littermates (B10.BR) and sensitized the animals to OVA via the airways in the absence of adjuvant. In normal B10.BR mice, sensitization to OVA resulted in increased levels of antigen-specific Igs, antigen-specific T cell responses, increased TH2-type cytokine production by T cells from PBLN, eosinophil infiltration of the peribronchial regions of the lungs, and development of AHR. Predictably, B cell-deficient mice failed to produce any antigen-specific Igs but showed normal T cell responses to antigen and TH2-type cytokine production after sensitization. Increased IL-5 production by T cells from the PBLN in B cell-deficient mice was accompanied by eosinophil infiltration in the lungs comparable to normal mice. Despite these changes after airway sensitization, B cell-deficient mice failed to develop any increases in AR, confirming the important role of allergen-specific antibodies or of B cells in the development of AHR. Transfer of OVA-specific IgE into B cell-deficient mice during the course of airway sensitization resulted in measurable serum levels of allergen-specific IgE and fully restored the capacity of these mice to develop AHR. These findings indicate that the development of AHR in this model of airway sensitization depends on the presence of allergen-specific IgE.
We have suggested previously the correlation between allergen-specific IgE production and the development of AHR in this model of airway sensitization. In these studies, we showed that mice from a low IgE responder strain (SJL), in contrast to BALB/c mice, were unable to produce increased levels of allergen-specific IgE or develop AHR after airway sensitization (16). Treatment of BALB/c mice with soluble IL-4 receptor (sIL-4R) during sensitization decreased IgE production and inhibited the development of AHR (23). Treatment of BALB/c mice with IFN-γ (18) or transfer of IFN-γ-producing CD8+ T cells (19) into BALB/c mice during sensitization to OVA also reduced IgE production and prevented alterations in airway reactivity. IFN-γ is the major antagonist of IL-4 in the regulation of IgE production (24) and inhibits eosinophil infiltration of the airways in allergen-sensitized mice (25, 26). Treatment of mice with anti-IL-4 antibody not only reduces IgE production but also prevents airway eosinophilia and development of AHR in allergen-sensitized mice (27). However, these manipulations did not allow us to distinguish effects on airway function secondary to reduced IgE production or as a result of decreases in eosinophil airway inflammation. This study demonstrates that allergen-sensitized, B cell-deficient mice develop normal eosinophil infiltration of the airways but no AHR unless they receive antigen-specific IgE. This result does not exclude the possibility that other isotypes of allergen-specific Igs might restore AHR in B cell-deficient mice. In fact, we have shown in normal BALB/c mice that passive sensitization with allergen-specific IgE and IgG1, but not IgG2a or IgG3, followed by airway challenge with OVA induces eosinophil infiltration and AHR (22).
It is well recognized that the T–B cell interaction is required for the activation of B cell Ig production (24). However, the role of B cells in T cell activation and priming remains less clear. Several reports suggest that B cells play an important role in T cell responses. Long term treatment of neonatal mice with anti-μ antibody inhibits the development of functional B cells and prevents T cell proliferative responses in vivo and in vitro (28, 29). In contrast, in experiments using adoptive transfer of lymphocytes into severe combined immunodeficient mice, the presence or absence of B cells was without effect on priming of naive T cells (30, 31). The recent availability of B cell-deficient μMt−/− mice (20) enabled us to address these issues in a more direct fashion. Epstein et al. (32) showed that T cell activation was identical in normal and in B cell-deficient mice. We reported recently, likewise using μMt−/− mice, that B cells are not essential for the induction of peripheral T cell tolerance to protein antigen or for T cell death after in vivo superantigen stimulation (33). These data were confirmed in a study from Phillips et al. (34) that showed successful T cell tolerance induction in μMt−/− mice. Finally, two recently published studies show induction and maintenance of long lasting T cell memory in μMt−/− mice, suggesting that B cells, serving as APC, are not essential (35, 36).
In the present study, we demonstrate that T cells from B cell-deficient mice are sensitized to antigen and produce similar levels of cytokines in vitro after allergic airway sensitization to OVA. Eosinophil infiltration of the lungs, which was similar in B cell-deficient and in normal mice, was only associated with increased airway reactivity in combination with active induction (in normal mice) or passive provision (in μMt−/− mice) of antigen-specific IgE. This suggests that two distinct signals, IgE and eosinophils, are required for the development of AHR in this model. This suggestion is supported by our previous studies of passive sensitization; BALB/c mice passively sensitized with anti-OVA IgE developed increased airway reactivity only if repeatedly challenged with allergen via the airways, leading to significant eosinophil airway infiltration (22). In contrast, athymic BALB/c nu/nu mice failed to develop AHR or eosinophil inflammation after passive sensitization and repeated (up to 10) airway challenges. However, additional treatment of passively sensitized nude mice with IL-5 before airway challenge leads to significant airway inflammation and increased airway reactivity (37), supporting the concept of two essential, but distinct, signals for the development of AHR.
The exact mechanism underlying eosinophil recruitment and activation is unclear. The role of IL-5 for eosinophil maturation, differentiation, and activation has been well established (38). More controversial is the role of IgE in eosinophil infiltration and activation. Some reports show that eosinophils express different types of IgE receptors that are involved in the cytotoxic activity of these cells (6, 7). In contrast, others have demonstrated that degranulation of eosinophils is independent of IgE but relates to IgG antibody (39). In a mouse model of allergic sensitization, it was suggested that IgE via CD23 on APC enhances TH2-type T cell responses and thus induces eosinophil infiltration (12). These data were not confirmed in our present study. The cytokine profile of sensitized T cells and the extent of the eosinophilic inflammation of the lungs were equal in normal and B cell-deficient mice, indicating no primary role for IgE in eosinophil infiltration of the airways in this model. Moreover, the numbers of eosinophils in the peribronchial regions of the lungs were similar in both mouse strains, and activation of eosinophils delineated by electron microscopy (data not shown) was not different. However, we cannot exclude some enhancing role of IgE on eosinophil recruitment and/or function, as suggested by Eum et al. (40).
In conclusion, we show that B cell-deficient mice have normal T cell responsiveness to allergen, TH2-type cytokine production, and eosinophil infiltration of the airways after allergic airway sensitization. In contrast to normal wild-type mice, μMt−/− mice fail to develop AHR unless provided with allergen-specific IgE. These studies demonstrate that B cell-deficient mice have other sources of cells serving as APC for the activation of T cells and directly indicate the role of antigen-specific IgE in the development of AHR in this model of allergen-induced sensitization.
Acknowledgments
We thank Dr. Gary Larsen and Joan Loader (National Jewish Center, Denver) for their help in carrying out these studies and Drs. G. Gleich (Mayo Clinic, Rochester, MN) and J. Lee (Mayo Clinic, Scottsdale, AZ) for the rabbit anti-mouse MBP antibody. The assistance of Ms. Diana Nabighian in the preparation of this manuscript is gratefully acknowledged. This work was supported by Grants AI-29704 and HL-36577 (E.W.G.) from the National Institutes of Health. E.H. is a fellow of the Deutsche Forschungsgemeinschaft (Ha 2162/1–1) and the recipient of the 1996 Janssen Research Award of the American Academy of Allergy, Asthma, and Immunology.
Footnotes
Abbreviations: TH2, T helper 2; OVA, ovalbumin; AHR, airway hyperreactivity; PBLN, peribronchial lymph nodes; FCS, fetal calf serum; APC, antigen-presenting cell; ES50, frequency leading to 50% of maximal contractions; IL, interleukin; IFN, interferon; HBSS, Hanks’ balanced salt solution; MBP, major basic protein.
References
- 1.Burrows B, Martinez F D, Halonen M, Barbee R A, Cline M G. N Engl J Med. 1989;320:271–277. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 2.Sears M R, Burrows B, Flannery E M, Herbison G P, Hewitt C J, Holdaway M D. N Engl J Med. 1991;325:1067–1071. doi: 10.1056/NEJM199110103251504. [DOI] [PubMed] [Google Scholar]
- 3.Galli S J. N Engl J Med. 1993;328:257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- 4.Galli S J, Gordon J R, Wershil B K. Curr Opin Immunol. 1991;3:865–872. doi: 10.1016/s0952-7915(05)80005-6. [DOI] [PubMed] [Google Scholar]
- 5.Bradding P, Roberts J A, Britten K M, Montefort S, Djukanovic R, Mueller R, Heusser C H, Howarth P H, Holgate S T. Am J Respir Cell Mol Biol. 1994;10:471–480. doi: 10.1165/ajrcmb.10.5.8179909. [DOI] [PubMed] [Google Scholar]
- 6.Capron M, Prin L. Springer Semin Immunopathol. 1990;12:327–348. doi: 10.1007/BF00225322. [DOI] [PubMed] [Google Scholar]
- 7.Truong M J, Gruart V, Liu F T, Prin L, Capron A, Capron M. Eur J Immunol. 1993;23:3230–3235. doi: 10.1002/eji.1830231228. [DOI] [PubMed] [Google Scholar]
- 8.Bousquet J, Chanez P, Lacoste J Y, Barneon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, Michel F B. N Engl J Med. 1990;323:1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 9.Kehry M R, Yamashita L C. Proc Natl Acad Sci USA. 1989;86:7556–7560. doi: 10.1073/pnas.86.19.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujiwara H, Kikutani H, Suematsu S, Naka T, Yoshida K, Tanaka T, Suemura M, Matsumoto N, Kojima S, Kishimoto T, Yoshida N. Proc Natl Acad Sci USA. 1994;91:6835–6839. doi: 10.1073/pnas.91.15.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flores-Romo L, Shields J, Humbert Y, Graber P, Aubry J P, Gauchat J F, Ayala G, Allet B, Chavez M, Bazin H, Bonnefoy J Y. Science. 1993;261:1038–1041. doi: 10.1126/science.8351517. [DOI] [PubMed] [Google Scholar]
- 12.Coyle A J, Wagner K, Bertrand C, Tsuyuki S, Bews J, Heusser C H. J Exp Med. 1996;183:1303–1310. doi: 10.1084/jem.183.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renz H, Smith H R, Henson J E, Ray B S, Irvin C G, Gelfand E W. J Allergy Clin Immunol. 1992;89:1127–1138. doi: 10.1016/0091-6749(92)90296-e. [DOI] [PubMed] [Google Scholar]
- 14.Renz H, Bradley K, Saloga J, Loader J, Larsen G L, Gelfand E W. J Exp Med. 1993;177:1175–1180. doi: 10.1084/jem.177.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saloga J, Renz H, Lack G, Bradley K L, Greenstein J L, Larsen G, Gelfand E W. J Clin Invest. 1993;91:133–140. doi: 10.1172/JCI116162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsen G L, Renz H, Loader J E, Bradley K L, Gelfand E W. J Clin Invest. 1992;89:747–752. doi: 10.1172/JCI115651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamelmann E., Oshiba A., Larsen K, Gleich, G., Lee, J. & Gelfand E. W. (1997) Am. J. Crit. Care Respir. Med., in press. [DOI] [PubMed]
- 18.Lack G, Renz H, Saloga J, Bradley K L, Loader J, Leung D Y, Larsen G, Gelfand E W. J Immunol. 1994;152:2546–2554. [PubMed] [Google Scholar]
- 19.Renz H, Lack G, Saloga J, Schwinzer R, Bradley K, Loader J, Kupfer A, Larsen G L, Gelfand E W. J Immunol. 1994;152:351–360. [PubMed] [Google Scholar]
- 20.Kitamura D, Roes J, Kuhn R, Rajewsky K. Nature (London) 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 21.Hamelmann E, Oshiba A, Paluh J, Bradley K, Loader J, Potter T A, Larsen G L, Gelfand E W. J Exp Med. 1996;183:1719–1730. doi: 10.1084/jem.183.4.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oshiba A, Hamelmann E, Takeda K, Bradley K L, Loader J E, Larsen G L, Gelfand E W. J Clin Invest. 1996;97:1398–1408. doi: 10.1172/JCI118560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renz H, Enssle K, Lauffer L, Kurrle R, Gelfand E W. Int Arch Allergy Immunol. 1995;106:46–54. doi: 10.1159/000236889. [DOI] [PubMed] [Google Scholar]
- 24.Coffman R L, Seymour B W, Lebman D A, Hiraki D D, Christiansen J A, Shrader B, Cherwinski H M, Savelkoul H F, Finkelman F D, Bond M W, Mosmann T R. Immunol Rev. 1988;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima H, Iwamoto I, Yoshida S. Am Rev Respir Dis. 1993;148:1102–1104. doi: 10.1164/ajrccm/148.4_Pt_1.1102. [DOI] [PubMed] [Google Scholar]
- 26.Iwamoto I, Nakajima H, Endo H, Yoshida S. J Exp Med. 1993;177:573–576. doi: 10.1084/jem.177.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corry D B, Folkesson H G, Warnock M L, Erle D J, Matthay M A, Wiener-Kronish J P, Locksley R M. J Exp Med. 1996;183:109–117. doi: 10.1084/jem.183.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janeway C A, Jr, Ron J, Katz M E. J Immunol. 1987;138:1051–1055. [PubMed] [Google Scholar]
- 29.Ron Y, Sprent J. J Immunol. 1987;138:2848–2856. [PubMed] [Google Scholar]
- 30.Sunshine G H, Jimmo B L, Ianelli C, Jarvis L. J Exp Med. 1991;174:1653–1656. doi: 10.1084/jem.174.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ronchese F, Hausmann B. J Exp Med. 1993;177:679–690. doi: 10.1084/jem.177.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Epstein M M, Di Rosa F, Jankovic D, Sher A, Matzinger P. J Exp Med. 1995;182:915–922. doi: 10.1084/jem.182.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vella A T, Scherer M T, Shultz L, Kappler J W, Marrack P. Proc Natl Acad Sci USA. 1996;93:951–955. doi: 10.1073/pnas.93.2.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips J A, Romball C G, Hobbs M V, Ernst D N, Shultz L, Weigle W O. J Exp Med. 1996;183:1339–1344. doi: 10.1084/jem.183.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Rosa F, Matzinger P. J Exp Med. 1996;183:2153–2163. doi: 10.1084/jem.183.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asano M, Ahmed R. J Exp Med. 1996;183:2165–2174. doi: 10.1084/jem.183.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamelmann, E., Oshiba, A., Schwarze, J., Bradley, K., Loader, J., Larsen, G. L. & Gelfand, E. W. (1997) Am. J. Respir. Cell Mol. Biol., in press. [DOI] [PubMed]
- 38.Drazen J M, Arm J P, Austen K F. J Exp Med. 1996;183:1–5. doi: 10.1084/jem.183.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaneko M, Swanson M C, Gleich G J, Kita H. J Clin Invest. 1995;95:2813–2821. doi: 10.1172/JCI117986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eum S Y, Haile S, Lefort J, Huerre M, Vargaftig B B. Proc Natl Acad Sci USA. 1995;92:12290–12294. doi: 10.1073/pnas.92.26.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]