Abstract
Using defined oligomers of IgE, our group previously studied the quantitative relationship between the aggregation of the high affinity receptors for IgE (FcɛRI) and the earliest signals initiated by such aggregation: the phosphorylation of tyrosines on the receptor. Notably, at certain doses of the oligomers such phosphorylation reached a plateau level well before the aggregation of the receptors had reached a maximum. These findings and others led us to propose that aggregates of the receptor were competing for a limited amount of the critical kinase—thought to be Lyn in this system. This paper describes a test of this proposal. We incubated cells with two distinguishable IgEs and examined the effect of aggregating one or the other or both types on the phosphorylation. When receptors binding antigen-specific IgE were aggregated with polyvalent antigen, they became rapidly phosphorylated as expected. Remarkably, however, FcɛRI that had already been phosphorylated by the binding of dimers of IgE, became dephosphorylated simultaneously. Furthermore, when the antigen-driven aggregates were dissociated with hapten, the phosphorylation pattern reverted to that seen prior to the addition of antigen: as the antigen-driven aggregates became dephosphorylated, the receptors stably aggregated by the bound oligomers became rapidly rephosphorylated. Dephosphorylation of oligomer-driven aggregates was also partially reversed during the “spontaneous” dephosphorylation of the antigen-driven receptors seen at longer times after addition of antigen. Thus signal transduction in this system is in part regulated by the shuttling of limited amounts of the kinase that initiates the cascade of phosphorylations.
When aggregated by ligand, the receptor with high affinity for IgE (FcɛRI), like many other plasma membrane receptors, initiates a complex cascade of biochemical perturbations that lead to immediate and delayed cellular responses (1). In the case of FcɛRI, the ligand is usually a multivalent antigen that reacts with multiple FcɛRI bearing monomeric IgE of the appropriate specificity. In this and in many related systems, an initial phosphorylation of tyrosines on the cytoplasmic domains of the receptor by an associated membrane-bound Src family kinase is the likely proximal consequence (2). For the RBL rat cell line of mucosal-type mast cells used in this study, there is good evidence that this kinase is Lyn (3).
We recently observed that under certain conditions of stimulation, phosphorylation of the receptors reaches a plateau well before aggregation of additional receptors has ceased (4). Based on these and other experimental results, we hypothesized that the premature leveling off of phosphorylation resulted from competition between aggregated receptors for limiting amounts of the initiating Src family kinase (4).
We have now developed a protocol to test this suggestion. A proportion of receptors were stably aggregated by dimers of rat IgE prior to aggregation by antigen of other receptors on the same cells bearing antigen-specific [anti-2,4-dinitrophenyl (DNP)] mouse IgE. In some instances the latter aggregates were dissociated by adding monovalent hapten a few minutes later. The receptors were individually analyzed by immunoprecipitating them with species-specific anti-IgE. The purpose of these and other experiments was to see whether discrete aggregates of receptors competed for a limited amount of tyrosine kinase activity.
MATERIALS AND METHODS
Reagents and Cells.
Anti-DNP monoclonal mouse IgE (5) was purified as described (6). Rat IgE of unknown specificity was purified from immunocytoma IR162 (7). We used mouse antiphosphotyrosine antibody PY20 conjugated with horseradish peroxidase (Transduction Laboratories, Lexington, KY), ECL chemiluminescence reagents and hyperfilm ECL were from Amersham, and 12% polyacrylamide gels (1.5 mm) were from NOVEX (San Diego). RBL-2H3 cells were cultured as described previously (8). All of our studies were on cells in suspension.
Preparation and Purification of IgE Oligomers.
IgE oligomers were prepared as described previously (9) using dimethylsuberimidate. The oligomers of IgE were separated by chromatography on a Superose 6 column (Pharmacia). The composition (multiplicity) of each preparation of oligomer was determined by PAGE (in the absence of SDS).
Sensitization and Activation of Cells.
Cells were detached with trypsin and washed with medium. In some instances, the cells were partially sensitized with DNP-specific mouse IgE. 125I-labeled IgE was used. An aliquot of cells was incubated with sufficient mouse IgE to saturate the receptors to calculate the percent occupancy of the partially saturated cells. The cells were washed with buffer A (150 mM NaCl/5 mM KCl/25 mM Pipes/5.4 mM glucose/1 mM MgCl2/0.5 mM CaCl2, pH 7.2/0.1% BSA). They were then resuspended in buffer A at 6.25 × 106/ml and reacted with rat IgE oligomers or with antigen [DNP-conjugated BSA (10)] at 37°C. At appropriate times, 0.8 ml of the sample (5 × 106 cells) was mixed with 0.4 ml of ice-cold 3× solubilization buffer (3 mM phenylmethylsulfonyl fluoride/30 μg/ml aprotinin/30 μg/ml leupeptin/30 μg/ml pepstatin A/3 mM Na3VO4/15 mM Na4P2O7/150 mM NaF/6 mM iodoacetate/15 mM EDTA/1.5% Triton X-100/150 mM NaCl/150 mM Tris, pH 7.6), placed on ice, vortexed, and kept at 4°C for 30 min on a nutator. In some cases 100 μM hapten (DNP-ɛNH2caproate) was added to assure that the solubilization of the antigen aggregated receptors was sufficiently rapid to avoid spurious dephosphorylation and to enhance recovery (S.-Y Mao and H.M., unpublished results).
Sample Preparation and Analysis.
Ten μg of rabbit anti-rat IgE serum, which had been absorbed with mouse IgE and mouse IgG, and 30 μl of a 50% suspension of protein A-Sepharose (Pharmacia) were added to the detergent-lysate of cells at 4°C for 3 hr. The supernatant was subsequently immunoprecipitated with 10 μg of goat anti-mouse IgE and with 30 μl of a 50% suspension of protein G-Sepharose (Pharmacia) when necessary. The protein A or G was washed 3 times with above solubilization buffer (1×) on ice and boiled for 5 min with 20 μl of 2× SDS sample buffer. The denatured protein was collected, and phosphotyrosine in each sample was analyzed as described previously (11). In all of the results where quantitative comparisons are presented, the amount of phosphotyrosine associated with the receptor represents the sum of the amount on the β and γ subunits. Bound 125I-labeled oligomeric IgE was simultaneously monitored as described (12).
RESULTS
Premature Plateauing of Phosphorylation of Receptors.
We confirmed that the new preparations of oligomer prepared for the experiments reported here produced the same discordance between aggregation and phosphorylation that we had observed previously. The binding of 0.5 nM IgE dimers to the cells ([FcɛRI] = 1.64nM) was measured along with determinations of the resulting phosphorylation of tyrosines on the β and γ subunits of FcɛRI. As observed previously, receptor-associated phosphotyrosine reached its maximum level well before the binding (and formation of aggregates (see figure 7 in ref. 4) by the oligomers had ceased.
One way to explain these results that we had not previously assessed would be if only a fraction of the oligomers of IgE effectively stimulate phosphorylation of the FcɛRI and that those oligomers bind substantially faster than the remaining ineffectual ones. We tested this possibility directly. Cells were incubated with 125I-labeled dimeric rat IgE at a ratio of approximately 0.5 molecules of total IgE per molecule of FcɛRI, and the suspension was sampled at 1, 3, and 9 min. After the latter sample had been removed, the cell suspension was centrifuged, and a portion of the supernatant was then added to a fresh batch of cells such that both the new cells and the supernatant were at the same concentration as in the original suspension. This second incubation mixture was then sampled at appropriate times. All cell samples were washed, counted for radioactivity to determine the number of IgE that had bound, and solubilized with detergent. The FcɛRI were immunoprecipitated and subjected to SDS/PAGE. The relative amounts of phosphotyrosine on the subunits was quantitated as described in Materials and Methods.
The results of this experiment are shown in Table 1. The supernatant from the initial incubation contained about 70% of the original counts. By direct experiment, bindability of the preparation was estimated to be ≈55% (see legend to Table 1), so that one calculates that about 50% of the bindable dimers were depleted. Comparing the kinetics of binding of the starting preparation (column 2) with those observed for the depleted preparation (column 5) it appears that as much as two-thirds of the bindable oligomers may have been bound during the initial incubation. Notably, the data in columns 4 and 7 demonstrate that the dimers remaining in the depleted supernatant were just as effective in triggering phosphorylation of FcɛRI as the preparation as a whole.
Table 1.
Effect of depleting rapidly binding dimers on the ability of the remainder to initiate phosphorylation of receptor
| Time, min | Unfractionated preparation
|
Depleted preparation
|
||||
|---|---|---|---|---|---|---|
| Bound dimer, cpm | PY, OD units | PY per bound dimer | Bound dimer, cpm | PY, OD units | PY per bound dimer | |
| 1 | 1483 ± 43 | 501 ± 27 | 0.34 ± 0.02 | ND | ND | |
| 3 | 2840 ± 144 | 1757 ± 3.8 | 0.62 ± 0.03 | 1421 ± 59 | 829 ± 55 | 0.58 ± 0.05 |
| 9 | 5467 ± 200 | 2760 ± 8.9 | 0.50 ± 0.02 | 2616 ± 233 | 1501 ± 286 | 0.57 ± 0.12 |
| 15 | ND | ND | 3495 ± 185 | 1778 ± 315 | 0.51 ± 0.09 | |
RBL-2H3 cells at 6.25 × 106/ml (≈3 × 10−9 M receptors) were reacted with 0.3 μg/ml of 125I-labeled rat IgE oligomer (≈1.5 × 10−9 M total IgE, of which ≈55% was determined to be bindable in a separate experiment) in a total volume of 15 ml. At the times indicated, duplicate samples of 0.8 ml were taken for immunoprecipitation and duplicate 0.05 ml samples were taken for measuring the amount of bound IgE. After the sampling at 9 min, the remaining suspension was centrifuged and the supernatant was added to freshly prepared cells. This second incubation was estimated to contain the same concentration of receptors as in the first, but only 1.05 × 10−9 M of total IgE based on the radioactive counts. Phosphotyrosines on the β and γ subunits of the receptors were quantitated (see Materials and Methods). The data are the averages of two samples and the range is shown. The values have not been corrected for the small amount of non-specifically bound oligomers; the relatively low value for the 1-min time point in the initial incubation (top of column 4) is likely accounted for by the fact that this value in particular would be affected by the nonspecifically bound material. ND, not determined.
These results effectively rule out the possibility that the failure to observe the expected concordance between aggregation of the receptors and their phosphorylation at later times is simply related to heterogeneity in the preparation of oligomeric IgE.
Phosphorylation of Receptors Aggregated by Oligomers of IgE and by Multivalent Antigen.
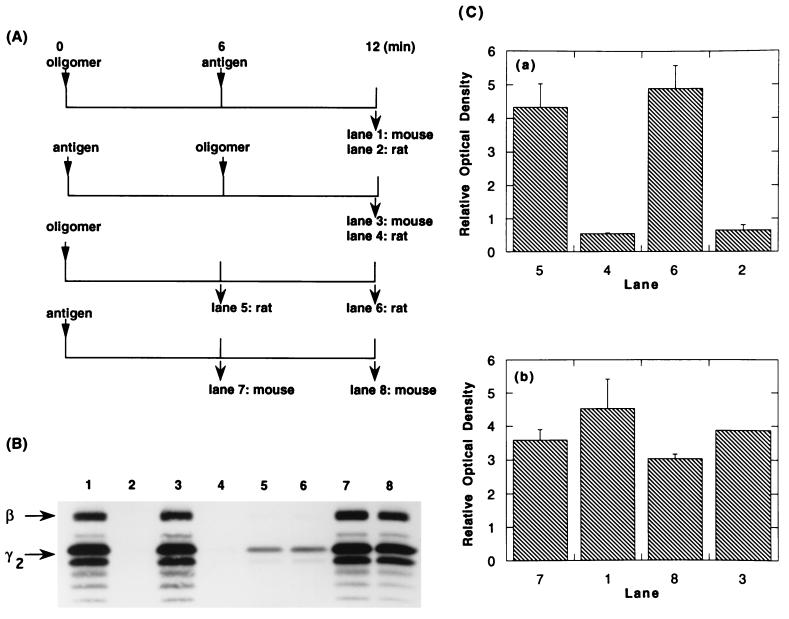
RBL-2H3 cells were incubated with monomeric antigen-specific mouse IgE and then washed free of unbound immunoglobulin. The concentrations of the reactants and the time of incubation were established to allow for approximately 50% of the receptors to be occupied. The occupancy with mouse IgE actually achieved was determined using 125I-labeled IgE directly. The cells were then reacted with covalently linked oligomers of rat IgE. At the desired times, the cells were solubilized, and the rat and mouse IgEs (with their bound receptors) were separately immunoprecipitated (Fig. 1A). Typical results from such a protocol are shown in lanes 5 and 6 of Fig. 1B. In the immunoprecipitates prepared 6 min after the addition of oligomers, the receptors associated with the rat oligomers demonstrated phosphorylation of tyrosines on the β and γ subunits (lane 5); after an additional 6 min, there was only a modest increase [Fig. 1 B, lane 6, and C, part a). In contrast, the unaggregated receptors associated with mouse IgE showed barely detectable phosphorylation of their subunits (data not shown).
Figure 1.
Effect of aggregating IgE-FcɛRI with antigen on phosphorylation of receptors previously aggregated by IgE oligomer. (A) Protocols for stimulating cells and immunoprecipitating their receptors. FcɛRI on RBL-2H3 cells were partially (52%) saturated with DNP-specific monomeric mouse IgE and reacted at 6.25 × 106 cells/ml with rat IgE oligomer (0.3 μg/ml) or antigen (DNP6-BSA, 100 ng/ml) or both. At each time point, the total IgE was immunoprecipitated with species-specific anti-IgE, and the phosphotyrosine on the subunits of the receptor was analyzed. (B) Autophotograph of a Western blot using antiphosphotyrosine antibodies. Each lane contains approximately 5 × 106 cell equivalents. (C) Densitomeric quantitation of the blot shown in B. The bars show the averages of two samples, and the ranges of the duplicates are shown. The ordinate values are graphed on a relative scale; the absolute ratio of the data for b:a was as 15:1. In numerous experiments of this type the ratio approximated 10:1.
In other samples, DNP-BSA was added 6 min after the addition of oligomers (Fig. 1A top line). As expected, the immunoprecipitates of the mouse IgE showed a high level of phosphorylated tyrosines in the β and γ subunits (Fig. 1B, lane 1). Remarkably, however, the receptors that had been stably aggregated by the oligomers of rat IgE were dephosphorylated (Fig. 1 B and C, lane 2 vs. lane 6). The competition by the stronger signal (aggregation by antigen) was also demonstrated by the protocol shown in line 2 of Fig. 1A. That is, when cells were first reacted with antigen, the oligomers of IgE failed to stimulate phosphorylation of the receptors (Fig. 1 B and C, lane 4 vs. lane 5). These studies also showed that the more limited aggregation induced by the IgE dimers failed to inhibit the signal generated by the more extensive aggregation induced by the multivalent antigen (Fig. 1 B and C, lane 1 vs. lane 7 and lane 3 vs. lane 8). Two additional experiments using the same protocol gave the same results.
In Fig. 1, the phosphotyrosine associated with the β subunit of those receptors bound to the dimeric IgE is not seen clearly, although the original autophotograph showed a low but definite phosphorylation on this subunit. However, in over 20 experiments where the β/γ ratio of phosphotyrosine on receptors stimulated with the two types of stimuli could be quantitatively compared, the ratio was 2- to 3-fold lower when the receptors were aggregated with dimer rather than by antigen. (That this difference was real and not an artifact related to differences in the absolute level of phosphorylation was confirmed in a few instances by loading gels used for Western blotting with different cell equivalents so as to equalize the absolute level of phosphotyrosine.)
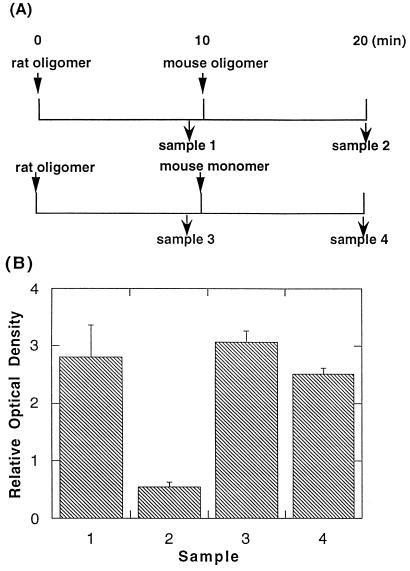
The explanation of our original observations that we wished to test in the present studies would predict that “competition” between equivalent stimuli should be bidirectional. We tested this experimentally as shown in Fig. 2A. After addition of oligomers of rat IgE, the cells were reacted with an excess of oligomer of mouse IgE. PAGE (without SDS) confirmed that the oligomers were of equivalent multiplicity (almost exclusively dimers), and functional studies confirmed that they induced comparable levels of phosphotyrosines on the receptors (data not shown). It is apparent from the results in Fig. 2B that the dimeric mouse IgE was able to reduce the phosphotyrosine on the receptors aggregated previously with the dimeric rat IgE. The same results were obtained in a duplicate experiment.
Figure 2.
Competition between oligomers of IgE. (A) Protocols for stimulating cells and immunoprecipitating their receptors. RBL-2H3 cells (6.25 × 106/ml) were reacted with 0.3 μg/ml of rat IgE oligomer. After 10 min, 3 μg/ml of mouse IgE oligomer was added. As a control, 3 μg/ml monomeric mouse IgE was added instead. At t = 9 and 20 min, rat IgE was immunoprecipitated and the phosphotyrosines on the bound FcɛRI were analyzed. (B) Densitomeric quantitation of the blot for the experiment shown in A. The bars show the averages of two samples, and the ranges of the duplicates are shown.
Phosphorylation, Dephosphorylation, and Rephosphorylation of Receptors.
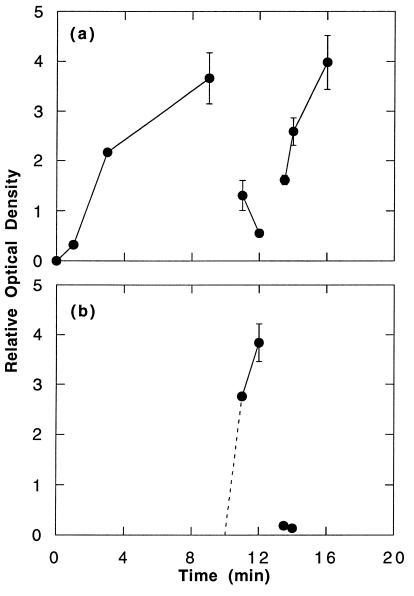
We tested to see if the dephosphorylation of the oligomer-bound receptors induced by aggregation of other receptors with antigen was reversible. The protocol was similar to that shown in line 1 of Fig. 1A except that after the addition of the polyvalent antigen a monovalent ligand (DNP-εNH2caproate) was added. As expected, the addition of antigen induced rapid phosphorylation of the appropriate receptors, and this was rapidly reversed by disaggregating antigen-bound receptors with hapten (Fig. 3b) (13, 14). What was striking was that the population of receptors that had been stably aggregated by the rat IgE oligomers were first dephosphorylated and then rephosphorylated after addition of the antigen and hapten, respectively (Fig. 3a). The rephosphorylation following the addition of hapten proceeded more rapidly than the initial phosphorylation that followed addition of oligomers at the beginning of the protocol (Fig. 3a), but in this and several similar experiments returned more or less to the level that existed before the addition of antigen. Because in these protocols no new receptor aggregates were permitted to form (see legend to Fig. 3), the rephosphorylation that was observed must have occurred on the stable, oligomer-induced aggregates that had been formed before addition of antigen. Two additional experiments using the same protocol gave the same results.
Figure 3.
Time course of the phosphorylation of receptors in the presence of antigen stimulation and subsequent addition of hapten. RBL-2H3 cells (6.25 × 106/ml), which had been partially sensitized with DNP-specific mouse IgE, were reacted with 0.3 μg/ml rat IgE oligomers at t = 0. At t = 10 min, DNP6-BSA (100 ng/ml) and monomeric rat IgE (1 μg/ml) were added, and at t = 13 min, excess hapten (100 μM) was added. Hapten (100 μM final) was also added to the solubilization buffer to promote solubilization of antigen-bound receptors. Recovery of the receptors was assessed by the recovery of 125I-IgE oligomer. Binding of oligomer was monitored simultaneously, and it was confirmed that binding of oligomer stopped after the addition of monomeric IgE. At each time point, rat and mouse IgE were precipitated with species-specific anti-IgE, and the receptors bound to them were analyzed for phosphotyrosine. The data are the average of two samples. (a) Time course of the phosphorylation of receptors bound by rat IgE oligomers. (b) Time course of the phosphorylation of receptors bound to mouse DNP-specific IgE. In these experiments, the relative phosphorylation of the receptors associated with the mouse (b) and rat IgE (a) approximated 8:1 (cf. Fig. 1 legend)
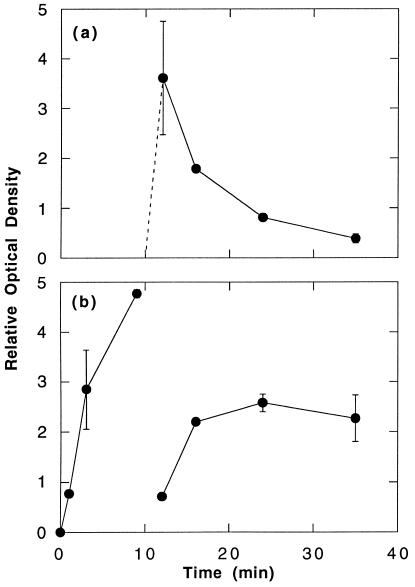
Receptors aggregated with relatively high concentrations of a multivalent antigen also dephosphorylate spontaneously, i.e., in the absence of a disaggregating stimulus (refs. 13 and 14 and Fig. 4a). We were interested to see whether this spontaneous dephosphorylation of the highly aggregated receptors would allow for rephosphorylation of the receptors stably aggregated by IgE oligomers as was the case with the disaggregation-induced dephosphorylation. This was indeed observed (Fig. 4b). The kinetics of rephosphorylation appeared to parallel the rate of dephosphorylation of the more extensively aggregated receptors, but in this instance, the final level of phosphotyrosine achieved never reached that existing before the addition of antigen. Three additional experiments using the same protocol gave the same results.
Figure 4.
Spontaneous dephosphorylation of receptors after stimulation with antigen. Two separate incubation mixtures were prepared in duplicate. In each at t = 0, RBL-2H3 cells (6.25 × 106/ml), which had been partially sensitized with DNP-specific mouse IgE, were reacted with rat IgE oligomer (0.3 μg/ml). At t = 10 min, DNP18.5-BSA (1 μg/ml) and 10 μg/ml monomeric rat IgE (protocol a) or monomeric dansyl-specific mouse IgE (protocol b) was added. At each time point, the cells were solubilized. The IgEs were immunoprecipitated with species-specific anti-mouse IgE (protocol a) or anti-rat IgE (protocol b). Recovery of receptors was confirmed by recovery of [125I]-IgE dimer. The immunoprecipitated receptors were assessed for phosphotyrosine. The data are the average of two samples. (a) Time course of the phosphorylation of receptors bound to anti-DNP mouse IgE. (b) Time course of the phosphorylation of receptors bound to rat IgE oligomers. The ordinate values are graphed on a relative scale; the absolute ratio of the data in a:b approximated 7:1.
DISCUSSION
The studies described in this paper were prompted by earlier ones in which we observed a progressive divergence between the extent to which FcɛRI was aggregated and its subsequent phosphorylation (4). It appears that those receptors that were aggregated late in the reaction were somehow less effective. Using a mechanistic model we had proposed for how the Lyn kinase phosphorylates the receptor when the latter is aggregated (15, 16), we offered a specific explanation (4): “… kinases that… [are] pre-associated with receptors… [are] capable of dissociating and binding to newly created phosphotyrosines on other proteins. Since Lyn kinase is only weakly associated with unaggregated receptors but is recruited to phosphorylated tyrosines on aggregated FcɛRI, the creation of new high affinity binding sites that could compete for Lyn might reduce the number of pre-associated Lyn-receptor complexes available late in the response. … such newly formed aggregates, lacking associated kinase would be ineffective.” We have now experimentally tested this explanation.
We first eliminated one trivial alternative explanation, i.e., that the receptors that were aggregated late in the reaction were not phosphorylated because the later binding oligomers of IgE were not in the correct orientation to induce productive aggregates of FcɛRI. The data in Table 1 show that this explanation is not supported by the experimental results. Preparations of oligomers depleted of the most rapidly binding ones are quantitatively indistinguishable from the initial preparation in their ability to induce phosphorylation of the receptors’ tyrosines.
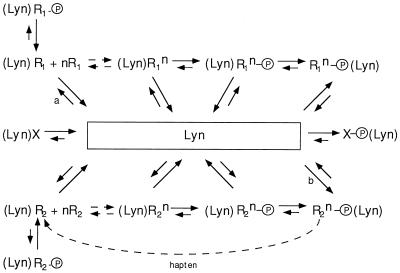
We next set up experimental conditions that would accentuate the competition between aggregates for limiting amounts of the kinase. We observed that receptors aggregated by a strong stimulus became phosphorylated at the same time as those receptors that had been aggregated by dimeric IgE were dephosphorylated. These results are consistent with the dimerized FcɛRI interacting with Lyn as shown in the upper portion of Fig. 5. Here R1 refers to the receptors that bind the rat IgE dimers. Some of these will be weakly associated with Lyn, (Lyn)R1, and when aggregated with other R1, (nR1), will initiate the phosphorylation of tyrosines on the receptors in the aggregate ((Lyn)R1n − P). The phosphorylation of the receptors recruits additional molecules of Lyn (15, 16). This phosphorylation-dependent interaction of Lyn with the receptor is denoted by the species R1n − P (Lyn). Each of the species of receptors is shown interacting reversibly with each other and with a pool of Lyn. The constitutive action of cellular phosphatases also creates a dynamic balance between the phosphorylated and unphosphorylated counterparts of each species that is associated with Lyn.
Figure 5.
Kinetic scheme for interaction between receptors and a common pool of Lyn kinase. In this figure, Lyn placed to the left of the receptor Ri refers to the constitutively associated kinase; Lyn placed to the right of phosphorylated Ri − P refers to recruited kinase (15, 16). In the latter species, no constitutively associated kinase is shown simply to avoid complicating the figure. Whether such species continue to interact with Lyn at their presumed site of constitutive interaction is not known. Our schematic representation is also not meant to rule out the possibility that Lyn bound to the phosphorylated receptors may be different than the Lyn bound constitutively. X refers to any non-FcɛRI that may be able to interact with Lyn constitutively or after phosphorylation or both. n to the left of Ri refers to monomeric receptors where n ≥ 1; n as a superscript refers to an aggregate of Ri where n ≥ 2. P refers to phosphotyrosine. The slanted arrows refer to the disposition of Lyn; the vertical and horizontal arrows to the disposition of the entire complex. The horizontal arrows on the left are shown as dashed because the direction of the equilibrium will depend upon the binding constant of the aggregating stimulus for the IgE (if the stimulus is antigen) or for the receptor (if the stimulus is oligomer).
In the experiment under discussion, addition of antigen promotes reaction b. Possibly, some phosphorylated downstream targets (X − P) can also compete for Lyn. With a finite pool of Lyn, that kinase constitutively associated with R1 through reaction a will be diminished to the point where the generation and build-up of the species (Lyn)R1n − P becomes negligible. The prompt dephosphorylation of the small oligomers of receptor, in this instance because of competition from other aggregates, is consistent with the results from other experimental protocols that demonstrate indirectly (refs. 11, 14, and 17 and S.-Y Mao and H.M. unpublished results) that aggregates are no less susceptible to dephosphorylation than monomeric receptors.
Although in the initial experiments the dimerized receptors were pitted against larger aggregates driven by a multivalent antigen, the experiment depicted in Fig. 2 indicates that dimers can produce the same effect providing that there is a sufficient number. In that sense, there is nothing special about the antigen-induced aggregates; it is the number and size of the competing aggregates that will determine the ability to sequester the limited amount of Lyn, not the nature of the aggregating stimulus per se.
Returning to the experiments involving the use of antigen-induced aggregates, addition of hapten, as in the experiments depicted in Fig. 3, depolymerizes the competing species R2n − P (Lyn), and its rapid dephosphorylation (and the corresponding dephosphorylation of any X − P (S.-Y Mao and H.M. unpublished results) is expected to restore the pool of Lyn to its original size so that the stable polymer R1n can recover its share of Lyn and rephosphorylate. It is notable that the rephosphorylation of the stable oligomers of receptor proceeded more rapidly than the initial phosphorylation (Fig. 3). Likely this is because the rate of the initial phosphorylation is limited by the kinetics of dimer formation. According to the scheme proposed in Fig. 5, the kinetics of the rephosphorylation of the predimerized receptors is governed by the hapten-induced depolymerization and then dephosphorylation of R2n − P (Lyn), by the dissociation of Lyn, and by its reassociation with the dimers R1n. The rephosphorylation of the preexisting oligomers is consistent with our previous results that demonstrated that at least modest-sized aggregates of FcɛRI are subject to repeated cycles of phosphorylation, dephosphorylation, and rephosphorylation over considerable periods of time (11). The rapidity of the rephosphorylation also shows that even if aggregation of FcɛRI can stimulate phosphatase activity in the cells, as one group has reported (18), that increase does not appear to diminish the steady-state level of phosphotyrosines on the receptors.
The mechanism by which more extensive aggregates are spontaneously dephosphorylated relatively rapidly remains obscure. Clearly, our observation that other smaller aggregates become rephosphorylated under these conditions (Fig. 4b) indicates that the phenomenon is real and is not due to an experimental artifact whereby heavily aggregated receptors are simply more difficult to recover in their phosphorylated state. The rephosphorylation of the dimerized receptors in the face of the dephosphorylation of the antigen-driven aggregates is most easily understood if the dephosphorylation is accompanied by a release of the Lyn that the latter complexes recruited. However, whether this dissociation is a consequence of the dephosphorylation or vice versa is unclear. That the rephosphorylation of the smaller aggregates is only partial suggests that one is observing an instance of “heterologous desensitization”—a phenomenon that has been described in other instances by monitoring later steps in the reactions stimulated by FcɛRI (19, 20).
Finally, we comment briefly on an incidental observation related to the relative phosphorylation of β and γ. From direct analysis using incorporation with 32P, it is now known that these phosphorylations occur almost exclusively on the two canonical tyrosines in the immunoreceptor tyrosine-based activation motifs (ITAMs) within the cytoplasmic domains of these subunits (V. Pribluda, C. Pribluda, and H.M., unpublished results). If these residues were equally susceptible to phosphorylation, the predicted ratio would be 1:2 since each β and each of the two γ subunits contain a single ITAM. However, the direct analysis showed a lesser incorporation into the amino-terminal tyrosine within the ITAM of the β subunit than in the other tyrosines. In the current experiments we regularly observed an even greater difference in the relative phosphorylation of tyrosines on β when the receptors were aggregated by dimer compared with the larger aggregates that result by the addition of multivalent antigen. A plausible explanation is as follows. All of these phosphorylations occur by a process of transphosphorylation by receptor-bound Lyn kinase (15, 16) so that phosphorylation of the β subunits depends on Lyn associated with coaggregating receptors. There is evidence that Lyn preferentially associates with the β subunit (21, 22), and we surmise that since the molecular weight of the entire C-terminal cytoplasmic domain of the β subunit is less than one-tenth that of Lyn, the latter partially blocks access to the canonical tyrosines on β for steric reasons. Therefore, in a dimer the Lyn associated with the single coaggregating receptor can phosphorylate the β subunit of its partner (and vice versa) from only a single orientation. In a multireceptor complex, the less accessible tyrosines should be approachable from many more directions.
Acknowledgments
We thank Dr. R. Siraganian for a careful reading of the manuscript and helpful comments. This work was supported in part by National Institutes of Health Grant GM35556 and National Science Foundation Grant HRD9627118 and was performed in part under the auspices of the National Institutes of Health and the U.S. Department of Energy.
Footnotes
Abbreviation: DNP, 2,4-dinitrophenyl.
References
- 1.Däeron, M. (1997) Annu. Rev. Immunol., in press.
- 2.Seed B, Kolanus W, Romeo C, Xavier R. Adv Exp Med Biol. 1994;365:111–119. doi: 10.1007/978-1-4899-0987-9_12. [DOI] [PubMed] [Google Scholar]
- 3.Eiseman E, Bolen J B. Nature (London) 1992;355:78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- 4.Wofsy C, Kent U M, Mao S-Y, Metzger H, Goldstein B. J Biol Chem. 1995;270:20264–20272. doi: 10.1074/jbc.270.35.20264. [DOI] [PubMed] [Google Scholar]
- 5.Liu F T, Bohn J W, Ferry E L, Yamamoto H, Molinaro C A, Sherman L A, Klinman N R, Katz D H. J Immunol. 1980;124:2728–2737. [PubMed] [Google Scholar]
- 6.Holowka D, Metzger H. Mol Immunol. 1982;19:219–227. doi: 10.1016/0161-5890(82)90334-0. [DOI] [PubMed] [Google Scholar]
- 7.Bazin H, Querinjean P, Beckers A, Heremans J F, Dessy F. Immunology. 1974;26:713–723. [PMC free article] [PubMed] [Google Scholar]
- 8.Barsumian E L, Isersky C, Petrino M G, Siraganian R P. Eur J Immunol. 1981;11:317–323. doi: 10.1002/eji.1830110410. [DOI] [PubMed] [Google Scholar]
- 9.Segal D M, Taurog J D, Metzger H. Proc Natl Acad Sci USA. 1977;74:2993–2997. doi: 10.1073/pnas.74.7.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farah F S, Kern M, Eisen H N. J Exp Med. 1960;112:1195–1210. doi: 10.1084/jem.112.6.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kent U M, Mao S Y, Wofsy C, Goldstein B, Ross S, Metzger H. Proc Natl Acad Sci USA. 1994;91:3087–3091. doi: 10.1073/pnas.91.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulczycki A, Jr, Metzger H. J Exp Med. 1974;140:1676–1695. doi: 10.1084/jem.140.6.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paolini R, Jouvin M-H, Kinet J-P. Nature (London) 1991;353:855–858. doi: 10.1038/353855a0. [DOI] [PubMed] [Google Scholar]
- 14.Pribluda V S, Metzger H. Proc Natl Acad Sci USA. 1992;89:11446–11450. doi: 10.1073/pnas.89.23.11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pribluda V S, Pribluda C, Metzger H. Proc Natl Acad Sci USA. 1994;91:11246–11250. doi: 10.1073/pnas.91.23.11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamashita T, Mao S-Y, Metzger H. Proc Natl Acad Sci USA. 1994;91:11251–11255. doi: 10.1073/pnas.91.23.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adamczewski M, Paolini R, Kinet J-P. J Biol Chem. 1992;267:18126–18132. [PubMed] [Google Scholar]
- 18.Hampe C S, Pecht I. FEBS Lett. 1994;346:194–198. doi: 10.1016/0014-5793(94)00471-4. [DOI] [PubMed] [Google Scholar]
- 19.MacGlashan D W, Jr, Lichtenstein L M. J Immunol. 1981;127:2410–2414. [PubMed] [Google Scholar]
- 20.Weetall M, Holowka D, Baird B. J Immunol. 1993;150:4072–4083. [PubMed] [Google Scholar]
- 21.Jouvin M H, Adamczewski M, Numerof R, Letourneur O, Valle A, Kinet J P. J Biol Chem. 1994;269:5918–5925. [PubMed] [Google Scholar]
- 22.Kihara H, Siraganian R P. J Biol Chem. 1994;269:22427–22432. [PubMed] [Google Scholar]