Abstract
A fundamental goal of current strategies to develop an efficacious vaccine for AIDS is the elicitation of broadly reactive cytotoxic T lymphocyte (CTL) reactivities capable of destroying virally infected targets. Recent application of recombinant canarypox ALVAC/HIV-1 vectors as vaccine immunogens in HIV-1,-noninfected volunteers has produced CTL responses in a significant number of vaccinees. Using a newly developed targeting strategy, we examined the capacity of vaccine-induced CTL to lyse autologous targets infected with a diverse group of viral isolates. CTL derived from recipients of a canarypox ALVAC/HIV-1 gp160 (MN) vaccine were found capable of lysing autologous CD4+ lymphoblasts infected with the prototypic LAI strain of HIV-1. When tested against autologous targets infected with primary HIV-1 isolates representing genetically diverse viral clades, CTL from ALVAC/gp160 recipients showed both a broad pattern of cytolysis in which viruses from all clades tested were recognized as well as a highly restricted pattern in which no primary isolates, including clade B, were lysed. Differences in the HLA haplotypes of the volunteers immunized with the envelope vector might be a major determinant of the relative breadth of their CTL response. In contrast to ALVAC/gp160 vaccinees, recipients of the ALVAC/HIV-1 immunogen containing envelope as well as gag and protease genes consistently had CTL reactivities effective against a spectrum of primary isolate-infected targets. These studies demonstrate for the first time that clade B-based canarypox vaccines can elicit broad CTL reactivities capable of recognizing viruses belonging to genetically diverse HIV-1 clades. The results also reinforce the impact of viral core elements in the vaccine as well as the pattern of major histocompatibility complex class I allelic expression by the vaccine recipient in determining the relative breadth of the cellular response.
Foremost among the many obstacles confounding the development of an efficacious preventive vaccine for AIDS is the elicitation of broadly reactive immune responses capable of recognizing the genetically diverse viral quasispecies that now exist worldwide. This has been particularly difficult with respect to generation of virus-neutralizing antibodies using envelope subunit immunogens that, although effective against laboratory isolates (1), failed to neutralize primary HIV-1 isolates that more closely resemble the transmissible agents (2). Cellular reactivities that recognize highly conserved epitopes within virus structural and regulatory elements (3) might provide much broader immunologic specificities. Present day vaccine efforts are clearly aimed at eliciting such broad cellular reactivities in the form of major histocompatibility complex (MHC) class I-restricted, CD8+ cytotoxic T lymphocytes (CTL). Although the correlates of immune protection against HIV-1 infection have not yet been clearly defined, several lines of evidence suggest that CTL might represent an important component of protective immunity. First, CTL appear to be temporally correlated with the clearance of the virus during the acute phase of infection (4–6), and an inverse correlation has been demonstrated between the level of CTL reactivities and virus load during HIV-1 infection (7). Second, a decline in both anti-HIV CTL activity and precursor frequency routinely accompanies disease progression (8, 9), in contrast to the course of long term nonprogressors, who maintain a persistent level of anti-Gag CTL reactivity (10). Finally, HIV-exposed but seronegative individuals as well as uninfected children born to HIV-1-infected mothers have exhibited anti-HIV CD8+ CTL reactivity as a unique sign of virus exposure (11–13), suggesting that CTL are an important correlate to be considered.
Phase I clinical trials of candidate AIDS vaccines completed over the past 3–5 years have confirmed that envelope subunit vaccines, although capable of inducing high titered humoral reactivities, were extremely inefficient in eliciting CD8+ CTL (14–16). Second generation vaccines using live, recombinant, poxvirus constructs have proven to be far more potent CTL immunogens (17–19). In recently instituted protocols, canarypox (ALVAC)-based vectors have been shown to elicit CTL activity in a significant number of volunteers (20–22), particularly in conjunction with a “prime boost” strategy involving initial vector immunization followed by a subunit boost. In the present study, we examined for the first time the ability of CTL elicited by clade B-based vaccines to lyse autologous CD4+ lymphocytes infected with HIV-1 primary isolates representing six genetically diverse virus clades.
MATERIALS AND METHODS
Subjects and Study Protocols.
Peripheral blood mononuclear cell (PBMC) samples were randomly selected from volunteers enrolled in the National Institute of Allergy and Infectious Diseases/National Institutes of Health-sponsored AIDS Vaccine Evaluation Group (AVEG) protocols 012B and 022 who had developed a detectable CTL response to the vaccine immunogen. Protocol 012B consisted of two immunizations with a recombinant canarypox ALVAC-HIV-1 gp160MN vector (vCP125; Pasteur–Merieux Connaught Laboratories) followed by either two additional vCP125 injections or two HIV-1 envelope subunit boosts with HIV-1SF2 rgp120 (Biocine, Emeryville, CA). For protocol 022, a recombinant canarypox ALVAC-HIV-1 vector (vCP205; Pasteur–Merieux Connaught Laboratories) comprised of the following HIV-1 genes was used: gp120MN, the transmembrane portion of gp41LAI, gagLAI, and protease. The immunization schedule consisted of either three or four vector injections during the first 6 months, followed by two HIV-1 envelope subunit boosts with either HIV-1SF2 rgp120 (Biocine) alone or in combination with vCP205.
HIV-1 Subtypes.
Six different primary HIV-1 isolates [92RW009 (clade A), 92TH026 (clade B), 92BR025 (clade C), 92UG035 (clade D), 92TH021 (clade E), and 93BR029 (clade F)] were used to infect CD4+ T lymphocytes obtained from the volunteers to generate HIV-1-infected autologous target cells for the CTL assay. All of the six primary isolates were of the nonsyncytium-inducing (NSI) biological phenotype. These reagents were provided by James Bradac (Division of AIDS, National Institute of Allergy and Infectious Diseases/National Institutes of Health) through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases/National Institutes of Health (23).
Recombinant Vaccinia Viruses.
Autologous stimulator cells for in vitro stimulation (IVS) as well as autologous B lymphocyte cell line (BLCL) targets for the CTL assays were infected with recombinant vaccinia viruses (multiplicity of infection = 5:1) expressing the following extrinsic gene inserts: vP1170 (Western Reserve parent control), vP1174 (HIV-1MN env gp160), vDK1 (HIV-1LAI gag), and vP1291 (HIV-1MN env gp120+gp41 transmembrane protein; HIV-1LAI gag without protease). The vDK1 construct was a generous gift from D. Kuritzkes (University of Colorado, Health Sciences Center), and all of the other vectors were kindly provided by J. Tartaglia (Virogenetics, Troy, NY).
Depletion of Lymphocyte Subsets.
Samples of venous blood, anti-coagulated in preservative-free heparin, were received from the AVEG clinical sites within 24 h, and lymphocytes were prepared as described (24). Anti-CD4 and anti-CD8 mAb-coated magnetic microspheres (Dynal, Oslo) were used to selectively deplete effector cell populations for the CTL assay. Goat anti-mouse IgG-coated beads were used as controls for the manipulations. Cellular depletions were performed as described (24), and the depleted populations were used in CTL assays according to their predepletion concentration. Lymphocyte depletion efficiency was analyzed by flow cytometry and was generally found to be greater than 90%. Anti-CD4 antibody-coated beads also were used with the same procedure to enrich HIV-1-infected p24+/CD4− lymphocyte target cells for the CTL assay (see below).
IVS.
Twenty-four million PBMC were used as responder cells resuspended at 1 × 106 cells/ml in RPMI 1640 medium (GIBCO) supplemented with 5% human AB serum (Advanced Biotechnologies, Columbia, MD) and antibiotics. Six million cells were used as stimulator cells. The stimulator cells were infected either with vP1174 (protocol 012B) or vP1291 (protocol 022) for 90 min at 37°C in 15-ml polypropylene tubes (Sarstedt), washed once, recombined with the remaining PBMC, and cultured in T-75 flasks (Costar) in the presence of 330 units/ml of interleukin 7 (IL-7) (Genzyme). If positive for CTL activity after 12–14 days, cryopreserved, autologous PBMC collected from a previous visit were infected with the appropriate recombinant vaccinia virus and used at a responder-to-stimulator ratio of 4:1. Cells were cocultured at 1 × 106 cells/ml in RPMI 1640 medium (GIBCO) supplemented with 10% human AB serum (Advanced Biotechnologies), antibiotics, and IL-7. Twenty-five units per milliliter of recombinant IL-2 (Hoffman–La Roche) was added to the cell culture at days 3, 7, and 10 during the first and second stimulations.
Vaccinia-Infected Targets.
Autologous, Epstein–Barr virus-transformed B lymphocytes (BLCL) were used as targets in the CTL assay after infection with recombinant vaccinia–HIV constructs. In brief, 1 × 106 BLCL were infected (multiplicity of infection, 5:1) for 90 min at 37°C with the vP1170, vP1174, and vDK1 constructs. Cells were subsequently washed and labeled overnight with 100–200 μCi (1 Ci = 37 GBq) of sodium chromate (51Cr; DuPont) in 1 ml of RPMI 1640 medium (GIBCO) supplemented with 20% fetal calf serum and antibiotics (culture medium) at 37°C and 5% CO2. Unlabeled BLCL infected with the vP1170 control vector were prepared in a similar manner and were used as cold target competitors for anti-vac reactivity in the CTL assays.
HIV-Infected Targets.
Frozen, autologous PBMC were thawed and counted for viability. Cells were resuspended in 4 ml of PBS supplemented with 0.01 mM EDTA and 0.5% human albumin and dispensed into a T-25 CD4 MicroCELLector flask (Applied Immune Systems, Menlo Park, CA) primed according to the manufacturer’s procedures. After 1 h of incubation at room temperature, the nonadherent cells were removed by gentle washes. Adherent cells were activated for 3 days in culture medium with anti-CD3 mAb 100 ng/ml (Orthoclone OKT3; Ortho Biotech, Raritan, NJ), anti-CD28 mAb 100 ng/ml (Becton Dickinson) in the presence of 100 units/ml IL-2. The cells were removed from the flask, assessed for viability, and resuspended at 2 × 106/ml in culture medium supplemented with 100 units/ml IL-2. One million cells were infected with 1 × 105 tissue culture 50% infective dose of the different clades of NSI HIV stock for 2 h at 37°C in 5% CO2. The cells were transferred in a final volume of 1 ml without washing into a 12-well culture plate. Cells were refed on days 3 and 5 with culture medium, adjusting the concentration to 1.5 × 106 cells/ml. On day 7, the cells were washed, resuspended in fresh medium, and radiolabeled overnight with 100–200 μCi of 51Cr at 37°C and 5% CO2. Flow cytometric analyses revealed that, even under optimal conditions, only 20–40% of the autologous CD4 lymphoblasts was positive for intracellular p24 expression, and all of the p24+ cells were contained in the CD4− population. Therefore, just before the setup of the CTL assays, the labeled cells were washed twice and subsequently enriched for infected cells by depletion of uninfected, CD4+ cells with anti-CD4-coated beads (Dynal) as described above. After removal of the beads, target cells were washed, counted for viability, and adjusted to the desired concentration in RPMI 1640 medium (GIBCO) supplemented with 10% fetal calf serum and antibiotics (assay medium).
CTL Assay.
In vitro-stimulated lymphocytes were tested against HIV-recombinant vaccinia-infected, autologous BLCL after the first IVS and also against the autologous, HIV-infected lymphocytes after the second IVS. Effector cells were treated with the magnetic beads as described above. Undepleted (i.e., monoclonal anti-mouse IgG-treated), in vitro-stimulated lymphocytes were resuspended in assay medium and were used as effectors at effector-to-target (E/T) ratios of 100:1 and 50:1 after the first IVS. Depending on the cell recovery after the second IVS, the E/T ratio was either 50:1 or 40:1. Subset-depleted populations (anti-CD4 and anti-CD8-treated cells) were resuspended to the same volume as undepleted samples. Each E/T ratio was tested in triplicate. Undepleted and depleted populations were tested against recombinant vaccinia/HIV-1-infected, autologous BLCL in the presence of control vaccinia-infected, autologous, cold target cells using a ratio of unlabeled-to-labeled cells of 20:1. On the day of the assay, 51Cr-labeled targets were washed, counted, resuspended in assay medium, and plated at a concentration of 2500 viable cells/well in round bottomed, 96-well plates. 51Cr-labeled target cells plus medium or 0.5% Triton X-100 served as controls for spontaneous (SR) and maximum 51Cr release (MR), respectively. After a 4-h incubation at 37°C and 5% CO2, cell-free supernatants were harvested and counted on a Top-Count Microplate Scintillation Counter (Packard). The percentage-specific lysis was calculated according to the formula [(cpm experimental release − cpm SR)/(cpm MR − cpm SR)] × 100. SR values were ≤38% of MR. The SE for each triplicate did not exceed 10% of the mean value.
RESULTS
Vaccine-Induced Anti-HIV-1 CTL.
In a manner analogous to other viral vaccines (25–27), the measurement of HIV-1 vaccine-induced CTL requires IVS of CTL precursors. We therefore used a standard IVS strategy capable of activating anti-HIV-1 CTL precursors obtained from infected patients. This strategy (see Materials and Methods) has been used in the assessment of vaccine-induced CTL in conjunction with a number of phase I clinical protocols conducted by the AVEG. To date, envelope subunit protocols, even in the context of novel adjuvants, have elicited very few MHC class I-restricted CD8+ CTL responses. Live vector vaccine protocols using recombinant poxvirus constructs have, on the other hand, produced detectable CTL responses in a significant number of vaccinees (17, 19, 21, 22).
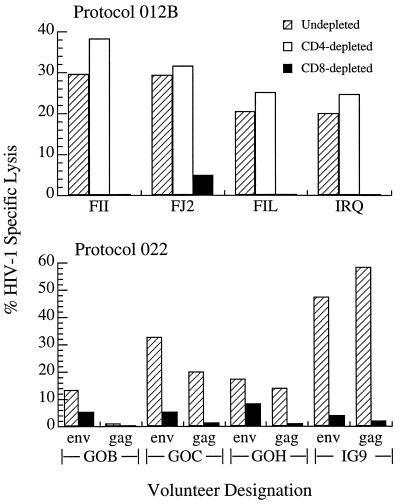
One recently completed protocol (AVEG 012B) and one ongoing protocol (AVEG 022) were conducted with canarypox constructs expressing full length HIV-1 gp160 (ALVAC vCP125) or gp120 plus the transmembrane portion of gp41, full length gag, and protease (ALVAC vCP205), respectively. A representative sampling of the CTL responses elicited by these vectors is depicted in Fig. 1. For all AVEG studies, an operational, two-tiered definition of a positive CTL response has been used, namely (i) a 10% or greater difference in the percentage-specific lysis above that of the control target and (ii) a 50% or greater depletion in lytic activity after removal of CD8+ cells. At the timepoints tested, all four of the protocol 012B volunteers had received two ALVAC vCP125 priming immunizations followed by two SF2 rgp 120 subunit boosts whereas the 022 volunteers had received only ALVAC vCP205 immunizations and no subunit boosting. CTL assays were performed 2 weeks after the last immunization. Anti-envelope CTL activities were apparent in all of the volunteers depicted, and three of the four protocol 022 vaccinees also exhibited significant anti-gag reactivity. All of the CTL activity was contained within the CD8+ lymphocyte population, as shown by the elimination of lytic reactivity after depletion of CD8+ cells.
Figure 1.
The percentage of HIV-1-specific lysis against vaccinia/Env- and vaccinia/Gag-infected, autologous BLCL is reported subtracting the background lysis of control vaccinia-infected targets. Results for volunteers FII, FJ2, FIL, and IRQ are reported at E/T = 40:1 and for volunteers G0B, G0C, G0H, and IG9 at E/T = 50:1. (Upper) The CTL activities are directed against env determinants only. (Lower) The CTL are directed against both env and gag determinants.
CTL Reactivity Against an HIV-1 Prototypic Isolate.
As with HIV-1-infected patients, the analysis of anti-viral CTL reactivities in vaccine recipients was performed using a targeting strategy first reported by Walker et al. (28) involving acute infection of autologous BLCL with recombinant poxvirus vector constructs expressing specific HIV-1 genes. This is because routine measurement of CTL reactivity against HIV-1-infected, autologous lymphocyte targets is difficult, due mainly to the fact that the postinfection peak of viral expression immediately precedes the onset of virus-induced cytopathology and declining cell viability, greatly confounding the use of such targets in cytotoxicity assays. However, if this and other problems could be overcome, the use of targets infected with diverse HIV-1 isolates would be very useful in determining the breadth of CTL activity.
With this goal in mind, we developed a standardized infection protocol and monitored infection by expression of intracellular p24 in CD4+ lymphoblasts. In preliminary studies, we found that virus expression usually reached maximal levels by 5–7 days postinfection, before a decline in overall cell viability (data not shown). However, maximal intracellular p24 expression varied greatly depending on which viral stocks were used and seldom reached levels of more than 40% of the population. Therefore, to enrich for HIV-1-infected p24+ target cells, we removed the p24−/CD4+ population with anti-CD4 magnetic microspheres just before using them as targets (see Materials and Methods).
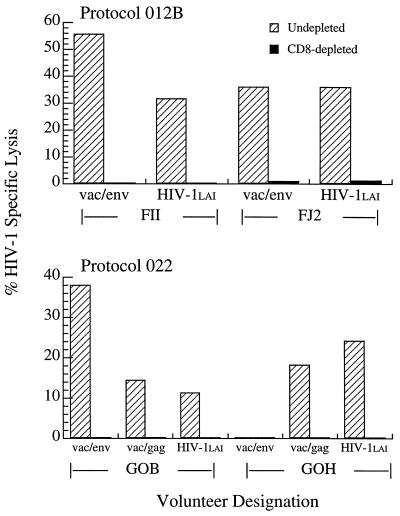
To study reactivity against HIV-1-infected targets, effector cells from CTL positive screening assays were restimulated and retested for CTL activity against both recombinant vaccinia construct-infected, autologous BLCL as well as HIV-1LAI-infected, autologous CD4+ lymphoblast targets. Representative results of these analyses are shown in Fig. 2. Clearly, the envelope-specific CTL derived from volunteers enrolled in protocol 012B exhibited high lytic reactivity against autologous CD4+ lymphoblast targets infected with HIV-1LAI. Likewise, CTL cultures of two protocol 022 CTL volunteers, one with both anti-env and anti-gag reactivities (G0B) and the other with only anti-gag activity (G0H), also recognized HIV-1LAI-infected targets. Thus, CTL generated in response to a clade B-based immunogen effectively lysed autologous cells infected with a clade B primary isolate (HIV-1LAI).
Figure 2.
The percentage of HIV-1-specific lysis against vaccinia/Env-infected or vaccinia/Gag-infected, autologous BLCL targets or HIV-1LAI-infected, autologous CD4+ lymphoblasts is reported subtracting the background lysis of control vaccinia-infected, autologous BLCL targets or uninfected, autologous CD4+ lymphoblast targets, respectively. Results for volunteers FII and FJ2 are reported at E/T = 40:1 and for volunteers G0B and G0H at E/T = 50:1.
CTL Activity Against Diverse HIV-1 Subtypes.
To date, all of the candidate AIDS vaccine strategies that have been tested in phase I clinical trials through the AVEG use HIV-1 clade B-based immunogens. Given the broad genetic diversity of HIV-1 isolates worldwide, coupled with the geographic localization of distinct viral clades (23), a major question to be addressed is whether clade B-based vaccines would have utility in areas where nonclade B strains are endemic. At the level of CTL reactivity, the question focuses on the potential breadth of CTL effector function. To address this, we next tested the specificity of vaccine-induced CTL against autologous CD4+ lymphoblasts infected with representative primary HIV-1 isolates from clades A through F. Previous studies have determined that the “transmitted virus” most likely has a NSI biologic phenotype (29, 30), so we used NSI isolates for these CTL studies. Once again, noninfected CD4+ lymphoblasts were removed from our target cell population just before performing the CTL assays.
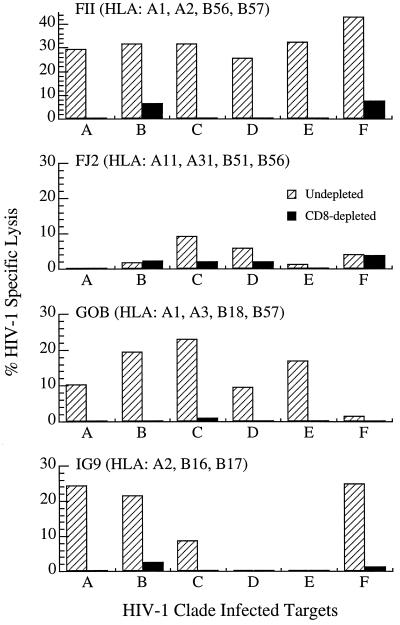
As shown in Fig. 3, the two canarypox ALVAC/HIV-1 gp160 protocol 012B volunteers (FII and FJ2) exhibited distinctly different patterns of CTL reactivity against primary isolate-infected lymphoblast targets. CTL derived from volunteer FII showed a broad pattern of activity, lysing targets infected with clade A, B, C, D, E, and F isolates. Volunteer FJ2-derived CTL, on the other hand, showed a highly restricted pattern of reactivity in which none of the primary isolate-infected targets, including those infected with clade B, were recognized (i.e., ≤10% specific lysis). Flow cytometric analysis of intracellular p24 levels in the infected CD4+ lymphoblasts (data not shown) revealed comparable expression in both the FII and FJ2 sets of targets, thus eliminating the possibility that the lack of cytolytic activity by FJ2 effectors was due to suboptimal infection of the various target cell populations. An important difference in these two volunteers is the composition of their MHC class I alleles, which act as restricting elements for CTL.
Figure 3.
The percentage of HIV-1-specific lysis against autologous CD4+ lymphoblasts infected with primary HIV-1 isolates representing the designated clades is reported subtracting the background lysis against uninfected, autologous CD4+ lymphoblast targets. Results for volunteers FII and FJ2 are reported at E/T = 40:1 and for volunteers G0B and IG9 at E/T = 50:1
The CTL reactivities from the two protocol 022 volunteers depicted in Fig. 3 (i.e., G0B and IG9) who were immunized with a gp120+TM+gag/protease canarypox vector recognized select populations of primary HIV-1-infected targets. Both G0B and IG9 effector cell populations lysed CD4+ lymphoblasts infected with clade A and B primary isolates. CTL from volunteer G0B also lysed clade C- and E-infected targets, and IG9 CTL showed strong activity against clade F. Thus, both a gp160-based canarypox immunogen (protocol 012B) as well as one containing additional gag/protease genes (protocol 022) could elicit CTL reactivities capable of recognizing autologous CD4+ lymphocytes infected with genetically diverse HIV-1 primary isolates. An obvious variable in determining the relative breadth of these CTL responses is the expression of MHC class I alleles capable of restricting the presentation of target epitopes, as illustrated most clearly in the patterns of lysis seen with CTL from the ALVAC/gp160-immunized volunteers FII and FJ2. Similarly, differences in the CTL activity of the multi-gene construct recipients against the various primary viral isolates might be largely governed by HLA haplotypes.
DISCUSSION
Among the principal goals in the development of an effective prophylactic AIDS vaccine is the selection of an immunogen capable of eliciting broadly reactive CTL activities. The present studies demonstrate, for the first time, that CTL elicited in response to two different HIV-1 clade B-based canarypox vector vaccines are capable of recognizing and lysing autologous CD4+ cells infected with genetically diverse primary HIV-1 isolates. These findings suggest that vaccine-induced CTL are not constrained by existing barriers that have prevented the exploitation of neutralizing antibodies in vaccine development, namely (i) their inability to neutralize field isolates on their natural target cells and (ii) poor success in the induction of antibodies that effectively cross-neutralize diverse strains of HIV-1 (2).
Conventional screening of CTL reactivities from both infected patients and vaccinees is performed using autologous, Epstein-Barr virus transformed BLCL acutely infected with recombinant vaccinia targeting vectors expressing specific HIV-1 genes, in most cases based on the clade B prototypic HIV-1LAI or HIV-1MN isolates (31, 32). We sought an alternative targeting strategy that was more relevant and amenable to measurement of CTL breadth against genetically diverse primary HIV-1 isolates. The use of virally infected, autologous CD4+ lymphoblasts more closely approximates the naturally occurring CTL target and eliminates concerns that transformed B cell lines infected with recombinant constructs might present and process antigens in a different manner than the more biologically relevant, infected CD4 cell (33). The relatively low infection rate of primary isolates for the autologous CD4+ lymphoblast targets in the present study was overcome by taking advantage of the diminished CD4 expression on infected cells resulting from either intracellular complexing of CD4 and gp160 (34) or down-regulation by nef (35). Uninfected CD4+ cells were removed before use in the CTL assay, thus assuring virus expression in the majority of lymphoblast targets.
The extent and breadth of vaccine-induced CTL activity depends on the ability of cells present in the vaccinee to process and present linear peptide sequences from the vaccine antigens in association with MHC molecules to CTL. Because of extensive variation in MHC allelic expression in the population, recognition of CTL epitopes is not uniform, as is evident from the studies of the two subjects immunized with the single envelope gene product. In contrast to the broad pattern of CTL reactivity in subject FII, CTL from FJ2 failed to lyse autologous cells infected with the clade B primary isolate in our target panel as well as any of the primary isolate-infected targets, despite showing greater than 30% lysis of HIV-1LAI-infected cells. One possible explanation for this lack of reactivity is the MHC class I haplotype of this ALVAC/HIV-1MN gp160 vaccinee, in particular the expression of the HLA-A11 allele. To date, the only HLA-A11-restricted CTL epitope present in the HIV-1 envelope encompasses amino acids 315–329, a portion of the third hypervariable (V3) domain (36). The V3 regions of HIV-1MN (i.e., the immunogen) and HIV-1LAI are reasonably homogenous in most of this region with respect to amino acid sequence. The primary HIV-1 isolate selected as the clade B representative in our targeting panel, on the other hand, has a number of amino acid changes throughout this portion of the V3 region (37), which could dramatically affect binding and presentation in the context of HLA-A11. Thus, although we have not yet mapped the fine epitope(s) recognized by this vaccinee’s CTL, a predominant HLA-A11-restricted anti-V3MN reactivity would be consistent with the CTL specificity findings. This serves to highlight the influence of the host’s MHC class I haplotype on the induction of CTL specificities against genetically diverse viral variants.
The pattern of highly restricted CTL reactivity shown by the ALVAC/gp160-immunized volunteer (FJ2) illustrates the potential shortcomings of such single gene immunogens. Although there was variation in the CTL responses against different targets, both individuals immunized with the multi-gene vector displayed CTL activity against cells infected with multiple HIV-1 isolates. The rationale for inclusion of gag components in the canarypox vector used in the 022 protocol was based on studies in HIV-1-infected patients that showed that a number of well conserved determinants in HIV-1 p24 served as dominant CTL epitopes with multiple MHC class I-restricting alleles (38, 39). Thus, elicitation of anti-gag CTL responses should provide broader overall CTL reactivities than immunogens based on envelope alone. In addition to the two volunteers shown in Fig. 3, similarly broad patterns of CTL responses have been seen in two additional protocol 022 recipients tested thus far (data not shown). Recent studies have also suggested that anti-gag CTL reactivities were maintained in HIV-1-infected patients categorized as “long term nonprogressors” (10), thus implying that such responses might represent an important component of protective immunity. Future vaccine strategies, some already in phase I clinical testing, will incorporate additional HIV-1 genes, such as pol and nef, which have been shown to also encode a significant mosaic of determinants recognized by CTL in the context of MHC class I (3). It is hoped that each of these will result both in added breadth in the CTL response as well as a greater response rate among vaccinees. Whether or not it is realistic to expect broad CTL responses in all vaccine recipients can only be addressed by these ongoing studies.
In the case of a virus such as HIV-1, one must contend with an extensive amount of intra- and interclade variation as well as genetic recombination between clades (40) that further complicates the design of an efficacious vaccine, particularly with respect to the issue of the required breadth for a protective immune response. Although only a limited number of subjects have been studied thus far, the extent of CTL killing of genetically diverse targets induced by these vaccines is surprising. When considering the widely differing patterns of cytolysis by CTL from single gene envelope vector recipients in which extensive cross-clade killing is seen in one individual and not in the other, it might be suggested that the dominant factor conferring immunologic breadth is not so much the variation within the virus but rather the MHC class I haplotype of the host. It is for this reason that one cannot extrapolate the present findings to predict the relative cross-clade responses these vaccines might elicit in other areas of the world. Either of the two vaccines might fail to induce such reactivities in areas such as Thailand or Uganda, for example, because of differences in the frequencies of MHC class I alleles in the population that are capable of presenting conserved viral determinants. Such information can only be obtained by studying the specific vaccine in question in the specific geographic region in which cross-clade CTL will be tested as a prelude to eventual efficacy trials.
The present findings of cross-clade CTL reactivities among uninfected vaccine recipients open the door for in-depth studies into the characteristics of vaccine-induced CTL, which might best translate into overall vaccine efficacy. For example, recent studies by Berzofsky and colleagues (41) suggest that high avidity CTL might be especially well suited for HIV-1-specific clearance. At present, we have not yet begun to examine the relative avidity of vaccinee CTL for specific peptide-defined target determinants. Additionally, we have focused attention on conventional measurements of vaccine-induced cellular responses (i.e., the cytolytic activity of CD8+ lymphocytes). Previous work from our laboratory strongly suggests that this important subpopulation of lymphocytes can also exhibit anti-viral effector activity by noncytolytic means (42). In our studies of clones derived from the CD8+ cells of HIV-1-infected patients, we found that both CTL clones as well as clones devoid of detectable CTL activity could suppress HIV-1 in an MHC class I- independent, noncytolytic manner. Therefore, lytic reactivity might only represent one aspect of immune protection effected by CD8+ cells. Studies aimed at examining noncytolytic CD8+ cellular suppression of HIV-1 by vaccine-induced effector populations have recently been initiated in our laboratory.
Although live vector vaccine constructs might represent potent CTL immunogens, they might fall short of envelope subunit vaccines in eliciting humoral responses. Thus, the combination of live vector priming followed by envelope subunit boosting represents a marriage of two well defined, individual strategies resulting in the generation of multiple immune responses, each capable of adding to the potential overall efficacy of such vaccines (43). The challenge of broadening virus-neutralizing reactivities against primary HIV-1 isolates still remains, but the presence of cross-clade CTL responses among vaccinees, especially against nonenvelope determinants such as gag, might represent an important component of protective immunity.
Acknowledgments
We gratefully acknowledge the valuable contributions of the AVEG vaccine volunteers, clinic coordinators, and support staff. Additional recognition goes to Kelly Corr, Gene Vann, Sharon Brenner–Jones, and Jason Johnson in the CTL laboratory at Duke University as well as to Cindy Berend and Janet Ottinger in the Duke Center for AIDS Research for their technical contributions to the present studies and, finally, to Jim Demarest for his critical review of the manuscript. We dedicate this manuscript to the memory of Dr. Kathelyn S. Steimer, whose untiring devotion to the field of HIV-1 vaccine development served as an inspiration to everyone who knew her. These studies were supported by the following grants and contracts from the National Institute of Allergy and Infectious Diseases/National Institutes of Health: 2R01-AI29852, N01-AI65305, 5P30-AI28662.
Footnotes
Abbreviations: MHC, major histocompatibility complex; CTL, cytotoxic T lymphocytes; PBMC, peripheral blood mononuclear cells; AVEG, AIDS Vaccine Evaluation Group; NSI, nonsyncytium-inducing; BLCL, B lymphocyte cell line; IL, interleukin; IVS, in vitro stimulation; SR, spontaneous release; MR, maximum release; ALVAC, designation given to the canarypox-based vector; E/T, effector-to-target.
References
- 1.Mascola J R, Snyder S W, Weislow O S, Belay S M, Belshe R B, Schwartz D H, Clements M, Dolin R, Graham B S, Gorse G J, Keefer M C, McElrath M J, Walker M C, Wagner K F, McNeil J G, McCutchan F E, Burke D S. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 2.Van Cott T C, Bethke F R, Burke D S, Redfield R R, Birx D L. J Immunol. 1995;155:4100–4110. [PubMed] [Google Scholar]
- 3.McMichael A, Walker B. AIDS. 1994;8:S155–S173. [Google Scholar]
- 4.Koup R A, Safrit J F, Cao Y, Andrews C A, McLeod G, Borkowski W, Farthing C, Ho D D. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safrit J T, Andrews C A, Zhu T, Ho D D, Koup R A. J Exp Med. 1994;179:463–472. doi: 10.1084/jem.179.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B A. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ariyoshi K, Cham F, Berry N, Jaffar S, Sabally S, Corrah T, Whittle H. AIDS. 1995;9:555–559. doi: 10.1097/00002030-199506000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Pantaleo G, De Maria A, Koenig S, Butini L, Moss B, Baseler M, Lane H C, Fauci A S. Proc Natl Acad Sci USA. 1990;87:4818–4822. doi: 10.1073/pnas.87.12.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmichael A, Jin X, Sisson P, Borysiewicz L. J Exp Med. 1993;177:249–256. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P M, Eeftinck-Schattenkerk J-K M, Osterhaus A D M E, Scuitemaker H, Miedema F. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowland-Jones S, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T, Takiguchi M, Schultz T, McMichael A, Whittle H. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 12.Cheynier R, Langlade-Demoyen P, Marescot M-R, Blanche S, Blondin G, Wain-Hobson S, Griscelli C, Vilmer E, Plata F. Eur J Immunol. 1993;22:2211–2217. doi: 10.1002/eji.1830220905. [DOI] [PubMed] [Google Scholar]
- 13.Rowland-Jones S, Nixon D S, Aldhous M C, Gotch F, Aryoshi K, Hallam N, Kroll J S, Froebel K, McMichael A J. Lancet. 1993;341:860–861. doi: 10.1016/0140-6736(93)93063-7. [DOI] [PubMed] [Google Scholar]
- 14.Gorse G J, Keefer M C, Belshe R B, Matthews T J, Forrest B D, Hsieh R H, Koff W C, Hanson C V, Dolin R, Weinhold K J, Frey S E, Ketter N, Fast P. J Infect Dis. 1996;173:330–339. doi: 10.1093/infdis/173.2.330. [DOI] [PubMed] [Google Scholar]
- 15.Graham, B. S., Keefer, M. C., McElrath, M. J., Gorse, G. J., Schwartz, D. H., Weinhold, K. J., Matthews, T. J., Esterlitz, J. R., Sinangil, F. & Fast, P. E. (1996) Ann. Intern. Med. 270–279. [DOI] [PubMed]
- 16.Keefer M C, Graham B S, McElrath M J, Matthews T J, Stablein D M, Corey L, Wright P F, Lawrence D, Fast P E, Weinhold K J, Hsieh R H, Chernoff D, Dekker C, Dolin R. AIDS Res Hum Retroviruses. 1996;12:683–693. doi: 10.1089/aid.1996.12.683. [DOI] [PubMed] [Google Scholar]
- 17.Cooney E L, McElrath J M, Corey L, Hu S-L, Collier A C, Arditti D, Hoffman M, Coombs R W, Smith G E, Greenberg P D. Proc Natl Acad Sci USA. 1993;90:1882–1886. doi: 10.1073/pnas.90.5.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham B S, Matthews T J, Belshe R B, Clements M L, Dolin R, Wright P F, Gorse G J, Schwartz D H, Keefer M C, Bolognesi D P, Corey L, Stablein D M, Esterliz J R, Hu S-L, Smith G E, Fast P E, Koff W C. J Infect Dis. 1993;167:533–537. doi: 10.1093/infdis/167.3.533. [DOI] [PubMed] [Google Scholar]
- 19.McElrath M J, Corey L, Berger D, Hoffman M C, Klucking S, Dragavon J, Peterson R, Greenberg P D. J Infect Dis. 1994;169:41–47. doi: 10.1093/infdis/169.1.41. [DOI] [PubMed] [Google Scholar]
- 20.Pialoux G, Excler J-L, Riviere Y, Gonzalez-Canali G, Feuillie V, Coulad P, Gluckman J-C, Matthews T J, Meigneir B, Kieny M P, Gonnet P, Diaz I, Meric C, Paoletti E, Tartaglia J, Salomon H, Plotkin S. AIDS Res Hum Retroviruses. 1995;11:373–381. doi: 10.1089/aid.1995.11.373. [DOI] [PubMed] [Google Scholar]
- 21.Egan M A, Pavlat W A, Tartaglia J, Paoletti E, Weinhold K J, Clements M L, Siliciano R F. J Infect Dis. 1995;171:1623–1627. doi: 10.1093/infdis/171.6.1623. [DOI] [PubMed] [Google Scholar]
- 22.Fleury B, Janvier G, Pialoux G, Buseyne F, Robertson M N, Tartaglia J, Paoletti E, Kieny M P, Excler J L, Riviere Y. J Infect Dis. 1996;174:734–738. doi: 10.1093/infdis/174.4.734. [DOI] [PubMed] [Google Scholar]
- 23.WHO Network for HIV Isolation and Characterization. AIDS Res Hum Retroviruses. 1994;10:1327–1343. doi: 10.1089/aid.1994.10.1327. [DOI] [PubMed] [Google Scholar]
- 24.Ferrari, G., King, K., Rathbun, K., Place, C. A., Packard, M. V., Bartlett, J. A., Bolognesi, D. P. & Weinhold, K. J. (1995) Clin. Exp. Immunol. 101. [DOI] [PMC free article] [PubMed]
- 25.McMichael A J, Gotch F M, Noble G R, Beare P A. N Engl J Med. 1983;309:13–17. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- 26.Issekutz T B. Clin Exp Immunol. 1984;56:515–523. [PMC free article] [PubMed] [Google Scholar]
- 27.Graham S, Green C P, Mason P D, Borysiewicz L K. J Gen Virol. 1991;72:1183–1186. doi: 10.1099/0022-1317-72-5-1183. [DOI] [PubMed] [Google Scholar]
- 28.Walker B D, Chakrabarti S, Moss B, Paradis T J, Flynn T, Durno A G, Blumberg R S, Kaplan J C, Hirsch M S, Schooley R T. Nature (London) 1987;328:345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- 29.Wolinsky S M, Wike C M, Korber B T M, Hutto C, Parks W P, Rosenblum L L, Kunstman K J, Furtado M R, Munoz J L. Science. 1992;255:1134–1137. doi: 10.1126/science.1546316. [DOI] [PubMed] [Google Scholar]
- 30.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 31.Chakrabarti S, Brechling K, Moss B. Mol Cell Biol. 1985;5:3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Earl P L, Hueghin A W, Moss B. J Virol. 1990;64:2448–2451. doi: 10.1128/jvi.64.5.2448-2451.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennink J R, Yewdell J W. Curr Top Microbiol Immunol. 1990;163:153–184. doi: 10.1007/978-3-642-75605-4_6. [DOI] [PubMed] [Google Scholar]
- 34.Bedinger P, Moriarty A, von Borstel R C, II, Donovan N J, Steimer K S, Littman D R. Nature (London) 1988;334:162–165. doi: 10.1038/334162a0. [DOI] [PubMed] [Google Scholar]
- 35.Garcia J V, Miller A D. Nature (London) 1991;350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 36.Achour A, Lemhammedi S, Picard O, M’Bika J P, Zagury J F, Moukrim Z, Willer A, Beix F, Burny A, Zagury D. AIDS Res Hum Retroviruses. 1994;10:19–25. doi: 10.1089/aid.1994.10.19. [DOI] [PubMed] [Google Scholar]
- 37.Korber B T M, Walker B D, Moore J P, Brander C, Koup R, Haynes B F, Myers G. HIV Molecular Immunology Database 1995. Los Alamos, NM: Theoretical Biology and Biophysics; 1995. [Google Scholar]
- 38.Nixon D, Townsend A, Elvin J, Rizza C, Gallwey J, McMichael A. Nature (London) 1988;336:484–487. doi: 10.1038/336484a0. [DOI] [PubMed] [Google Scholar]
- 39.Nixon D F, McMichael A J. AIDS. 1991;5:1049–1059. [PubMed] [Google Scholar]
- 40.Robertson D L, Sharp P M, McCutchan F E, Hahn B H. Nature (London) 1995;374:124–127. doi: 10.1038/374124b0. [DOI] [PubMed] [Google Scholar]
- 41.Alexander-Miller M A, Leggatt G R, Berzofsky J A. Proc Natl Acad Sci USA. 1996;93:4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toso, J. F., Chen, C. H., Mohr, J. R., Piglia, L., Oei, C., Ferrari, G., Greenberg, M. L. & Weinhold, K. J. (1995) J. Infect. Dis. 172. [DOI] [PubMed]
- 43.Weinhold K J, Rathbun K, Humphrey W, Corr K, Berend C, Ferrari G. In: Dixieme Colloque Des Cent Gardes. Girard M, Dodet B, editors. Paris: Elsevier; 1996. pp. 261–267. [Google Scholar]