Abstract
Matrix metalloproteinases (MMPs) classically have been implicated in basement membrane destruction associated with late-stage tumor cell invasion and metastasis. However, recent studies have demonstrated that one MMP family member, matrilysin, is expressed in a high percentage of early-stage human colorectal tumors. We analyzed matrilysin expression in benign intestinal tumors from mice heterozygous for the ApcMin allele (Min/+) and found that the mRNA was induced in the majority (88%) of these adenomas. Protein was detected in the tumor cells, where, surprisingly, it was predominantly immunolocalized to the lumenal surface of dysplastic glands rather than the basement membrane or extracellular matrix. To address the role of matrilysin in Min intestinal tumorigenesis, we generated Min/+ mice deficient in this MMP by gene targeting and homologous recombination. The absence of matrilysin resulted in a reduction in mean tumor multiplicity in Min/+ animals of approximately 60% and a significant decrease in the average tumor diameter. Based on these findings, we conclude that matrilysin is a suppressor of the Min phenotype, possibly by functioning in a capacity independent of matrix degradation. These results argue for the use of MMP inhibitors in the treatment and prevention of early-stage colon cancer.
Keywords: knockout, Min, colon cancer, extracellular matrix, APC
Inhibitor studies have demonstrated that destruction of basement membrane components and connective tissue by matrix metalloproteinases (MMPs) is critical for tumor cell invasion and dissemination (reviewed in ref. 1). The MMPs compose a family of structurally similar metal-dependent enzymes that includes the collagenases, gelatinases A and B, the stromelysins, matrilysin, metalloelastase, and the membrane-type metalloproteinases. In many of the neoplastic lesions that have been examined in humans, the expression of most MMPs is restricted to the stromal component (reviewed in ref. 2). However, matrilysin (MMP-7, pump-1; EC 3.4.24.23) localizes primarily to the tumor cells in lesions of epithelial origin (reviewed in ref. 3) and can be detected readily in normal epithelium of specific glandular organs in both humans (4) and mice (5). Matrilysin mRNA has been detected in human adenomas, as well as carcinomas and adenocarcinomas, of the breast and colon (6–11). Furthermore, ectopic expression of matrilysin cDNA in a colorectal carcinoma cell line was found to increase its tumorigenicity in nude mice but did not affect metastasis (12). Taken together, these results suggest that matrilysin may contribute to early tumor development, particularly in the gastrointestinal tract.
The Min (multiple intestinal neoplasia) mouse has proven to be a powerful model system to study molecules involved in the progression of intestinal adenomas. It has been determined that a nonsense autosomal dominant germline mutation in the adenomatous polyposis coli (Apc) gene (the ApcMin allele) induces spontaneous intestinal tumors in these mice, providing a model that closely mimics the human hereditary colon cancer syndrome, familial adenomatous polyposis (13, 14). Congenic C57BL/6-Min mice heterozygous for this mutant allele (Min/+) develop numerous benign tumors throughout the intestinal tract, although with less frequency in the large bowel, and have an average life span of 119 days (13, 15). We have used this model system to examine MMP expression in benign tumors and to address the potential role of matrilysin in intestinal tumorigenesis. Matrilysin-deficient mice were generated by targeted mutagenesis and mated to Min mice to assess tumor development in the absence of functional matrilysin protein.
MATERIALS AND METHODS
In Situ Hybridization and Immunohistochemical Analysis.
Intestines from congenic C57BL/6-Min (Min/+) mice (The Jackson Laboratory) were fixed in 4% paraformaldehyde, and tumors were excised and embedded in paraffin. In situ hybridization was performed on 5-μm sections as described (8). 35S-labeled riboprobes (antisense and sense) were generated using selected fragments of mouse MMP cDNAs as described (5, 16). Sections of mouse postpartum uterus were used as positive controls for all experiments, and the specificity of the probes was previously verified (5, 16). Slides were exposed at 4°C for 2 weeks and counterstained with hematoxylin, and were considered positive if hybridization of the antisense probe exceeded that of the sense control.
For generation of a polyclonal antibody against mouse matrilysin, a 325-bp fragment from the 3′ end of the cDNA (in pGEM7-MMATAH; ref. 5) was amplified using primers 5′-GCTCTAGACTCTTCTGTTCCCGGTACT-3′ (forward; XbaI site underlined) and 5′-TGTTGATGTCTCGCAACTTC-3′ (reverse). This segment of the cDNA (base pairs 722–842) encodes the carboxyl-terminal 40 aa of the protein and excludes the conserved zinc-binding domain. The resulting product was cleaved with XbaI and PvuII (63 bp after stop codon at position 842) and subcloned into pGEM7Zf(+) cut with XbaI and SmaI. The 175-bp XbaI–HindIII fragment was then ligated in frame into the polylinker of the glutathione S-transferase fusion protein vector pGEX-KG (17). Fusion protein was produced following induction of bacterial transformants with isopropyl 1-thio-β-d-galactoside and was purified from cell lysates by glutathione-agarose (Sigma) chromatography. Antiserum was generated by injection of 0.1 mg of fusion protein in Freund’s adjuvant into two New Zealand White rabbits (Research Genetics, Huntsville, AL). Fusion protein was coupled to Affigel 10 (Bio-Rad) for affinity purification of the antiserum using standard techniques (18). Immunohistochemistry was performed as described (16) with the following modifications. Tissues were fixed in Bouin’s fixative and embedded in paraffin. Five-micrometer sections were incubated in 40 μg/ml proteinase K for 30 min at room temperature. The affinity-purified anti-mouse matrilysin antibody (3.0 μg/ml) was incubated overnight at 4°C, and control slides were incubated with the same concentration of rabbit IgG (Sigma). Biotinylated anti-rabbit IgG secondary antibody (Vector Laboratories) was used at a dilution of 1:5000, and matrilysin was visualized using the Vectastain ABC kit (Vector Laboratories) and TrueBlue substrate (Kirkegaard & Perry Laboratories). Sections were counterstained with Contrast Red.
Mapping and Targeted Disruption of the Matrilysin Gene.
The matrilysin locus (Mmp7; Mouse Genome Database accession no. MGD-CREX-626) was mapped using the BSS backcross panel [(C57BL/6JEi × SPRET/Ei)F1 × SPRET/Ei; The Jackson Laboratory Backcross DNA Panel Map Service; ref. 19]. DNA from 94 N2 progeny was digested with TaqI and subjected to Southern blotting using a radiolabeled 750-bp EcoRI–SphI fragment containing exon 1 (probe A) to detect recombinants by a restriction fragment length polymorphism.
A 6.5-kb BamHI genomic fragment previously cloned from a 129/Sv library (pGEM7-MuPB1; ref. 5) was used to generate the targeting construct. A 550-bp EcoRV–StuI fragment spanning exons 3 and 4 was replaced with a 1.6-kb phosphoglycerate kinase–neomycin cassette (20). Insertion of the phosphoglycerate kinase–neomycin cassette resulted in the removal of 95 nt from the coding sequence and left approximately 1 kb and 4.5 kb of 5′ and 3′ flanking sequence, respectively. The herpes simplex virus-thymidine kinase cassette, derived from pMC1-tk (21), was inserted 3′ of exon 6 in the reverse transcriptional orientation. The construct (100 μg) was linearized with NotI before electroporation of R1 embryonic stem (ES) cells (generously provided by J. Rossant, University of Toronto, Toronto). Colonies resistant to both G418 and gancyclovir (Syntex, Palo Alto, CA) were screened by restriction enzyme digestion and Southern blotting. Six targeted clones were obtained (targeting frequency of 0.9%), three of which were injected into C57BL/6 blastocysts after karotype analysis. Culture and manipulation of ES cells were performed as described (22).
Genotyping of ES Cell Clones and Mice.
ES cell and tail DNAs were extracted using conventional techniques. Analysis of the ApcMin mutation was performed as described using PCR amplification and Southern analysis (14). A 618-bp fragment of Apc was amplified using primers 5′-GCCATCCCTTCACGTTAG-3′ (forward) and 5′-TTCCACTTTGGCAGCATAAGGC-3′ (reverse) and probed with a 32P-labeled oligonucleotide (5′-ACAGAAGTTAGGAGAGAGA-3′) containing the Min point mutation (T → A; nucleotide 2549). Blots were hybridized at 45°C and washed at 53°C as described (14). Characterization of the Mmp7 genotype was performed by Southern blotting using either BstEII and probe A (described above) or BamHI and probe B (a 0.5-kb HindIII–EcoRI genomic fragment encompassing the exon 2 sequence).
Northern Blot Analysis.
Total cellular RNA was extracted from the entire small intestine using the guanidinium/acid phenol method (23). Fifteen micrograms of RNA was separated by denaturing gel electrophoresis in Mops buffer and was transferred to nitrocellulose. After Northern blotting, the samples were analyzed for matrilysin expression using a 3′ matrilysin cDNA probe which has been described previously (MMAT2; ref. 5).
Analysis of Tumor Formation.
mmp7+/− F1 mice were backcrossed to C57BL/6 mice to the N4 generation. These N4 mice were mated to Min/+ mice to generate Min/+; mmp7+/− N5 (>96% C57BL/6) progeny. Subsequent Min/+; mmp7−/− N5F1 mice produced from N5 intermatings were compared with Min/+ animals for tumor number and diameter. Animals were housed under identical conditions and were killed at 17 weeks of age by CO2 asphyxiation. Intestinal tissue was fixed overnight at 4°C in 4% paraformaldehyde and then transferred to 70% ethanol before analysis. In all cases, tumors from the small and large intestines were scored blindly under a dissecting microscope (×10 magnification) with the diameter and location documented. At the magnification used, all tumors observed were ≥1.0 mm in diameter. To avoid making any assumptions about tumor distribution, the nonparametric Wilcoxon Rank Sum test was used to analyze the statistical significance of all data.
RESULTS
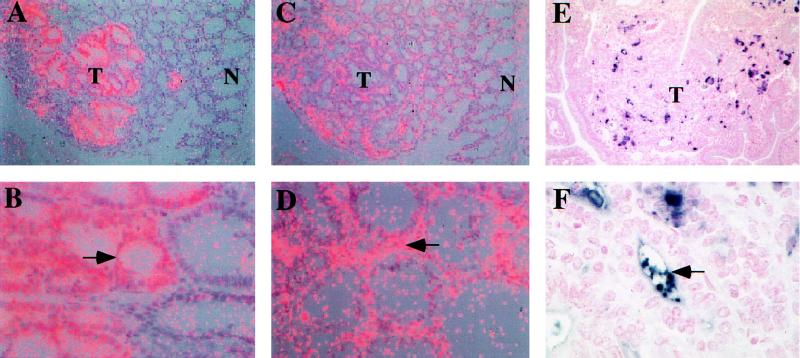
In human colonic neoplasms, matrilysin mRNA has been found in approximately 60% of adenomatous lesions; a low percentage of these tumors also express gelatinase B and interstitial collagenase (9). We examined both colonic and small intestinal adenomas from Min/+ mice for MMP expression and localization. Matrilysin mRNA was detected in a high percentage (88%) of Min adenomas and was localized by in situ hybridization to epithelial-derived tumor cells (Fig. 1 A and B). In contrast, the mRNAs of other MMPs were present in fewer tumors (60–65% for gelatinase A and stromelysin-2, and approximately 50% for stromelysin-1 and collagenase) and were localized exclusively to tumor-associated stroma (Fig. 1 C and D, for example). No correlation was observed between the pattern of MMP expression and the size or histological appearance of tumors. Immunohistochemical analysis using a polyclonal antibody against mouse matrilysin showed a heterogenous pattern of staining in the dysplastic epithelium of Min intestinal adenomas (Fig. 1 E and F). Surprisingly, apical and lumenal localization of matrilysin protein in these tumors was clearly evident (Fig. 1F). Although matrilysin expression was induced in Min tumor cells arising in both the small and the large bowel, neither matrilysin mRNA nor protein was detectable in the normal colonic mucosa. In the small intestine, matrilysin is normally localized to the specialized Paneth cells at the base of the crypts but is absent from other cell lineages (5).
Figure 1.
Localization of matrilysin and gelatinase A in Min/+ tumors. In situ hybridization of matrilysin (A and B) and gelatinase A (C and D). Photomicrographs were taken using a double exposure with a red filter on dark field illumination so that the silver grains appear pink. (A) In a Min colonic adenoma, matrilysin mRNA is expressed by tumor (T) epithelium but not normal (N) colonic mucosa. (×125.) (B) Matrilysin mRNA localized to the dysplastic epithelium of a small intestinal tumor (arrow) and is absent in normal intestinal glandular epithelium. (×400.) (C) Gelatinase A mRNA is expressed in the tumor (T) compared with the adjacent normal (N) mucosa in a serial section of the colonic tumor shown in A. (×125.) (D) Gelatinase A mRNA localizes within the stroma (arrow) of a colonic tumor. (×250.) Immunolocalization of matrilysin protein (E and F). Matrilysin protein was visualized with TrueBlue color substrate (dark blue to purple), and nuclei were counterstained with Contrast Red. (E) Sporadic localization of matrilysin protein in a small intestinal tumor (T). (×160.) (F) Matrilysin immunoreactivity is frequently observed within the lumen of glandular structures (arrow). (×640.)
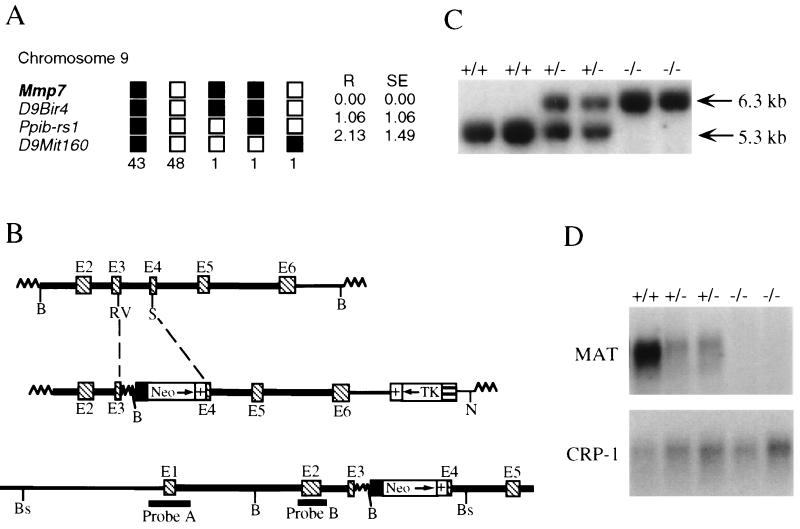
To determine if the induction of matrilysin in benign Min lesions plays a causal role in the development of these tumors, matrilysin-deficient animals were generated by gene targeting and homologous recombination in ES cells. The gene was cloned from a strain 129/Sv genomic library (5) and was mapped to the proximal end of chromosome 9 using The Jackson Laboratory interspecific backcross panel [(C57BL/6JEi × SPRET/Ei)F1 × SPRET/Ei; Fig. 2A; ref. 19]. Both matrilysin (Mmp7) and the MMP metalloelastase (Mmel; ref. 24) have now been mapped to this same region in different crosses, and interstitial collagenase (Mmp1) has also been localized to chromosome 9 (25). The strategy used for targeting the matrilysin gene is outlined in Fig. 2B. Male chimeras resulting from blastocyst injection of three independently targeted ES cell clones were mated with C57BL/6 females, and germline transmission of the recombined allele (Mmp7m1Vu) occurred in two lines. F1 heterozygous progeny were mated to produce homozygous animals, and Southern blot analysis of the F2 progeny (Fig. 2C) showed that all genotypes were produced in the expected Mendelian ratio. Furthermore, pups homozygous for Mmp7m1Vu (mmp7−/−) were born without any obvious defects and were suckled and weaned normally. To demonstrate that disruption of the gene resulted in a null mutation, total RNA was isolated from the small intestine, where matrilysin expression is normally restricted to the Paneth cells (5), and analyzed by Northern blotting (Fig. 2D). Matrilysin transcript was undetectable in tissue from mmp7−/− animals, whereas mmp7+/− mice in general showed decreased levels of the mRNA in comparison to mmp7+/+ mice (Fig. 2D), although some variability was noted (data not shown). Adult mmp7−/− mice are overtly normal and appear to have average life spans when compared with wild-type animals.
Figure 2.
Mapping and targeted disruption of the matrilysin gene. (A) The BSS backcross panel [(C57BL/6JEi × SPRET/Ei)F1 × SPRET/Ei] from The Jackson Laboratory Backcross DNA Panel Map Service (19) was used to map the matrilysin locus (Mmp7). The segregation patterns of Mmp7 and several linked loci (D9Bir4, Ppib-rs1, and D9Mit160) among the backcross offspring are depicted by the columns of boxes, where each column represents the chromosome inherited from the (C57BL/6JEi × SPRET/Ei)F1 parent. C57BL/6JEi and SPRET/Ei alleles are indicated by the filled and open boxes, respectively. The number at the bottom of each column is the number of offspring with that haplotype. Recombination distances in cM (R) and standard errors (SE) are indicated to the right of the columns. The Chromosome Committee map places the metalloelastase locus (Mmel) at the same offset as D9Bir4. D9Bir3 may be the only mapped locus more proximal than Mmp7. (B) Structure of genomic segment, targeting vector, and disrupted allele. The top diagram depicts the 6.5-kb BamHI (B) genomic fragment containing exons 2–6 (E2-E6; hatched boxes) that was used to generate the targeting construct. Introns, extragenic sequence, and plasmid sequence are denoted by the heavy, thin, and wavy lines, respectively. As shown in the middle diagram, the phosphoglycerate kinase–neomycin cassette containing the phosphoglycerate kinase promoter (filled box), neomycin phosphotransferase cDNA (Neo), and a polyadenylylation signal (+) was inserted using the EcoRV (RV) and StuI sites (S) indicated. The herpes simplex virus-thymidine kinase (TK) cassette, composed of the TK promoter (barred box) and a polyadenylylation signal (+), was used for negative selection in the presence of gancyclovir. The construct was linearized at the unique NotI (N) site. The bottom diagram shows the expected structure of the targeted allele following electroporation into R1 ES cells and selection for doubly resistant colonies. The locations of the BamHI and BstEII (Bs) restriction sites and fragments (probes A and B) used for Southern blotting are also indicated. (C) Southern analysis of F2 progeny from heterozygote matings. Tail DNA was digested with BstEII and Southern blotted using probe A, which detects bands of 6.3 and 5.3 kb corresponding to the targeted (−) and wild-type (+) alleles, respectively. (D) Northern blot analysis of small intestinal RNA. Total RNA was extracted from the entire small intestine of wild-type (+/+), heterozygous (+/−), and homozygous (−/−) F2 animals and was subjected to Northern blotting using a 3′ matrilysin cDNA probe as described (MMAT2; ref. 5). As a loading control, the blot was also probed with radiolabeled cryptdin-1 (CRP-1) cDNA, which detects an abundant message of approximately 450–480 bp in the small intestine (26).
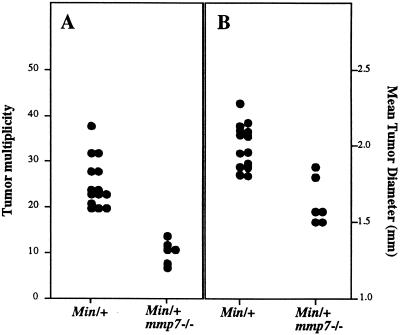
It is known that different genetic backgrounds alter the severity of the Min phenotype (15). Although one modifier of Min locus, Mom1, has been described in other strains (15), the 129/Sv strain has been reported to demonstrate a “Min-susceptible” phenotype similar to that of C57BL/6 (27). Nevertheless, to reduce the contributions of potential suppressive modifiers from the 129/Sv strain, mmp7+/− F1 progeny were backcrossed with C57BL/6 mice to the N4 generation before mating to Min/+ animals. mmp7−/− N5F1 mice heterozygous for the Min mutation were analyzed for the presence of intestinal tumors at approximately 17 weeks of age (Fig. 3). Control mice (Min/+) developed an average of 25.4 tumors per animal. Min animals that lacked matrilysin (Min/+; mmp7−/−) developed an average of 10.5 tumors per animal, which represents a decrease of 58% (P < 0.0006 by a nonparametric statistical test; Fig. 3A). In addition, the average tumor diameter per animal was reduced by 20% (from 2.0 to 1.6 mm; P < 0.003; Fig. 3B). This modest decrease in tumor diameter could in fact represent a substantial reduction in tumor volume if the decrease is assumed to occur in all dimensions. The observed reduction in tumor size did not appear to be due to a lack of angiogenesis because no obvious difference in the degree of vascularization was observed between large and small Min/+; mmp7−/− tumors as determined by immunohistochemistry for von Willebrand factor (data not shown). Min/+; mmp7+/− animals (also N5F1 generation) developed approximately the same average number of tumors as Min/+ (22.4; n = 7), and this mean was also significantly different from Min/+; mmp7−/− (P < 0.02) animals.
Figure 3.
Effect of the matrilysin null mutation on tumor multiplicity and diameter in Min/+ animals. Min/+; mmp7−/− N5F1 (>96% C57BL/6) mice were generated as described in Materials and Methods. These mice were analyzed in comparison to Min/+ animals for tumor number (A) and diameter (B). The mean and median numbers of tumors for Min/+ were 25.4 and 24 (range = 20–38, n = 14), respectively, and for Min/+; mmp7−/−, the numbers were 10.5 and 11 (range = 7 to 12, n = 6), respectively (P < 0.0006). The mean diameters of tumors per mouse for Min/+ and Min/+; mmp7−/− were 2.0 and 1.6 mm, respectively (P < 0.003). The nonparametric Wilcoxon Rank Sum test was used to analyze the statistical significance of all data.
Using a genetic approach, we demonstrate that matrilysin is required for the development of the majority of Min intestinal neoplasias. However, our findings also suggest that Min tumorigenesis involves both matrilysin-dependent and independent mechanisms because a subset of tumors (42% of the Min/+ control) is able to develop in the absence of matrilysin. To assess whether the stromal MMPs that are expressed in Min/+ tumors may be involved in development of lesions in Min/+; mmp7−/− mice, we examined these tumors by in situ hybridization for expression of MMP mRNAs. While only 62% of tumors from Min/+ mice expressed gelatinase A in the surrounding stroma, the mRNA for this stromal MMP was present in all the tumors analyzed from Min/+; mmp7−/− animals (Table 1). Such an increase (38%) was not observed for other stromal MMPs, including stromelysin-1, stromelysin-2, and collagenase (Table 1). Although the functional significance of this observation is not clear, it is possible that the ability of initiated cells to induce gelatinase A in surrounding stroma provides a growth advantage over cells not capable of inducing this MMP. Additional experiments using genetic or pharmacological means of altering MMP activity are required to address the contribution of other MMPs to intestinal tumor formation.
Table 1.
Expression of MMPs in Min intestinal adenomas as determined by in situ hybridization analysis
| MMP | No. of positive tumors per total no. of tumors (%)
|
|
|---|---|---|
| Min/+ | Min/+; mmp7−/− | |
| Matrilysin | 22/25 (88) | —/— (—) |
| Gelatinase A | 13/21 (62) | 16/16 (100) |
| Stromelysin-1 | 11/21 (52) | 9/16 (56) |
| Stromelysin-2 | 17/26 (65) | 12/15 (80) |
| Collagenase | 14/26 (53) | 7/15 (46) |
| Stromelysin-3 | 0/21 (—) | 0/15 (—) |
DISCUSSION
Studies using MMP inhibitors have demonstrated a role for extracellular matrix degradation in late-stage tumor progression and the acquisition of an invasive and metastatic phenotype (1). The results described here extend the involvement of one MMP, matrilysin, to the development of benign lesions in the Min model of intestinal neoplasia. Similar to observations in human adenomas (8–11), matrilysin mRNA was expressed in the majority (88%) of benign Min tumors. Using the matrilysin-deficient mouse, we have demonstrated that functional matrilysin protein is required for the formation of a significant portion (58%) of Min intestinal adenomas. Our results do not rule out a role for matrilysin in invasion and metastasis but significantly expand the contributions of MMPs in tumor progression to effects on premalignant cells.
Based on our genetic experiments, we propose that the matrilysin locus (Mmp7) constitutes a modifier locus of the ApcMin allele. Other known modifiers of the Min phenotype include the Mom1 locus, for which the phospholipase A2 gene (Pla2 g2a) has been proposed as a candidate (28), and the DNA cytosine methylase locus (Dnmt; ref. 27), although in both cases, the specific mechanism by which these gene products affect tumorigenesis has not yet been defined. In contrast, molecular alterations that have been strongly implicated in colorectal tumor progression (29), such as activating mutations in K-ras and inactivation of p53, have had minimal effects on Min-induced tumor formation (30–32). Recent studies have shown that exogenous agents such as nonsteroidal anti-inflammatory drugs (33) and the Bowman–Birk serine protease inhibitor (34) can reduce tumor multiplicity in Min mice, but the specific targets of these compounds have not been identified. The addition of matrilysin to the list of known modifiers of the Min phenotype provides further opportunities to investigate the molecular mechanisms underlying early stages of intestinal neoplasia using a well characterized gene product.
The mechanism by which matrilysin is able to promote tumor development in this system is not yet defined. Because tumor formation was analyzed at only one time point in our studies, we cannot clearly distinguish between matrilysin-mediated effects on tumor initiation and tumor growth. However, the reported reduction in tumor diameter and preliminary data showing that Min/+; mmp7−/− mice examined as late as 6 months of age can develop more than 50 tumors (data not shown) supports the idea that matrilysin contributes to tumor growth rate. A potential mechanism underlying this effect may be found in matrilysin’s accepted role as a matrix-degrading enzyme. Matrilysin is capable of degrading multiple components of the basement membrane, including type IV collagen, laminin, and entactin (3). The basement membrane conveys signals important to the maintenance of the epithelial phenotype and modulates cellular proliferation, differentiation, and apoptosis (16, 35–37). However, our immunolocalization results indicate that matrilysin is predominantly secreted apically, suggesting that its substrates may not be restricted to the extracellular matrix. Based on evidence linking MMPs to processing of tumor necrosis factor α, heparin-binding epidermal growth factor, and Fas ligand (38–40), we speculate that matrilysin may activate lumenal or membrane-bound cytokines or growth factors to perturb locally the growth of responsive cells. Alternatively, disorganization or loss of polarity of tumor epithelium may alter matrilysin secretion patterns and accessibility to potential substrates, thus modifying matrilysin’s ability to degrade the matrix and/or release associated growth factors. Further studies will be necessary to identify the specific matrilysin substrates that are critical to early- and late-stage intestinal tumor progression.
The potential relevance of our results to human familial and spontaneous colon cancer is provocative. Matrilysin expression has been demonstrated in over 90% of adenomas from familial adenomatous polyposis patients (T. Ishikawa, N. Takeuchi, and Y. Ichikawa, personal communication). In addition, greater than 60% of spontaneous human colonic adenocarcinomas exhibit APC mutations (41) and approximately 80% of spontaneous tumors analyzed express matrilysin (9). Taken together, these findings suggest that inhibition of matrilysin activity in human colorectal cancers may provide a protective effect. Preliminary experiments demonstrate that the broad-spectrum synthetic MMP inhibitor, batimastat (BB-94), produces a decrease in tumor number similar to that resulting from matrilysin ablation (unpublished results). This compound also reduces the growth, invasion, and metastasis of established colon cancer (42, 43). The findings reported here significantly alter current concepts of MMP contributions to tumor progression by extending them to very early stages in tumor development, and expand the application of MMP inhibitors to chemopreventative as well as therapeutic agents for colorectal cancer.
Acknowledgments
We thank L. Hargett for blastocyst injection; M. Barter and L. Maltais (The Jackson Laboratory) for helpful advice and assistance with the mapping data; Y. Shyr for biostatistical assistance; C. V. E. Wright for advice in generating the matrilysin fusion protein; J. Rossant (University of Toronto and Mount Sinai Hospital, Toronto) for the ES cells; British Biotech Pharmaceuticals Ltd. (Oxford) for the MMP inhibitor batimastat; and P. D. Brown (British Biotech Pharmaceuticals Ltd.), W. Dove (University of Wisconsin, Madison, WI), C. V. E. Wright, and Matrisian laboratory members for critical review of the manuscript. P.A.L. is an Associate and B.L.M.H. is an Investigator of the Howard Hughes Medical Institute. This work was supported by National Institutes of Health Grant CA 60867 (to L.M.M.), by Syntex Research, Palo Alto, CA, and by Vanderbilt Cancer Center Support Grant P30 CA68485. C.W. was supported by a postdoctoral fellowship from the American Cancer Society, and K.H. was supported by a predoctoral grant from the National Institutes of Health (T32-CA09592).
Footnotes
Abbreviations: MMP, matrix metalloproteinase; ES cell, embryonic stem cell.
References
- 1.MacDougall J R, Matrisian L M. Cancer Metastasis Rev. 1995;14:351–362. doi: 10.1007/BF00690603. [DOI] [PubMed] [Google Scholar]
- 2.Powell W C, Matrisian L M. In: Attempts to Understand Metastasis Formation: I. Metastasis-Related Molecules. Gunthert W, Birchmeier W, editors. Heidelberg: Springer; 1996. pp. 1–22. [Google Scholar]
- 3.Wilson C L, Matrisian L M. Int J Biochem Cell Biol. 1996;28:123–136. doi: 10.1016/1357-2725(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 4.Saarialho-Kere U K, Crouch E C, Parks W C. J Invest Dermatol. 1995;105:190–196. doi: 10.1111/1523-1747.ep12317104. [DOI] [PubMed] [Google Scholar]
- 5.Wilson C L, Heppner K J, Rudolph L A, Matrisian L M. Mol Biol Cell. 1995;6:851–869. doi: 10.1091/mbc.6.7.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heppner K J, Matrisian L M, Jensen R A, Rodgers W H. Am J Pathol. 1996;149:273–282. [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf C, Rouyer N, Lutz Y, Adida C, Loriot M, Bellocq J-P, Chambon P, Basset P. Proc Natl Acad Sci USA. 1993;90:1843–1847. doi: 10.1073/pnas.90.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonnell S, Navre M, Coffey R J, Matrisian L M. Mol Carcinog. 1991;4:527–533. doi: 10.1002/mc.2940040617. [DOI] [PubMed] [Google Scholar]
- 9.Newell K J, Witty J P, Rodgers W H, Matrisian L M. Mol Carcinog. 1994;10:199–206. doi: 10.1002/mc.2940100404. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimoto M, Itoh F, Yamamoto H, Hinoda Y, Imai K, Yachi K. Int J Cancer. 1993;54:614–618. doi: 10.1002/ijc.2910540415. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto H, Itoh F, Hinoda Y, Senota A, Yoshimoto M, Nakamura H, Imai K, Yachi A. Biochem Biophys Res Commun. 1994;201:657–664. doi: 10.1006/bbrc.1994.1751. [DOI] [PubMed] [Google Scholar]
- 12.Witty J P, McDonnell S, Newell K, Cannon P, Navre M, Tressler R, Matrisian L M. Cancer Res. 1994;54:4805–4812. [PubMed] [Google Scholar]
- 13.Moser A R, Pitot H C, Dove W F. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 14.Su L, Kinzler K W, Vogelstein B, Preisinger A C, Moser A R, Luongo C, Gould K A, Dove W F. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 15.Dietrich W F, Lander E S, Smith J S, Moser A R, Gould K A, Luongo C, Borenstein N, Dove W. Cell. 1993;75:631–639. doi: 10.1016/0092-8674(93)90484-8. [DOI] [PubMed] [Google Scholar]
- 16.Witty J P, Wright J, Matrisian L M. Mol Biol Cell. 1995;6:1287–1303. doi: 10.1091/mbc.6.10.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan K-L, Dixon J E. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- 18.Harlow E, Lane D. Antibodies: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. pp. 283–318. [Google Scholar]
- 19.Rowe L B, Nadeau J G, Turner R, Frankel W N, Letts V A, Eppig J T, Ko M S H, Thurston S J, Birkenmeier E H. Mamm Genome. 1994;5:253–274. doi: 10.1007/BF00389540. [DOI] [PubMed] [Google Scholar]
- 20.Rudnicki M A, Braun T, Hinuma S, Jaenisch R. Cell. 1992;71:383–390. doi: 10.1016/0092-8674(92)90508-a. [DOI] [PubMed] [Google Scholar]
- 21.Mansour S L, Thomas K R, Capecchi M R. Nature (London) 1988;336:348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- 22.Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. Vol. 2. Plainview, NY: Cold Spring Harbor Lab. Press; 1995. pp. 217–253. [Google Scholar]
- 23.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro S D, Griffin G L, Gilbert D J, Jenkins N A, Copeland N G, Welgus H G, Senior R M, Ley T J. J Biol Chem. 1992;267:4664–4671. [PubMed] [Google Scholar]
- 25.Schorpp M, Mattei M, Herr I, Gack S, Schaper J, Angel P. Biochem J. 1995;308:211–217. doi: 10.1042/bj3080211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouellette A J, Greco R M, James M, Frederick D, Naftilan J, Fallon J T. J Cell Biol. 1989;108:1687–1695. doi: 10.1083/jcb.108.5.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laird P W, Jackson-Grusby L, Fazeli A, Dickinson S L, Jung W E, Li E, Weinberg R A, Jaenisch R. Cell. 1995;81:197–205. doi: 10.1016/0092-8674(95)90329-1. [DOI] [PubMed] [Google Scholar]
- 28.MacPhee M, Chepenik K P, Liddell R A, Nelson K K, Siracusa L D, Buchberg A M. Cell. 1995;81:957–966. doi: 10.1016/0092-8674(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 29.Fearon E R, Vogelstein B. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 30.Kim S H, Roth K A, Moser A R, Gordon J I. J Cell Biol. 1993;123:877–893. doi: 10.1083/jcb.123.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dove W F, Luongo C, Connelly C S, Gould K A, Shoemaker A R, Moser A R, Gardner R L. Cold Spring Harbor Symp Quant Biol. 1994;59:501–508. doi: 10.1101/sqb.1994.059.01.055. [DOI] [PubMed] [Google Scholar]
- 32.Clarke A R, Cummings M C, Harrison D J. Oncogene. 1995;11:1913–1920. [PubMed] [Google Scholar]
- 33.Jacoby R F, Marshall D J, Newton M A, Novakovic K, Tutsch K, Cole C E, Lubet R A, Kelloff G J, Verma A, Moser A R, Dove W F. Cancer Res. 1996;56:710–714. [PubMed] [Google Scholar]
- 34.Kennedy A R, Beazer-Barclay Y, Kinzler K W, Newberne P M. Cancer Res. 1996;56:679–682. [PubMed] [Google Scholar]
- 35.Boudreau N, Sympson C J, Werb Z, Bissell M J. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sympson C J, Talhouk R S, Alexander C M, Chin J R, Clift S M, Bissell M J, Werb Z. J Cell Biol. 1994;125:681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Witty J P, Lempka T, Coffey R J, Jr, Matrisian L M. Cancer Res. 1995;55:1401–1406. [PubMed] [Google Scholar]
- 38.Gearing A J H, Beckett P, Christodoulou M, Churchill M, Clements J, et al. Nature (London) 1994;370:555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- 39.Kayagaki N, Kawasaki A, Ebata T, Ohmoto H, Ikeda S, Inoue S, Yoshino K, Okumura K, Yagita H. J Exp Med. 1995;182:1777–1783. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanzrein M, Garred O, Olsnes S, Sandvig K. Biochem J. 1995;310:285–289. doi: 10.1042/bj3100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Powell S M, Zilz N, Beazer-Barclay Y, Bryan T M, Hamilton S R, Thibodeau S N, Vogelstein B, Kinzler K W. Nature (London) 1992;359:235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 42.Watson S A, Morris T M, Robinson G, Crimmin M J, Brown P D, Hardcastle J D. Cancer Res. 1995;55:3629–3633. [PubMed] [Google Scholar]
- 43.Wang X, Fu X, Brown P D, Crimmin M J, Hoffman R M. Cancer Res. 1994;54:4726–4728. [PubMed] [Google Scholar]