Abstract
Recently, we developed a targeted cytotoxic analog AN-207 of luteinizing hormone-releasing hormone (LH-RH), consisting of an intensely potent derivative of doxorubicin, 2-pyrrolinodoxorubicin (AN-201) conjugated to carrier agonist [d-Lys6]LH-RH. In this study, we investigated the effects of cytotoxic analog AN-207, designed for targeted chemotherapy and radical AN-201 on pituitary function in rats. A selective damage to the pituitary gonadotroph cells was found at 1 week after a single i.v. injection of 150 nmol/kg AN-207, as evidenced by a 63% decrease in the LH-RH-stimulated release of LH in vitro. The release of growth hormone (GH) and thyrotropin (TSH), stimulated by GH-releasing hormone (GH-RH) and TSH-releasing hormone (TRH), respectively, was reduced by only 11–12%. In contrast, even a smaller dose of 75 nmol/kg of AN-201 nonselectively damaged pituitary function, reducing the stimulated release of LH, GH, and TSH by 57%, 74%, and 67%, respectively. Two weeks after administration, the LH-RH-stimulated LH release in vivo entirely normalized in the AN-207-treated rats, and only a 13% decrease in the LH response was found in the group given AN-201. GH and TSH responses to receptor-mediated stimuli with GH-RH and TRH were normal at 2 weeks in both treated groups. Neither cytotoxic compound caused changes in the concentration of pituitary LH, GH, or TSH, as determined by RIA at 1 week and 7 weeks after treatment. This study demonstrates that the cytotoxic LH-RH analog AN-207 exerts highly selective effects on the gonadotroph cells containing LH-RH receptors and is less toxic for other cells. Conversely, its cytotoxic radical AN-201 nonselectively damages the pituitary cells. The damaging effect of both cytotoxic compounds on pituitary functions is reversible. In view of its high selectivity and reduced toxicity, AN-207 could be a potential therapeutic agent for the treatment of tumors that possess receptors for LH-RH such as prostatic, mammary, ovarian, and endometrial cancers.
Keywords: targeted chemotherapy, pituitary hormones, growth hormone, thyrotropin, selective damage
Oncological uses of luteinizing hormone-releasing hormone (LH-RH) analogs are based on the inhibition of the pituitary-gonadal function, the suppression of gonadal steroid hormone secretion leading to inhibition of the growth of sex hormone-dependent tumors. (1–4) However, direct effects of LH-RH analogs on tumor cells may also play a role (5). There is evidence that specific membrane receptors for LH-RH are present in various tumors, such as human prostate cancer (5–7) and breast (8), ovarian (4, 9, 10), endometrial (9–11), and pancreatic (2, 3, 12) cancers. In patients afflicted with these malignancies, conventional chemotherapy results in a varying degree of response and is associated with severe toxic side effects (1). A modern approach to overcome the problem of nonselective toxic effects on normal cells is the targeting. This approach is based upon the selectivity of certain carrier molecules for specific binding sites in tumor tissues (1, 7, 11–14). Highly active cytotoxic agents can be conjugated to appropriate carrier molecules to achieve a more specific delivery to malignant cells, sparing healthy tissues (1). Because specific high affinity binding sites for LH-RH are present in about 52% of breast cancer specimens (8), in nearly 80% of ovarian and endometrial cancers (9), and in 86% of prostate cancers (5), targeted chemotherapy based on cytotoxic LH-RH analogs might be more efficacious and less toxic in these malignancies than conventional regimens of antineoplastic agents (1). However, before cytotoxic LH-RH analogs could be used clinically, a careful assessment is needed of their toxicity to the liver and hematopoietic system and of possible damage to pituitary cells.
Previously, various analogs of LH-RH containing antineoplastic radicals such as cisplatin, d-melphalan, anthraquinone derivatives, and methotrexate were designed, synthesized, and tested in our laboratory (1, 15, 16). Recently, we developed a series of highly active derivatives of doxorubicin (DOX) (17). Among them, 2-pyrrolino-DOX (AN-201) showed a potency 500-1000 times higher in vitro than its parent compound (17). This powerful cytotoxic agent was linked covalently to agonist [d-Lys6]LH-RH to form a cytotoxic LH-RH analog, AN-207 (18). The hybrid molecule thus obtained, fully retains cytotoxic activity of AN-201 as well as hormonal and binding properties of the peptide carrier in vitro (18). We have also shown that AN-207 can inhibit growth of various tumors including prostate cancers in rats (19).
In this study we examined the effects of AN-207 and its cytotoxic radical AN-201 at maximum tolerated doses (19) on the anterior pituitary function in rats, as demonstrated by receptor-mediated responses to hypothalamic-releasing hormones. We investigated the degree and selectivity of possible functional damage to the pituitary at various time periods after treatment with these cytotoxic compounds by testing the responsiveness of pituitary to LH-RH, growth hormone-releasing hormone (GH-RH), and thyrotropin (TSH)-releasing hormone (TRH) in vivo and in vitro.
MATERIALS AND METHODS
Peptides.
Cytotoxic radical AN-201 (2-pyrrolino-DOX), cytotoxic LH-RH analog AN-207 (AN-201 linked to [d-Lys6]LH-RH), and the carrier [d-Lys6]LH-RH were synthesized and characterized in our laboratory as reported (17, 18). LH-RH and GH-RH(1–29)NH2 were also synthesized in our laboratory, while TRH was obtained from Takeda (Osaka).
Experimental Procedure.
Female adult Sprague–Dawley rats (200–250 g) were used for all experiments. The rats were maintained under controlled conditions of lighting (12-h light, 12-h dark schedule) and temperature (24 ± 2°C) with free access to standard rat chow pellets and tap water.
Treatment and in vivo tests.
These experiments were designed to investigate the function of the pituitary gland at various time periods after the treatment with cytotoxic LH-RH analog AN-207 or its cytotoxic radical AN-201. In experiment 1, the rats were divided into four groups of 10 animals. Group 1 received a single i.v. injection of 150 nmol/kg AN-207, group 2 was injected with 75 nmol/kg AN-201, and control rats in groups 3 and 4 were treated with 150 nmol/kg carrier analog [d-Lys6]LH-RH or saline. All injections were performed under methoxyflurane (Metofane; Pittman-Moore, Mundelein, IL) anesthesia. The doses of AN-207 and AN-201 used were previously found to be the maximum tolerated doses inducing growth inhibition of Dunning R-3327-H, androgen dependent prostate cancer in rats (19). All compounds were dissolved in 0.9% sodium chloride and injected into the jugular vein. Body weights (BW) of the animals were recorded once a week, and vaginal smears were examined every day. Two, 4, and 6 weeks after the treatment, specific receptor-mediated responsiveness of pituitary to various releasing hormones was tested by injecting the mixture of 1 μg LH-RH, 1 μg GH-RH, and 1 μg TRH into the jugular vein of six rats in each group. Blood samples were obtained from the jugular vein under Metofane anesthesia before the injection (0 min) and 5, 60, and 180 min later. The volume of blood taken was replaced by saline. The blood was centrifuged, and sera were stored at −20°C until assayed for LH, GH, and TSH by RIA. Seven weeks after the treatment with the cytotoxic compounds, the rats were decapitated, and the pituitaries removed and homogenized. After extracting the protein content with 0.1 M HCl, the homogenates were centrifuged and supernatants stored at −20°C until assayed by RIA for LH, GH, TSH. The protein content of the supernatants was determined by the method of Bradford (20), and hormone concentrations of the pituitary were expressed as μg/mg protein. In experiment 2, four groups of five rats received the same treatment as in experiment 1, but the animals were sacrificed 1 week after the treatment, and LH, GH, TSH, and protein concentrations of the pituitaries were determined as described in experiment 1. This experiment was designed to investigate the effect of cytotoxic compounds AN-207 and AN-201 on the synthesis of LH, GH, and TSH at the time when the treated animals might show the greatest pituitary dysfunction as indicated by lowest BW.
In vitro tests.
The superfused pituitary system was used for these experiments (21). One week after a single injection of 150 nmol/kg cytotoxic LH-RH analog AN-207 or 75 nmol/kg cytotoxic radical AN-201, two animals in both treated groups showing the greatest loss of BW, and no estrous cycle was used for the experiments in vitro, because it was thought that the greatest possible damage to pituitary functions, caused by the cytotoxic compounds, occurred at that time. Two rats treated with [d-Lys6]LH-RH were used as controls. For the experiment performed at 3 weeks, the animals were selected randomly, and all of them showed a partial recovery of weight. Rats were sacrificed, and pituitaries were removed, cut into small pieces, and incubated with collagenase (type I, 0.5%; Worthington) for 50 min in a metabolic shaker. The cells were then dispersed, gently mixed with 1 ml of Sephadex G-10 that had been equilibrated with oxygenated tissue culture medium (medium 199; Sigma), and transferred into the superfusion chambers using two pituitaries of one group in each chamber. To assure stable baseline values, the cells were perfused with the enzyme-free medium 199 overnight. The collection of fractions was started next morning. At the beginning and the end of each experiment, the membrane-depolarizing K+ (50 mM KCl) was administered to obtain standard GH releases and check the amount of releasable GH in the cells. After the initial pulse of K+, 3-min pulses of 1 nM and 10 nM LH-RH were applied at 30-min intervals in each chamber. Sixty minutes after these pulses, the cells were exposed to 1 nM and 10 nM GH-RH at 30-min intervals, followed by subsequent pulses of 1 nM and 10 nM TRH 60 min later. Fractions (1 ml) of the superfusion medium were collected every 3 min. At the end of the experiment, the cells were extracted with 0.1 M HCl, and LH, GH, and TSH concentrations of the samples were determined by RIA.
Hormone Determinations.
Serum LH, GH, and TSH were determined by RIA using materials supplied by the National Hormone and Pituitary Program (Rockville, MD). Rat GH-RP-2/AFP-3190B, rat GH-I-6/AFP-5676B, and anti-rat GH-RIA-5/AFP-411S were used for GH; rat LH-RP-3/AFP-7187B, rat LH-I-9/AFP-10250C, and anti-rat LH-S-11/AFP-697071 P for LH; and rat TSH-RP-3/AFP-5512B, rat TSH-I-9/AFP-11542B, and anti-rat TSH-RIA 6/AFP-329691 Rb for TSH determinations.
Statistical Analysis of Data.
Results expressed as means ± SEM were evaluated by ANOVA; when the P value was <0.05, the analysis was completed using Duncan’s multiple range test. The superfusion data were analyzed with a computer program developed in our institute (21). Using this program we analyzed the peaks and calculated the amount of hormone secreted above the baseline.
RESULTS
Body Weights.
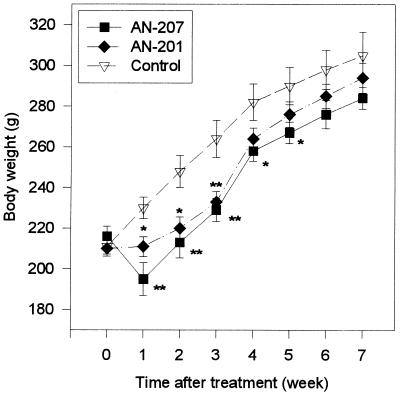
Cytotoxic LH-RH analog AN-207 at a dose of 150 nmol/kg caused a significant 15% decrease in the BW of rats as compared with controls injected with [d-Lys6]LH-RH or saline (P < 0.01), 1 week after the treatment. After the 1st week, the BW of the AN-207-treated animals increased gradually, and at the 2nd, 3rd, 4th, and 5th weeks relative losses of BW were 14%, 13%, 9%, and 8% (P < 0.05), respectively (Fig. 1). By the 6th and 7th weeks, BW of these rats did not differ significantly from the controls. Treatment with a 75 nmol/kg dose of cytotoxic radical AN-201 also caused significant 8%, 11%, and 12% decrease in the BW of rats at weeks 1, 2, and 3, respectively, as related to controls (P < 0.05, P < 0.05, and P < 0.01, respectively). The BW of these rats was similar to the controls by the 4th week. No significant differences in BW were found between the two groups treated with cytotoxic compounds at any time period (Fig. 1).
Figure 1.
BW of the rats treated with cytotoxic LH-RH analog AN-207, cytotoxic radical AN-201, and the carrier [d-Lys6]LH-RH (control). BW of rats given saline were similar to [d-Lys6]LH-RH control and are not shown. Data points represent mean ± SEM (n = 6–10). ∗, P < 0.05; ∗∗, P < 0.01 versus control.
Ovarian Cycles.
After a single injection of cytotoxic LH-RH analog AN-207, vaginal smears in 8 of 10 rats showed diestrus for 3–5 days. The estrous cycle returned 4–6 days after the treatment, and remained regular throughout the 7-week experiment. In 2 of 10 rats, the estrous phase of cycle did not appear by day 7 when they were sacrificed for in vitro experiment. Cytotoxic radical AN-201 did not alter the cycle in three rats and disturbed only the first cycle following the treatment in five animals. Estrous cycles of these five animals returned on days 5–7 and remained regular throughout the study. As in the group treated with AN-207, in 2 of 10 rats, the estrous phase did not return by the 7th day after treatment, when they were used for in vitro experiment. The ovarian cycle of rats in the group treated with [d-Lys6]LH-RH was also disturbed temporarily, but regular cycles returned on days 4–5 in all 10 rats. Treatment with saline did not disturb the estrous cycle of the rats.
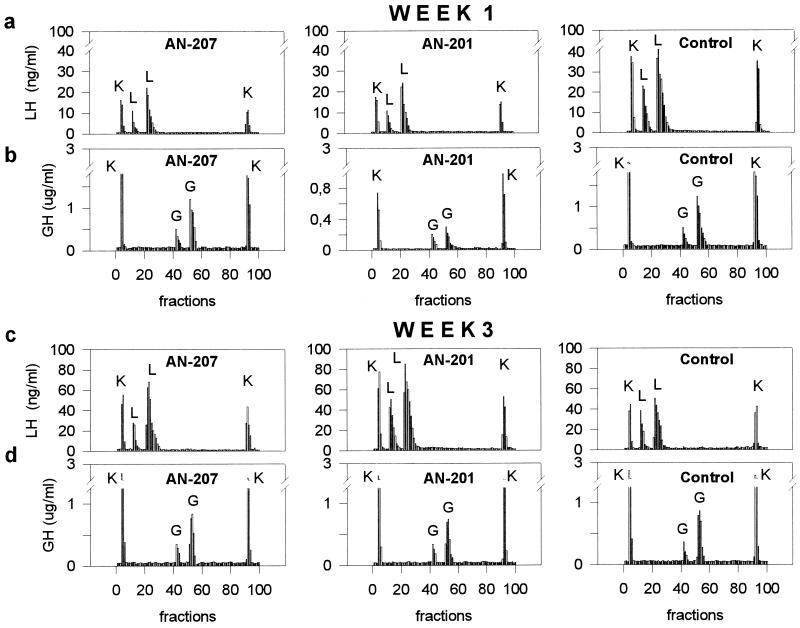
Pituitary Function Tested in Vitro at Weeks 1 and 3.
The results of the in vitro study are shown in Table 1 and Fig. 2.
Table 1.
LH, GH, and TSH responses in superfused rat pituitary cells to specific releasing hormone and nonspecific releasing challenges after an in vivo treatment with cytotoxic LH-RH analog (AN-207) and cytotoxic radical (AN-201)
| Groups | LH, ng
|
GH, μg
|
TSH, ng
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| SP | NSP | Content | SP | NSP | Content | SP | NSP | Content | |
| 1 week after treatment | |||||||||
| AN-207 | 93.6 | 64.6 | 1740 | 5.01 | 10.3 | 72.0 | 49.5 | 31.6 | 400 |
| AN-201 | 109 | 73.0 | 1681 | 1.45 | 3.30 | 33.6 | 18.6 | 14.5 | 175 |
| Control* | 253 | 154 | 2371 | 5.65 | 10.7 | 69.0 | 56.2 | 35.5 | 425 |
| 3 weeks after treatment | |||||||||
| AN-207 | 367 | 222 | 1826 | 4.17 | 9.46 | 76.3 | 41.5 | 27.4 | 352 |
| AN-201 | 574 | 283 | 1564 | 3.99 | 9.77 | 70.6 | 46.6 | 28.9 | 349 |
| Control* | 291 | 183 | 1796 | 4.37 | 9.47 | 74.9 | 45.6 | 30.2 | 384 |
Specific (SP) receptor-mediated release of LH, GH, and TSH was induced by LH-RH, GH-RH, and TRH, respectively. Data represent the total amount of hormones released by subsequent pulses of 1 nM and 10 nM releasing hormone. Nonspecific (NSP) release of LH, GH, and TSH was elicited by the membrane depolarizing agent K+ (50 mM KCl). Data represent the total amount of hormones released by 50 mM KCl at the beginning and the end of the superfusion experiment. The content equals the amount of hormones remaining in the pituitary cells at the end of the experiments.
Control rats were treated with the carrier [d-Lys6]LH-RH.
Figure 2.
LH (a and c) and GH (b and d) responses in superfused rat pituitary cells to specific releasing hormones and nonspecific releasing challenges at 1 week (a and b) and 3 weeks (c and d) after an in vivo treatment with cytotoxic LH-RH analog (AN-207), cytotoxic radical (AN-201), and the carrier [d-Lys6]LH-RH (control). The first and the last peak on the panels were evoked by a nonspecific stimulus with 50 mM KCl (K), the 2nd and 3rd peaks were elicited by 1 nM and 10 nM LH-RH (L) or GH-RH (G), respectively. Net integrated LH and GH releases are shown in Table 1.
LH release.
One week after the in vivo treatment with AN-207, the net integrated LH released by the pituitary cells in response to 1 nM and 10 nM LH-RH was decreased by 63% as compared with the control value (Fig. 2a). Treatment with AN-201 caused a 57% decrease in the LH response. LH responses to the nonspecific membrane depolarizing KCl were also diminished by 52% for AN-207 and by 53% in the case of AN-201 (Fig. 2a). The total LH content of the cells from rats treated with cytotoxic hybrid AN-207 was reduced by 27% and that from AN-201-treated rats was lowered by 29%. Three weeks after the treatment, the receptor-mediated LH response of the cells of rats given AN-207 increased by 26% and that of the AN-201-treated animals augmented by 97%. Nonspecific LH responses of the pituitary cells to KCl were also increased by 21% for AN-207 and by 55% for AN-201 (Fig. 2c). The residual content of LH in the pituitary cells of AN-207-treated rats was not significantly different from that of the control rats (>5%), and it was reduced by 13% in the cells of the AN-201-treated rats.
GH release.
One week after the treatment with cytotoxic hybrid AN-207 at a dose of 150 nmol/kg, there was only a 11% decrease in the GH-RH-induced GH release, and no significant change in the nonspecific GH release (<5%) (Fig. 2b) or the total GH content of the cells (<5%), as compared with control values. However, treatment with 75 nmol/kg of cytotoxic radical AN-201 suppressed the GH-RH-induced release of GH by 79%, the K+-induced nonspecific GH response by 69% (Fig. 2b), and lowered the residual content of GH in the cells by 51%. Three weeks after the treatment with the cytotoxic compounds, the GH responses of both treated groups returned to normal. At this time, differences of less than 10% were found in the receptor-mediated and the nonspecific GH responses of the cells between the treated and the control groups (Fig. 2d).
TSH release.
Pituitaries of rats treated with cytotoxic LH-RH conjugate AN-207 at 150 nmol/kg dose, showed only a 12% decrease in the specific TSH response to TRH and a 11% diminution of the nonspecific TSH release by K+, at 1 week after treatment. The TSH content was not significantly changed in these cells, as compared with controls (Table 1). However, treatment with cytotoxic radical AN-201 at a 75 nmol/kg dose caused significantly greater decrease in TSH release than the cytotoxic conjugate, reducing the receptor-mediated TSH response of the pituitary cells by 67% and the nonspecific TSH release by 59%. At the end of the experiment, a 59% decrease in the residual TSH content was found in these cells, as compared with controls (Table 1). Three weeks after the treatment, no significant differences could be observed in the TSH responses (>10%) or in the residual TSH content of the cells (>10%) between the three groups (Table 1).
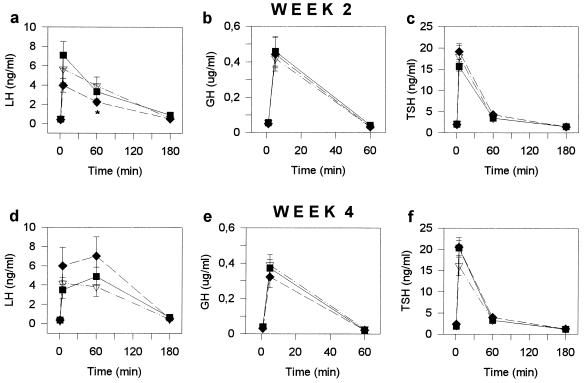
Pituitary Function Tested in Vivo at Weeks 2 and 4.
LH, GH, and TSH responsiveness of the pituitary to receptor-mediated stimuli in rats treated with cytotoxic LH-RH analog AN-207, cytotoxic radical AN-201, and control rats injected with [d-Lys6]LH-RH is represented in Fig. 3. The data obtained from rats injected with saline are not shown because they were the same as those in the carrier-treated group.
Figure 3.
Serum LH (a and d), GH (b and e), and TSH (c and f) responses to LH-RH, GH-RH, and TRH, respectively at 2 weeks (a–c) and 4 weeks (d–f) after a single injection of cytotoxic LH-RH analog AN-207 (▪) cytotoxic radical AN-201 (♦), and the carrier [d-Lys6]LH-RH (control) (▿) to female rats. Data points represent mean ± SEM of values from 8–10 rats per group. ∗, P < 0.05 versus control.
LH release.
Two weeks after a single injection of 150 nmol/kg cytotoxic LH-RH conjugate AN-207 or 75 nmol/kg cytotoxic radical AN-201 to adult female rats, no significant differences were found in the basal serum LH concentration in the treated groups as compared with control group (Fig. 3a). Similarly, the LH-RH-stimulated LH secretion in the treated rats did not differ significantly from that of the controls, as determined 5 min after a bolus injection of 1 μg LH-RH (Fig. 3a). Sixty minutes after the LH-RH challenge, a 13% decrease was found in the LH response of the group treated with AN-201 (P < 0.05), but no significant change could be seen in the AN-207-treated group, as compared with controls. LH levels in all rats returned to the basal values 180 min after the LH-RH injection (Fig. 3a). Four weeks after the treatment with the cytotoxic compounds, no significant differences were found in basal LH levels or LH-RH-induced LH responses between the three groups (Fig. 3d). However, a change could be seen in the time course of LH response between the treated and the control rats. Serum LH concentrations in rats treated with cytotoxic compounds were higher at 60 min than at 5 min after LH-RH challenge, while those of the control rats decreased between 5 and 60 min. LH levels of all animals returned to the basal values 180 min after the LH-RH injection (Fig. 3d). Six weeks after treatment, basal LH levels and LH responses to LH-RH were found to be normal in both groups treated with the cytotoxic compounds, compared with the control group (these data are not shown in Fig. 3).
GH release.
Single injections of cytotoxic hybrid AN-207 or cytotoxic radical AN-201 to rats did not change the basal GH secretion or the GH-RH-stimulated GH secretion measured 2 weeks or 4 weeks after administration (Fig. 3 b and e). GH responsiveness remained normal in both treated groups compared with controls at 6 weeks after treatment.
TSH release.
The TSH secretory function of the pituitary was also unaffected by treatment with the cytotoxic compounds at all time period tested (Fig. 3 c and f)
Pituitary LH, GH, and TSH Concentrations.
Table 2 shows the LH, GH, and TSH concentrations of pituitaries measured 1 and 7 weeks after a single i.v. administration of cytotoxic LH-RH analog AN-207, its cytotoxic radical AN-201, and the carrier [d-Lys6]LH-RH (control) to female rats. No significant differences could be found in the concentration of these pituitary hormones between the three groups at 1 or 7 weeks after treatment. These values were also similar to those found in rats injected with saline (data not shown).
Table 2.
Pituitary hormone concentrations after the treatment with cytotoxic LH-RH analog AN-207 or cytotoxic radical AN-201 in rats
| Group | Pituitary hormone concentrations, μg/mg
|
||
|---|---|---|---|
| LH | GH | TSH | |
| 1 week after treatment | |||
| AN-207 | 17.8 ± 3.35 | 141 ± 15.0 | 4.71 ± 0.75 |
| AN-201 | 16.3 ± 2.62 | 128 ± 16.8 | 4.43 ± 0.57 |
| Control* | 17.9 ± 1.31 | 119 ± 13.6 | 4.02 ± 0.38 |
| 7 weeks after treatment | |||
| AN-207 | 15.6 ± 1.62 | 113 ± 9.96 | 3.69 ± 0.35 |
| AN-201 | 15.9 ± 1.00 | 124 ± 9.83 | 4.28 ± 0.37 |
| Control* | 17.0 ± 2.10 | 110 ± 7.37 | 4.08 ± 0.33 |
Data represent mean ± SEM (n = 5–6 rats per group).
Control rats were treated with [d-Lys6]LH-RH.
DISCUSSION
Targeted cytotoxic LH-RH analogs have been developed in an endeavor to reduce the toxic side effects and increase the efficacy of antineoplastic agents by delivering them more selectively to tumor cells expressing receptors for LH-RH (1, 18). Because gonadotroph cells of pituitary possess high affinity receptors for LH-RH, it is important to determine whether these analogs would damage pituitary functions. It was previously shown that the cytotoxic LH-RH analog AN-207 used in this study fully preserves both the high binding affinity to LH-RH receptors and the powerful LH-releasing activity of the carrier molecule [d-Lys6]LH-RH, in rat pituitary cells (18). Because of the incorporation of the intensely potent 2-pyrrolino-DOX radical AN-201, the cytotoxic activity in vitro of AN-207 on MCF-7 human mammary carcinoma cell line possessing LH-RH receptors (22) was shown to be 500-1000 times higher than that of the hybrid AN-152 consisting of [d-Lys6]LH-RH linked to DOX (18). DOX is a bioreductive intercalating agent that inhibits DNA synthesis (23, 24), and its effect on cell membranes as the first target of action was also reported (25, 26). Because the cycle of the pituitary cells is rather long, the damaging effect of DOX and its derivatives may be detectable by an impairment of cell functions other than mitotic division. The results of this study show that AN-207 caused selective damage of the pituitary gonadotroph cell function 1 week after the treatment, diminishing the receptor-mediated LH release by 63%, while GH and TSH release were only reduced by 11–12%. The selectivity of this harmful effect could be also observed in the nonreceptor-mediated hormone release of the pituitary cells, since the K+-induced LH response was lowered by 52%, and GH and TSH response by only 4–11%. According to these results, it is likely that the dysfunction of the pituitary cells in LH release resulted from an injury to the cell membrane-mediated signaling mechanisms involved in LH release rather than from an intracellular impairment of the second messenger mechanisms. This conclusion is supported by the findings that DOX, the parent compound of AN-201, can interact with biomembranes and alter normal biochemical functions of the membranes, without entering the cells (25, 26). Based on our results, a temporary damage to gonadotroph cells might be the only side-effect on the pituitary expected in the case of clinical application of the cytotoxic analog AN-207. A functional damage to pituitary cells could be in any case alleviated by appropriate replacement therapy.
Cytotoxic radical AN-201 caused a nonselective damage to the pituitary, as shown by a similar reduction in the receptor-mediated release of LH, GH, and TSH as well as in the nonspecific release of these hormones. A similar selectivity of damage was shown in a previous study in vitro using pharmacological doses of early cytotoxic LH-RH analogs much less potent than AN-207 (27). The total immunoreactive hormone concentration of the pituitary was found to be essentially similar in the animals treated with AN-207 and AN-201 and control animals in vivo at 1 week after treatment. In view of this finding, it is likely that decreases in the residual hormone content in the cells of the superfusion study were caused by the loss of the injured cells during the preparative procedure (digestion with collagenase, mechanical dispersion) rather than a deficiency in the hormone synthesis. It has to be noted that the rats used for this superfusion experiment had the lowest BW due to toxicity, which could reflect the greatest dysfunction of pituitary cells. Our observation that the highest deprivation of hormone-releasing functions was observed in the GH (74%) and the smallest in the LH release (57%) is in agreement with the conclusion of a study in humans that GH cells are the most vulnerable of all the pituitary cells to irradiation (28).
The results of the present study also show that the toxic effect of cytotoxic LH-RH analog AN-207 and its cytotoxic radical AN-201 on the pituitary function is reversible. Two weeks after the treatment with these cytotoxic compounds, the receptor-mediated LH-releasing function of the pituitary of rats treated with AN-207 showed a complete recovery. At this time period, a slight decrease in LH response to LH-RH could still be found in the AN-201-treated rats. At 3 weeks following the treatment, a complete recovery as well as an increase of the LH secretory response was observed in vitro in the cells of both treated groups. Similar observations were made in recent studies in men, reporting that the LH response to an LH-RH test was exaggerated and the amplitude of LH pulses was also significantly elevated following chemotherapy with cytotoxic drugs (28). A greater responsiveness of the LH cells to LH-RH was considered to be a compensatory process in men after an impairment of the Leydig cell function due to chemotherapy (28). Such a compensatory process with an increase in LH response may have also occurred in our study in female rats. Although no significant differences were found in the LH-RH induced LH responses between treated and the control groups at 4 weeks after treatment, somewhat higher and more prolonged LH release could be seen in the groups treated with the cytotoxic compounds, signifying that the compensatory process was still in effect.
Another important inference from this work is that the toxic effect of the cytotoxic radical is greater than that of the cytotoxic conjugate. In spite of the fact that the i.v. dose of AN-207 was 2 times higher on molar basis than that of AN-201, no significant difference was found in the BW between the two treated groups. Similar results were obtained in our previous study, in which we administered these cytotoxic compounds by i.p. route to male rats (19) and in a preliminary study in female rats.
In conclusion, the cytotoxic LH-RH analog AN-207 is highly selective for the cells containing LH-RH receptors, and less toxic to other cells, while its cytotoxic radical AN-201 nonselectively injures various cells of the pituitary. The toxic effects of both cytotoxic compounds on the pituitary are completely reversible. Because of its high selectivity and reduced toxicity AN-207 should be more efficacious for treatment of tumors which possess receptors for LH-RH than its cytotoxic radical 2-pyrrolino-DOX AN-201.
Acknowledgments
We thank Prof. J. Engel (Asta Medica AG, Frankfurt am Main, Germany) for useful advice on possible clinical use of cytotoxic LH-RH analogs and Ms. Katalin C. Halmos and Ms. Elena Glotser for technical assistance. The gifts of materials for RIA from the National Hormone and Pituitary Program of the National Institute of Diabetes and Digestive and Kidney Diseases are greatly appreciated. The work described in this paper was supported by the Medical Research Service of the Veterans Affairs Department (to A.V.S.)
Footnotes
Abbreviations: DOX, doxorubicin; LH, luteinizing hormone; LH-RH, LH-releasing hormone; GH, growth hormone; GH-RH, GH-releasing hormone; TSH, thyrotropin; TRH, thyrotropin-releasing hormone; BW, body weight(s).
References
- 1.Schally A V, Nagy A, Szepeshazi K, Pinski J, Halmos G, Armatis P, Miyazaki M, Comaru-Schally A M, Yano T, Emons G. In: Treatment with Gn-RH Analogs: Controversies and Perspectives. Filicori M, Flamigni C, editors. Carnforth, U.K.: Parthenon; 1996. pp. 33–44. [Google Scholar]
- 2.Schally A V, Comaru-Schally A M. In: Cancer Medicine. 4th Ed. Holland J F, Frei E, Bast R C, Kufe D W, Morton D L, Weichselbaum R R, editors. Baltimore: Williams & Wilkins; 1997. pp. 1067–1086. [Google Scholar]
- 3.Schally A V. Anti-Cancer Drugs. 1994;5:115–130. [PubMed] [Google Scholar]
- 4.Yano T, Pinski J, Halmos G, Szepeshazi K, Groot K, Schally A V. Proc Natl Acad Sci USA. 1994;91:7090–7094. doi: 10.1073/pnas.91.15.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qayum A, Gullick W, Clayton R C, Sikora K, Waxman J. Br J Cancer. 1990;62:96–99. doi: 10.1038/bjc.1990.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fekete M, Redding T W, Comaru-Schally A M, Pontes A E, Connelly R W, Srkalovic G, Schally A V. Prostate. 1989;14:191–208. doi: 10.1002/pros.2990140302. [DOI] [PubMed] [Google Scholar]
- 7.Schally A V, Comaru-Schally A M, Gonzalez-Barcena D. Biomed Pharmacother. 1992;46:465–471. doi: 10.1016/0753-3322(92)90004-q. [DOI] [PubMed] [Google Scholar]
- 8.Fekete M, Wittliff J L, Schally A V. J Clin Lab Anal. 1989;3:137–147. doi: 10.1002/jcla.1860030302. [DOI] [PubMed] [Google Scholar]
- 9.Emons G, Schally A V. Hum Reprod. 1994;9:1364–1379. doi: 10.1093/oxfordjournals.humrep.a138714. [DOI] [PubMed] [Google Scholar]
- 10.Emons G, Ortmann O, Becker M, Irmer G, Springer B, Laun R, Holzel F, Schulz K D, Schally A V. Cancer Res. 1993;53:5439–5446. [PubMed] [Google Scholar]
- 11.Emons G, Schroder B, Ortmann O, Westphalen S, Schulz K D, Schally A V. J Clin Endocrinol Metab. 1993;77:1458–1464. doi: 10.1210/jcem.77.6.8263128. [DOI] [PubMed] [Google Scholar]
- 12.Schally A V, Radulovic S, Comaru-Schally A M. In: Experimental and Clinical Studies in Hormone Dependent Cancers. Mazzaferri E, Samaan N, editors. Boston: Blackwell; 1993. pp. 49–73. [Google Scholar]
- 13.Harris N, Dutlow C, Eidne K, Dong K-W, Millar R. Cancer Res. 1991;51:2577–2581. [PubMed] [Google Scholar]
- 14.Fitzgerald D, Pastan I. J Natl Cancer Inst. 1989;81:1455–1463. doi: 10.1093/jnci/81.19.1455. [DOI] [PubMed] [Google Scholar]
- 15.Pinski J, Schally A V, Yano T, Szepeshazi K, Halmos G, Groot K, Comaru-Schally A M, Radulovic S, Nagy A. Prostate. 1993;23:165–178. doi: 10.1002/pros.2990230209. [DOI] [PubMed] [Google Scholar]
- 16.Szepeshazi K, Schally A V, Halmos G, Szoke B, Groot K, Nagy A. Breast Cancer Res Treat. 1996;40:129–139. doi: 10.1007/BF01806208. [DOI] [PubMed] [Google Scholar]
- 17.Nagy A, Armatis P, Schally A V. Proc Natl Acad Sci USA. 1996;93:2464–2469. doi: 10.1073/pnas.93.6.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagy A, Schally A V, Armatis P, Szepeshazi K, Halmos G, Kovacs M, Zarandi M, Groot K, Miyazaki M, Jungwirth A, Horvath J. Proc Natl Acad Sci USA. 1996;93:7269–7273. doi: 10.1073/pnas.93.14.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jungwirth, A., Schally, A. V., Nagy, A., Pinski, J., Groot, K., Galvan, G., Szepeshazi, K. & Halmos, G. (1996) Cancer Res., in press.
- 20.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 21.Csernus V J, Schally A V. In: Neuroendocrine Research Methods. Greenstein B D, editor. London: Harwood; 1991. pp. 71–109. [Google Scholar]
- 22.Miller W R, Scott W N, Morris R, Fraser H M, Sharpe R M. Nature (London) 1985;313:231–233. doi: 10.1038/313231a0. [DOI] [PubMed] [Google Scholar]
- 23.Pigram W J, Fuller W, Hamilton L D. Nature (London) New Biol. 1972;235:17–19. doi: 10.1038/newbio235017a0. [DOI] [PubMed] [Google Scholar]
- 24.Moore W H. Science. 1977;197:527–532. doi: 10.1126/science.877572. [DOI] [PubMed] [Google Scholar]
- 25.Tritton T R, Yee G. Science. 1982;217:248–250. doi: 10.1126/science.7089561. [DOI] [PubMed] [Google Scholar]
- 26.Tritton T R. Pharmacol Ther. 1991;49:293–309. doi: 10.1016/0163-7258(91)90060-y. [DOI] [PubMed] [Google Scholar]
- 27.Rekasi Z, Szoke B, Nagy A, Groot K, Rekasi E S, Schally A V. Endocrinology. 1993;132:1991–2000. doi: 10.1210/endo.132.5.8477650. [DOI] [PubMed] [Google Scholar]
- 28.Shalet S M. Eur J Endocrinol. 1996;135:135–143. doi: 10.1530/eje.0.1350135. [DOI] [PubMed] [Google Scholar]