Abstract
The effects of selected heme analogues on heme oxygenase activity in tissues and on human and rabbit bone marrow hematopoietic colony growth were examined. Zinc protoporphyrin (ZnPP) and zinc mesoporphyrin (ZnMP), at concentrations ranging between 1 and 20 μM, produced significant inhibition of human and rabbit bone marrow erythroid (CFU-E, BFU-E) and myeloid (CFU-GM) colony growth. The growth inhibition produced by ZnPP or ZnMP was not overcome with exposure of cultures to elevated levels of the growth factors erythropoietin and granulocyte–macrophage colony stimulating factor. In contrast, tin protoporphyrin and tin mesoporphyrin did not display any significant bone marrow toxicity when used at similar concentrations. In other studies, differential effects of tin mesoporphyrin and ZnMP administered intravenously on kidney heme oxygenase were demonstrated. Chromium mesoporphyrin administered intravenously proved lethal to animals. These results indicate that exposure of bone marrow to ZnPP and ZnMP can be deleterious to hematopoietic cells and raise the possibility that ZnPP, which is endogenously formed and found in high concentration in red blood cells in lead-poisoned children, may itself participate in the bone marrow toxicity produced by this metal.
Hematopoietic cell growth and differentiation within the bone marrow microenvironment are dependent on a complex interplay of cells, cytokines, growth factors, and heme oxygenase (HO) activity, with the latter enzyme playing a major regulatory role in this system. Heme, a potent inducer of HO expression has been shown to have modulatory effects on hematopoiesis (1). A comprehensive study comparing the effects on hematopoietic cells of synthetic heme analogues, which inhibit HO activity has, not previously been undertaken. Information from this type of study is of special importance because of the clinical potential (2–8) of certain of these compounds.
In this study we compared the effects of tin and zinc porphyrins on hematopoietic cell growth and colony formation in animal and human bone marrow cultures. Such cell systems are especially vulnerable to the nature of their microenvironment and thus can provide sensitive indices of the deleterious potential of various chemical agents.
The results of this study indicate that zinc porphyrins are toxic to both myeloid and erythroid cell growth even at low concentrations. In contrast, tin porphyrins, even at high concentrations, displayed no toxic effects on hematopoiesis. In other experiments tin and zinc porphyrins were shown to have differing effects on renal HO activity when administered intravenously (i.v.). Chromium mesoporphyrin (CrMP) proved lethal to animals when administered by the iv route.
These findings provide additional examples of the differential effects of HO inhibitors on cell functions based on their central metal atom and on their route of administration. The inhibitory actions of zinc porphyrins on bone marrow cell growth represent newly identified deleterious properties of these metalloporphyrins and extend the range of cell systems in which zinc compounds display cellular toxicity. The bone marrow toxicity displayed by zinc protoporphyrin (ZnPP) also raises the possibility that this endogenously formed compound may be involved, in part, in the pathogenesis of the hematological abnormalities characterizing lead poisoning, a disorder in which high concentrations of ZnPP are found in red blood cells.
MATERIALS AND METHODS
Preparation of Cells.
Human bone marrow cells were obtained from the posterior iliac crest of normal donors. In all cases, informed consent was obtained. Adult New Zealand White rabbits were also used as bone marrow donors. Animals were sacrificed by anesthesia, femurs removed, and bone marrow flushed with Iscove’s modified Dulbecco’s medium (IMDM) (GIBCO). Bone marrow low-density nucleated cells were then separated by Histopaque (Sigma) density gradient centrifugation, washed, and resuspended in IMDM with 2% fetal calf serum (GIBCO). The nonadherent cells were separated by allowing adherent cells to attach to the bottom of Petri dishes over a 24-hr period of incubation.
Chemicals.
Heme analogues were obtained from Porphyrin Products (Logan, UT) and included tin mesoporphyrin (SnMP), tin protoporphyrin (SnPP), zinc mesoporphyrin (ZnMP), ZnPP, CrMP, protoporphyrin IX (PPIX), mesoporphyrin IX (MPIX), and heme. The heme analogues, except for ZnMP and ZnPP, were prepared and added to cultures as described (1). Zinc porphyrins were first dissolved in propylene glycol to give a final concentration of 10% (vol/vol) and then prepared as described (1).
Hematopoietic Colony Assays.
Bone marrow hematopoietic colonies were grown in methylcellulose cultures similar to previously described procedures (9, 10). Human and rabbit erythroid colonies (CFU-E, BFU-E) were grown in methylcellulose in the presence of erythropoietin (Epo, Amgen Biologicals). Myeloid colonies were grown in methylcellulose cultures in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF, Amgen). All cultures were then incubated at 37°C for 5–14 days after which colonies were scored.
Heme Oxygenase Assay.
Immediately after death, livers were perfused in situ with ice-cold saline. Liver, spleen, and kidney were removed and homogenized in three volumes of 0.1 M potassium phosphate buffer (pH 7.40) containing 0.25 M sucrose. Microsomal fractions were prepared from homogenates as described (11). The cytosolic fraction obtained from the livers of adult control animals served as a source of biliverdin reductase in the HO assay. HO activity, expressed as nanomoles of bilirubin formed per milligram of protein per hour, was determined as described (11). Protein concentrations were determined according to the method of Lowry et al. (12).
RESULTS
Effect of CrMP, SnMP, and ZnMP on Rabbit HO Activity.
Metalloporphyrins are potent inhibitors of HO activity in humans and animals and the route of administration of the metalloporphyrins may result in differences in distribution within the organism (13, 14). HO activity was measured in the liver, kidney, and spleen of rabbits administered SnMP, ZnMP, and CrMP i.v. at a dose of 10 μM/kg body weight (Table 1), a dose in excess of the dose of SnMP employed clinically (3). SnMP produced a significant decrease in enzyme activity in all three tissues; no enzyme activity was detectable in liver and kidney. ZnMP produced a significant decrease in HO activity in liver and spleen, but not in kidney. ZnMP, like SnMP, administration resulted in no detectable enzyme activity in liver. CrMP administered at the above dose resulted in the death of all animals, whereas the metalloporphyrin administered at a dose of 5 μM/kg body weight killed one-third of treated animals (results not shown). We therefore focused only on zinc and tin porphyrins in subsequent studies of the effects of heme analogues on bone marrow hematopoietic growth.
Table 1.
Effect of ZnMP and SnMP on tissue heme oxygenase activity
| Treatment | Heme oxygenase, nmol bilirubin formed per mg/hr*
|
||
|---|---|---|---|
| Liver | Spleen | Kidney | |
| Control | 0.70 ± 0.09 | 8.19 ± 2.0 | 1.16 ± 0.16 |
| SnMP | ND | 2.76 ± 0.90** | ND |
| ZnMP | ND | 2.90 ± 1.12** | 0.91 ± 0.18 |
Animals were injected i.v. with a dose of 10 μM/kg body weight and sacrificed 48 hr later. ND, no detectable activity.
Mean + SD.
P < 0.025 compared with control.
Effect of Metalloporphyrins on Rabbit Bone Marrow Growth.
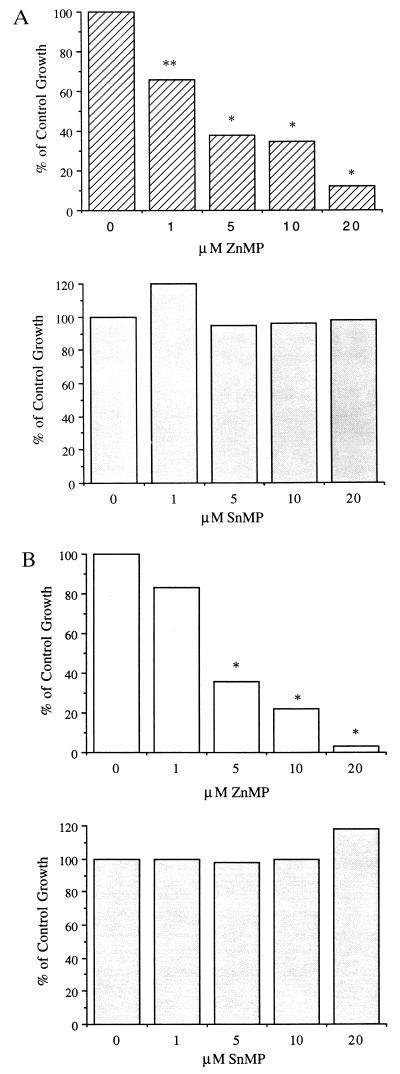
We examined the dose effects of different metalloporphyrins on rabbit bone marrow in vitro hematopoiesis. As shown in Figs. 1 and 2, ZnPP and ZnMP inhibited hematopoiesis at all concentrations examined (1–20 μM). SnMP and SnPP did not inhibit hematopoiesis (Figs. 1 and 2). ZnPP (5 μM), when added to BFU-E and CFU-GM cultures (Fig. 1 A and B), resulted in growth that was 41 and 56%, respectively, of control growth; when 20 μM of ZnPP was used, the growth was 25% and 1%, respectively, of control growth. Similarly, when 5 μM of ZnMP was included in BFU-E and CFU-GM cultures, the percent of control growth was 38% and 36%, respectively; when 20 μM of ZnMP was used, the percent of control growth was 12% and 3%, respectively (Fig. 2 A and B). Similar results were obtained for CFU-E growth in the presence of both zinc porphyrins. In contrast, when 20 μM of SnPP was included in BFU-E and CFU-GM cultures, the percent of control growth was 108% and 100%, respectively (Fig. 1 A and B); when 20 μM of SnMP was used, the percent of control growth was 100%, 98%, and 124%, respectively (Fig. 2 A and B). Thus, there were marked differences in the zinc and tin porphyrins with respect to their effects on bone marrow colony growth. In other experiments, mesoporphyrin IX itself was found to be toxic to bone marrow cultures at the concentrations tested (1–20 μM): protoporphyrin IX produced some toxicity at the highest concentration (20 μM) studied, though at lesser concentration protoporphyrin IX was less toxic than ZnPP. (results not shown).
Figure 1.
Dose effects of ZnPP and SnPP on rabbit bone marrow BFU-E (A) and CFU-GM (B) colony-forming units. Results represent percent of control growth. ∗, P < 0.001; ∗∗, P < 0.005.
Figure 2.
Dose effects of ZnMP and SnMP on rabbit bone marrow BFU-E (A) and CFU-GM (B) colony-forming units. Results represent percent of control growth. ∗, P < 0.001; ∗∗, P < 0.005.
Effect of Metalloporphyrins on Human Bone Marrow Growth.
We have previously shown that heme enhances in vitro erythropoiesis (1, 15). We therefore examined the dose effects of ZnPP and SnPP on human bone marrow erythroid colony growth (CFU-E, BFU-E). These results are presented in Table 2. Control numbers of CFU-E and BFU-E were 142 ± 9 and 94 ± 6 × 105 cells, respectively. SnPP at doses ranging from 1 to 20 μM had no effect on CFU-E or BFU-E erythroid growth. In contrast, ZnPP at doses ranging from 1 to 20 μM inhibited cell growth. Cultures containing 10 μM of ZnPP generated CFU-E and BFU-E at levels of only 49% and 36% of control growth, respectively; a more pronounced inhibition was seen with the highest dose (20 μM) of ZnPP examined (32% and 23% of control growth, respectively, Table 2). Thus, the data demonstrate that SnPP does not affect erythroid growth in the concentration range examined (1–20 μM), whereas ZnPP is a potent inhibitor of cell growth. Similar results were obtained with the mesoporphyrin derivatives of the metals (data not shown).
Table 2.
Dose effect of ZnPP and SnPP on human bone marrow erythroid CFU-E and BFU-E growth
| Additions | % of control growth
|
|
|---|---|---|
| CFU-E, 142 ± 9/5 × 105 cells | BFU-E, 94 ± 6/5 × 105 cells | |
| Epo alone | 100 | 100 |
| + ZnPP, μM | ||
| 1 | 80 | 79 |
| 5 | 64 | 51 |
| 10 | 49* | 36* |
| 20 | 32* | 23* |
| + SnPP, μM | ||
| 1 | 101 | 100 |
| 5 | 99 | 102 |
| 10 | 103 | 98 |
| 20 | 100 | 104 |
Human bone marrow cells were grown in methylcellulose cultures in the presence of 1–2 units per ml of Epo for CFU-E and BFU-E, respectively. Additions of analogues were made and then colonies scored after 7–14 days of incubation at 37°C. Control numbers of CFU-E and BFU-E are given as (M ± SEM), n = 6, and remaining results presented as percent of control growth.
P < 0.001.
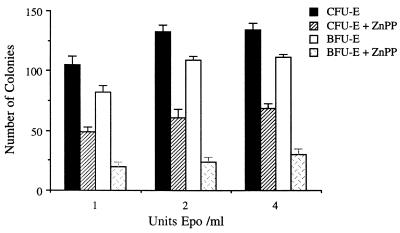
Since Epo is critical for the growth and development of erythroid colonies, we examined whether ZnPP growth inhibition could be overcome with increasing concentrations of Epo. Optimal CFU-E/BFU-E growth is usually obtained with 1–1.5 units of Epo per ml. ZnPP (10 μM) was inhibitory to CFU-E and BFU-E growth when cultures contained 1, 2, or 4 units of Epo per ml (Fig. 3). The highest concentration (6 units per ml) of Epo examined did not reverse the inhibition of growth caused by ZnPP (data not shown). Control CFU-E growth with 4 units of Epo per ml was 134 ± 10 colonies, whereas when ZnPP was included in cultures the CFU-E growth was only 72 ± 9 colonies (Fig. 3). Control BFU-E growth was 99 ± 9 colonies, whereas growth was only 35 ± 3 colonies for BFU-E cultures with ZnPP. ZnPP thus proved to be a potent inhibitor of in vitro erythropoiesis and its inhibitory effect could not be overcome with Epo.
Figure 3.
Dose effects of Epo on human bone marrow erythroid CFU-E and BFU-E growth in the presence of 10 μM ZnPP; M ± SEM, n = 6.
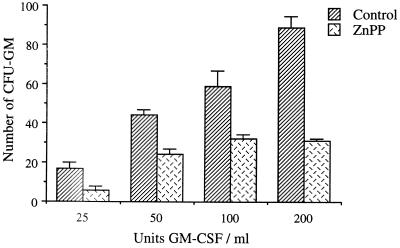
We then examined the effects of the metalloporphyrins on human myeloid CFU-GM growth. These results are shown in Fig. 4. The two analogues of zinc, ZnMP and ZnPP (10 μM), produced significant inhibition of CFU-GM growth; as shown in this figure, control CFU-GM growth was 104 ± 10 colonies, whereas colony growth for cultures containing ZnPP and ZnMP was only 17 ± 6 and 30 ± 5 colonies, respectively. Mesoporphyrin IX was even more toxic than ZnPP and ZnMP (data not shown). SnMP, in contrast, was without significant effect on colony growth. ZnPP inhibited bone marrow CFU-GM growth at all GM-CSF levels employed (Fig. 5). Control growth in the presence of 50 units per ml of GM-CSF was 44 ± 2 colonies, whereas when ZnPP was included, only 22 ± 2 colonies were generated. In a similar manner, control growth with 200 units per ml of GM-CSF was 80 ± 5, and only 33 ± 4 colonies when ZnPP was included in culture. ZnPP thus proved toxic to myeloid cell growth at all levels of GM-CSF tested.
Figure 4.
Effects of several metalloporphyrins (10 μM) on human bone marrow myeloid CFU-GM growth; M ± SEM, n = 6.
Figure 5.
Dose effects of GM-CSF on human bone marrow myeloid CFU-GM growth in the presence of 10 μM ZnPP; M ± SEM, n = 6.
DISCUSSION
The results of these studies demonstrate that ZnPP and ZnMP significantly inhibit in vitro hematopoiesis in both human and rabbit bone marrow. This effect was made manifest by a direct inhibition of erythroid (CFU-E, BFU-E) and myeloid (CFU-GM) growth when the compounds were included in cultures at concentrations ranging from 1 to 20 μM. Furthermore, elevated levels of hematopoietic growth factors did not reverse the inhibition of erythroid and myeloid cell growth produced by the zinc porphyrins. In contrast, SnPP and SnMP did not show any significant bone marrow toxicity when used at the same concentrations as ZnPP and ZnMP. These data thus identify important differences between tin and zinc porphyrins in relation to hematopoiesis and provide further evidence that the nature of the central metal atom in synthetic heme analogues profoundly influences the biological and toxicological properties of these compounds (16, 17). The suppressive effects of zinc porphyrins on hematopoiesis described in this report raise a caution about their proposed clinical use in newborns to control hyperbilirubinemia.
The mechanism of hematopoietic inhibition by zinc porphyrins is unclear; however, it is not restricted to a single lineage, since both myeloid and erythroid elements are affected. It is known that ZnPP is endogenously derived in lead poisoning and in certain anemias resulting in accumulation of the metalloporphyrin within red blood cells (18–20). Thus, accumulation of ZnPP within the cells of the bone marrow microenvironment could result in a direct toxic effect on progenitor cells. In this regard, the extensive incorporation (≈45% of the administered dose) of exogenous ZnPP in circulating red blood cells of primates indicates that this heme analogue has direct access to and enters into erythroid cells (8). In addition, ZnPP can act as an interleukin 1 antagonist (21–23), thus disturbing the cytokine network of the bone marrow stroma. It is also apparent from these studies that free protoporphyrin and mesoporphyrin have toxic effects on bone marrow cells raising the possibility that the mechanism of toxicity of zinc porphyrins may be due in part to release of the central zinc atom from the metal–porphyrin complex in situ, thus permitting the porphyrin macrocycle to exert a direct cell toxicity. Zinc porphyrins are known to be highly labile in this regard (24). In contrast, tin incorporated in the porphyrin ring structure to form either SnPP or SnMP is extremely stable and there is no known physiological mechanism by which the element can be removed from the porphyrin complex.
Finally, zinc itself can substantially alter normal cell functions; recent data have, for example, been reported that indicate that zinc is a potent inhibitor of bone resorption activity (25) and that zinc toxicity plays a significant role in neuronal ischemia leading to degeneration and cell death (26–30). Thus, if the metal is released from ZnPP or ZnMP intracellularly, the element itself could additionally contribute to the toxicity exerted by the free porphyrin macrocycle.
In this study, the degrees of inhibition of HO that resulted from the i.v. administration of SnMP and ZnMP compounds were similar in liver and spleen, whereas in kidney, SnMP was considerably more potent than ZnMP. Previous studies have indicated that SnMP is a more potent inhibitor of HO activity in vivo than ZnMP when administered by the subcutaneous route (14). These findings thus indicate that the route of administration of ZnMP may be important in determining the ability of zinc porphyrins to inhibit HO activity in various tissues. Preliminary in vivo experiments with rabbits (data not shown) have shown that administration of ZnMP, but not SnMP, produces a significant inhibition of hematopoiesis and progenitor cell mobilization, thus confirming in vivo the deleterious impact of ZnMP on bone marrow cells, in vitro, reported here.
CrMP administered i.v. at a dose of 10 μM/kg body weight resulted in the death of all treated animals; at a dose of 5 μM/kg body weight CrMP administration i.v. still proved lethal to a significant number (one-third) of the animals. Pathological examination of treated animals showed widespread lysis of liver cells. These results show that CrMP, while tolerated when administered by the subcutaneous route in animals, can in the same doses be lethal when administered by the i.v. route. In contrast, SnMP, administered i.v. to humans for prolonged periods of time and in very large cumulative dosage, has been shown to be without significant side effects (31, 32).
The findings reported in these studies bear on the suitability of the metalloporphyrins examined for use in humans, especially newborns, to regulate heme degradation to bilirubin for clinical purposes.
Acknowledgments
This work was supported in part by National Institutes of Health Grants ROI-HL-5438 and NO1-HD5-3234.
Footnotes
Abbreviations: HO, heme oxygenase; CrMP, chromium mesoporphyrin; ZnPP, zinc protoporphyrin; SnMP, tin mesoporphyrin; SnPP, tin protoporphyrin; ZnMP, zinc mesoporphyrin; Epo, erythropoietin.
References
- 1.Lutton J D, Chertkov J L, Levere R D, Abraham N G. Leuk Lymphoma. 1991;5:179–185. doi: 10.3109/10428199109068123. [DOI] [PubMed] [Google Scholar]
- 2.Kappas A, Drummond G S, Manola T, Petmezaki S, Valaes T. Pediatrics. 1988;81:485–497. [PubMed] [Google Scholar]
- 3.Valaes T, Petmezaki S, Henschke C, Drummond G S, Kappas A. Pediatrics. 1994;93:1–11. [PubMed] [Google Scholar]
- 4.Kappas A, Drummond G S, Henschke C I, Valaes T. Pediatrics. 1995;95:468–474. [PubMed] [Google Scholar]
- 5.Rodgers P A, Seidman D S, Wei P L, Dennery P A, Stevenson D K. Pediatr Res. 1996;39:1041–1049. doi: 10.1203/00006450-199606000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Stevenson D K, Rodgers P A, Vreman H J. Am J Dis Child. 1989;143:353–356. doi: 10.1001/archpedi.1989.02150150111027. [DOI] [PubMed] [Google Scholar]
- 7.Vreman H J, Ekstrand B C, Stevenson D K. Pediatr Res. 1993;33:195–200. doi: 10.1203/00006450-199302000-00021. [DOI] [PubMed] [Google Scholar]
- 8.Qato M K, Maines M D. Biochem J. 1985;226:51–57. doi: 10.1042/bj2260051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iscove N N, Sieber F, Winterhalter K H. Am J Cell Physiol. 1974;83:309–316. doi: 10.1002/jcp.1040830218. [DOI] [PubMed] [Google Scholar]
- 10.Lutton J D, Chertkov J L, Jiang S, Kappas A, Levere R D, Abraham N G. Am J Hematol. 1993;44:172–178. doi: 10.1002/ajh.2830440307. [DOI] [PubMed] [Google Scholar]
- 11.Drummond G S, Kappas A. Proc Natl Acad Sci USA. 1981;78:6466–6470. doi: 10.1073/pnas.78.10.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowry O H, Rosebrough N J, Farr A L, Randall R J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 13.Vallier H A, Rodgers P A, Stevenson D K. Pharmacol Lett. 1993;52:79–94. [Google Scholar]
- 14.Bundock E A, Drummond G S, Kappas A. Pharmacology. 1996;52:187–198. doi: 10.1159/000139383. [DOI] [PubMed] [Google Scholar]
- 15.Abraham N G, Lutton J D, Drummond G S, Kappas A. Cell Physiol Biochem. 1996;6:129–168. [Google Scholar]
- 16.Kappas A, Drummond G S. J Clin Invest. 1986;77:335–339. doi: 10.1172/JCI112309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drummond G S, Galbraith R A, Sardana M K, Kappas A. Arch Biochem Biophys. 1987;255:64–74. doi: 10.1016/0003-9861(87)90294-3. [DOI] [PubMed] [Google Scholar]
- 18.Hastka J, Lasserre J J, Schwarzbeck A, Hehlmann R. Clin Chem. 1994;40:768–773. [PubMed] [Google Scholar]
- 19.Granick J L, Sassa S, Kappas A. Adv Clin Chem. 1978;20:287–339. doi: 10.1016/s0065-2423(08)60025-6. [DOI] [PubMed] [Google Scholar]
- 20.Hastka J, Lasserre J J, Schwarzbeck A, Strauch M, Hehlmann R. Blood. 1993;81:1200–1204. [PubMed] [Google Scholar]
- 21.Yamasaki Y, Suzuki T, Yamaya H, Matsuura N, Onodera H, Kogure K. Neurosci Lett. 1992;142:45–47. doi: 10.1016/0304-3940(92)90616-f. [DOI] [PubMed] [Google Scholar]
- 22.Nagai H, Kitagaki K, Kuwabara K, Koda A. Agents Actions. 1992;37:273–283. doi: 10.1007/BF02028120. [DOI] [PubMed] [Google Scholar]
- 23.Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama Y, Kogure K. Stroke. 1995;26:676–690. doi: 10.1161/01.str.26.4.676. [DOI] [PubMed] [Google Scholar]
- 24.Greenbaum N L, Kappas A. Photochem Photobiol. 1991;54:183–192. doi: 10.1111/j.1751-1097.1991.tb02005.x. [DOI] [PubMed] [Google Scholar]
- 25.Moonga B, Dempster D. J Bone Miner Res. 1995;10:453–457. doi: 10.1002/jbmr.5650100317. [DOI] [PubMed] [Google Scholar]
- 26.Choi D W, Koh J Y. Neuroscience. 1994;60:1049–1057. doi: 10.1016/0306-4522(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 27.Choi D W, Yokoyama M, Koh J. Neuroscience. 1988;24:67–79. doi: 10.1016/0306-4522(88)90312-0. [DOI] [PubMed] [Google Scholar]
- 28.Csermely P, Szamel M, Resch K, Somogy J. J Biol Chem. 1988;263:6487–6490. [PubMed] [Google Scholar]
- 29.Koh J, Suh S W, Gwag B J, He Y, Hsu C Y, Choi D W. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- 30.Weiss J H, Hartley D M, Koh J Y, Choi D W. Neuron. 1993;10:43–49. doi: 10.1016/0896-6273(93)90240-r. [DOI] [PubMed] [Google Scholar]
- 31.Galbraith R A, Drummond G S, Kappas A. Pediatrics. 1992;89:175–182. [PubMed] [Google Scholar]
- 32.Kappas A, Drummond G S, Galbraith R A. Pediatrics. 1993;91:537–539. [PubMed] [Google Scholar]