Abstract
Sweet taste and nonnutritive suckling produce analgesia to transient noxious stimuli in infant rats and humans. The present study evaluated the pain-modulating effects of sucrose and suckling in a rat model of persistent pain and hyperalgesia that mimics the response to tissue injury in humans. Fore- and hindpaw withdrawal latencies from a 30° or 48°C brass stylus were determined in 10-day-old rats following paw inflammation induced by complete Freund’s adjuvant (CFA; 1:1 injected s.c. in a 0.01 ml volume). CFA markedly decreased escape latencies to both 48° and 30°C stimulation, thereby demonstrating thermal hyperalgesia and mechanical allodynia. The combination of nonnutritive suckling and sucrose (7.5%, 0.01–0.06 ml/min) infusion markedly increased escape latencies to forepaw stimulation in both CFA-treated and control rats. In contrast, intraoral sucrose and suckling did not increase hindpaw withdrawal latencies in either control or CFA-inflamed rats. The effect was specific to sweet taste because neither water nor isotonic saline infusion affected forepaw escape latencies. Parallel findings were obtained for CFA-induced Fos-like immunoreactivity (Fos-LI), a marker of neuronal activation. Fos-LI was selectively induced in cervical and lumbar regions ipsilateral to forepaw and hindpaw inflammation, respectively. Suckling-sucrose treatment significantly reduced Fos-LI at the cervical but not at the lumbar regions. These findings demonstrate: (i) the development of persistent pain and hyperalgesia in 10-day-old rats that can be attenuated by endogenous pain-modulating systems activated by taste and nonnutritive suckling; (ii) the mediation of the sucrose-suckling analgesia and antihyperalgesia at the spinal level; and (iii) a differential rostrocaudal maturation of descending pain-modulating systems to the spinal cord of 10-day-old rats. These findings may provide new clinical approaches for engaging endogenous analgesic mechanisms in infants following tissue injury and inflammation.
Keywords: analgesia, Freund’s adjuvant, pain modulation
Pain is a serious clinical problem in premature, newborn, and young infants that arises from medical or surgical intervention or traumatic injury. Although the commonly held view that infants do not perceive pain has been refuted (1), the fear that anesthetic agents produce respiratory depression, apnea, and hypotension in this population persists, and insufficient pain control remains an important clinical problem following common medical or surgical procedures (2). Recent behavioral studies demonstrating orogustatory and orotactile-induced analgesia hold considerable potential for the future management of pain in newborns through noninvasive means that engage endogenous analgesic systems (3, 4). In particular, calming, analgesia, and (in humans) bradycardia can be induced via sweet (sucrose, fructose, or glucose) or milk stimulation, but not lactose in both rat (5–8) and human infants (9–14).
The calming and analgesic effects of orogustatory stimulation appear to be opioid-mediated. Sucrose- and milk-induced calming and antinociception are naltrexone reversible in rats (5, 6), and neither sucrose calming nor bradycardia can be obtained in human infants whose mothers used methadone during pregnancy (11). These opioid-mediated analgesic effects are not due to infusions alone because they are not produced by ingestion of water or lactose (15). In rats, maternal contact and nonnutritive suckling of anesthetized dams that do not release milk also produce analgesia (7). However, this form of analgesia is not opioid-mediated (4).
In the present study, we evaluated the effectiveness of orogustatory-orotactile antinociception in a rat model of persistent inflammatory hyperalgesia that mimics inflammatory conditions in humans. Because very little is known about the neural targets that mediate the analgesic effect of gustatory and nonnutritive suckling, Fos protein expression was used as a measure of neuronal activity (16) to determine whether the behaviorally induced analgesia in rat pups was mediated in part at the level of the spinal dorsal horn.
METHODS
Timed-pregnant Sprague–Dawley rats (Harlan, Indianapolis) were individually housed in standard polypropylene cages and remained there with their litters until the time of study on the 10th postnatal day. Both male and female pups were used. To induce inflammation and hyperalgesia, complete Freund’s adjuvant (CFA; suspended in a 1:1 oil/saline emulsion) was injected s.c. (0.01 ml, 0.005 mg Mycobacterium tuberculosis) into the dorsum of one forepaw or hindpaw. This mimics the persistent response to tissue injury produced by surgical intervention and traumatic injury in humans (17). Hyperalgesia did not appear to be disruptive: CFA-inflamed rats presented normal levels of behavior and activity. Those procedures have been approved by the Animal Care and Use Committee of the University of Maryland at Baltimore Dental School.
A polyethelene cannula (PE 10; Clay Adams) was placed in the rats’ lower jaw (18) 2–6 h before the start of the experiment. The 10-cm cannulae had a small flange (1.5 mm in diameter) formed at the tip by gentle heating and then pressing against a cool, flat surface. Implantation was accomplished with the aid of an 8-cm length of curved wire (0.255 mm in diameter). One end was friction-fit to the nonflanged end of the cannula, the other inserted beneath the animal’s tongue and maneuvered out of the ventral surface of the jaw. The cannula was lubricated with mineral oil before cannulation. The flanged end was seated in the mouth under the tongue, allowing the infusion of testing solutions. The entire procedure is routinely accomplished in about 20 sec in nonanesthetized infant rats. The discomfort produced by the procedure was only momentary. The rats exhibited normal suckling behavior and fed properly after the procedure.
The experimental room was maintained at 30–32°C. The thermal test consisted of very lightly touching the rat’s paw with a brass stylus (2.5–3.0 mm diameter) heated to 48°C with a variable transformer (7). The latency with which the infant removed its paw from the stylus was determined with a stop-watch. To prevent possible tissue damage a 60-sec cut-off was used. A nonheated stylus at room temperature served as a mechanical stimulus.
Sucrose solution (7.5%, 0.22 M) was mixed on a weight/volume basis in distilled water. Water or isotonic saline was used as a control for the passage of fluid through the oropharynx. Solutions were delivered via an infusion pump (Stoelting) at a rate of 0.01–0.06 ml/min through a 1-ml hypodermic syringe with a 23-gauge needle that was attached to a length of PE-50 tubing. The tubing was friction-fit to the individual pup’s cannula.
For immunocytochemistry, rats were deeply anesthetized with sodium pentobarbital and perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate buffer at pH 7.4. The C7-8 or L4-5 spinal cord was removed, placed in the same fixative for 1–3 hr at 4°C, and transferred to 30% sucrose (wt/vol) in phosphate buffer for several days for cryoprotection. Thirty micron-thick sections were sliced on a cryostat at -20°C. Free-floating tissue sections were processed following the avidin and biotinylated horseradish peroxidase complex protocol with a Vector ABC kit (Vector Laboratories). Anti-Fos antibody was used at 1:20,000 dilution (Oncogene Science).
To examine the effects of nonnutritive suckling and intraoral sucrose on CFA-induced Fos-like immunoreactivity (Fos-LI), pups were divided into two groups. Rats in one group suckled their anesthetized mother and received intermittent sucrose infusion on a 10-min-on/10-min-off cycle. Maternal contact and sucrose infusion were initiated 30 min before CFA injection and continued for 2 h after CFA injection. Control rats were isolated from the dam and littermates for an equivalent period of time before and after the CFA injection. The number of Fos-labeled nuclei was determined without knowledge of treatment. Five to 10 sections from each rat were quantified.
Data are presented as mean ± SEM. Statistical comparisons were made by ANOVA or Student’s t test. P < 0.05 was considered statistically reliable.
RESULTS
Naive Rats.
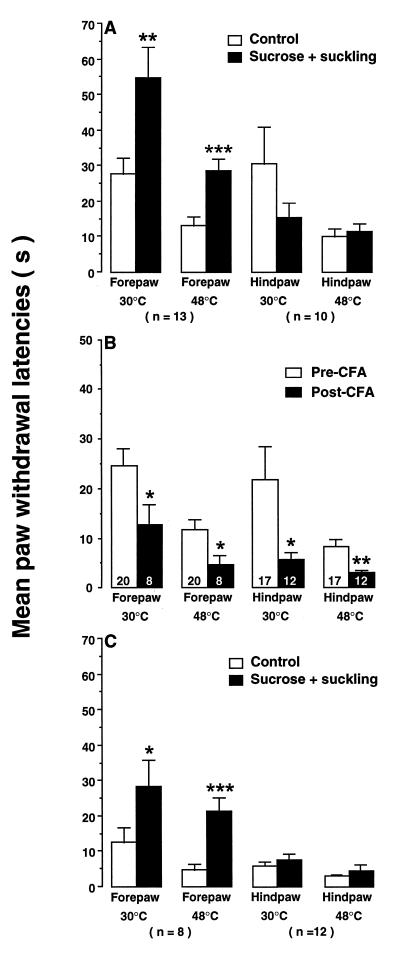
Ten-day-old rats exhibited well-developed forepaw and hindpaw withdrawal responses to both innocuous and noxious peripheral stimuli (Fig. 1A). The combination of nonnutritive suckling and intraoral sucrose markedly increased paw withdrawal latencies. In particular, 5–10 min of suckling in combination with 3 min of sucrose infusion doubled forepaw withdrawal latencies from 27.9 ± 4.3 to 54.8 ± 8.7 sec (n = 13, P < 0.01) to a 30°C stimulus and from 13.1 ± 2.7 to 28.7 ± 3.4 sec (n = 13, P < 0.001) to a 48°C stimulus (Fig. 1A). Forepaw withdrawal latencies remained elevated for 1–2 min after infusion termination. However, suckling-sucrose treatment did not influence hindlimb withdrawal latency. After the identical suckling and infusion episode, the hindpaw withdrawal latencies were not significantly different from control levels (Fig. 1A). The intraoral infusion of normal saline (n = 7) or distilled H2O (n = 6) without suckling did not produce any significant changes in paw withdrawal latencies from control values. These behavioral results confirm and extend previous findings that suckling and sucrose produce hypesthesia and analgesia in naive infant rats. It further suggested that there may be a differential maturation of descending inhibitory systems in 10-day-old rats.
Figure 1.
(A) Effects of suckling and intraoral sucrose on paw withdrawal latencies of naive 10-day-old rats. (B) Baseline paw withdrawal responses and CFA-induced hyperalgesia. The numbers in the bars indicate the number of rats in each group. All rats had an intraoral cannula. (C) Effects of suckling and intraoral sucrose on paw withdrawal latencies of forepaw-inflamed newborns. Note scale difference. Asterisks indicate significant differences from relevant control groups (open bars). ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001. Comparisons of filled bars for forepaw groups in C with open bars in A and B reveal that suckling-sucrose fully reversed the behavioral effects of CFA.
Rats with Inflamed Paws.
Withdrawal latencies in nontreated naive animals to a 30°C stimulus were 24.6 ± 3.3 sec (n = 20) and 21.7 ± 6.7 sec (n = 17) for forepaw and hindpaw, respectively. Response latencies in control rats to a noxious thermal stimulus (48°C) were 11.8 ± 2.0 sec (n = 20) and 8.3 ± 1.5 sec (n = 17) for forepaw and hindpaw, respectively (Fig. 1B). As in adult rats, CFA substantially reduced paw withdrawal latencies. Rats withdrew their inflamed forepaw with a latency of 12.8 ± 4.0 sec (n = 8, P < 0.05) from a 30°C stimulus and 4.8 ± 1.7 (n = 8, P < 0.05) from a 48°C stimulus. Withdrawal latencies of the inflamed hindpaws were also reduced to 5.8 ± 1.3 (n = 12, 30°C, P < 0.05) and 3.0 ± 0.4 sec (n = 12, 48°C, P < 0.01) (Fig. 1B). These findings strongly suggest that the neural mechanisms subserving thermal hyperalgesia and mechanical allodynia are functional by day 10.
In rats with unilateral forepaw inflammation, the combination of suckling plus sucrose infusion increased forepaw withdrawal latencies in response to thermal stimuli so that they equaled control (noninflamed) levels (Fig. 1). Response latency to a 30°C stimulus increased from 12.8 ± 4.0 to 28.5 ± 7.6 sec (n = 8, P < 0.05). Escape from noxious heating (48°C) was elevated 4-fold from 4.8 ± 1.7 to 21.5 ± 3.8 sec (n = 8, P < 0.001) (Fig. 1C). Suckling and sucrose infusion, however, did not influence withdrawal of the inflamed hindpaw. The modest increases in response latencies from 3.0 ± 0.4 to 4.7 ± 1.5 sec (n = 12) for 48°C and from 5.8 ± 1.3 to 7.6 ± 1.7 (n = 12) for 30°C were not statistically reliable (P > 0.05) (Fig. 1C).
Fos Protein Expression.
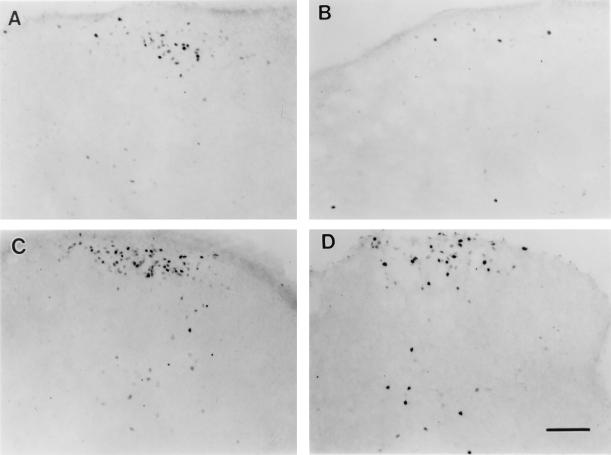
Fos-LI was induced in the somatotopically appropriate dorsal horn ipsilateral to the injection of CFA into forepaw or hindpaw (Fig. 2). The increased Fos-LI was predominantly located in the superficial laminae. A significant number of Fos-labeled neurons were also found in lamina V as well as laminae III/IV. Very few Fos-labeled nuclei were found contralateral to the side of inflammation. There was essentially no Fos-LI in the spinal cord of the naive rats. The induction of Fos-LI in the spinal dorsal horn suggests an increase in neuronal activity related to nociceptive processing following peripheral tissue inflammation.
Figure 2.
Photomicrographs illustrating the effects of intraoral sucrose and suckling on CFA-induced Fos-LI in the cervical (C7, A and B) and lumbar (L4, C and D) spinal cord of 10-day-old rats. The spinal dorsal horn ipsilateral to inflammation is shown with dorsum on top. (A and C) CFA-induced Fos-LI without intraoral sucrose and suckling. (B and D) CFA-induced Fos-LI with intraoral sucrose and suckling. (Bar = 0.1 mm.)
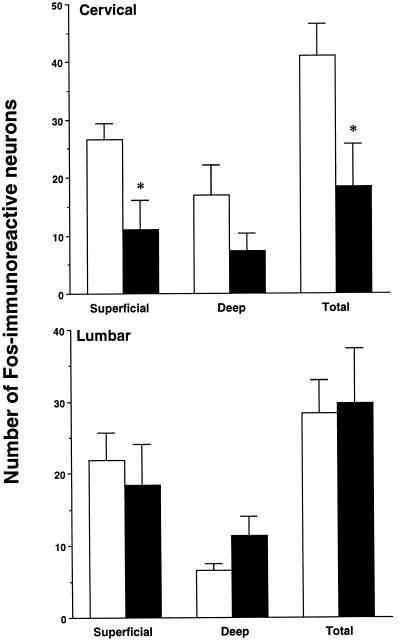
Suckling-sucrose infusion treatment caused a significant reduction in the number of Fos-positive nuclei in the cervical dorsal horn in forepaw-inflamed rats, when compared with the control pups (Figs. 2 and 3). The mean number of Fos-labeled nuclei per section decreased from 41.1 ± 5.5 (n = 3) to 18.5 ± 7.3 (n = 4) (P < 0.05). In contrast, the induction of Fos-LI in the lumbar dorsal horn after hindpaw inflammation was not significantly reduced by suckling and sucrose infusion (Figs. 2 and 3). Fos expression on the contralateral spinal cord was not affected by suckling and sucrose infusion.
Figure 3.
The effects of intraoral sucrose and suckling on the number of Fos-immunoreactive neurons in the cervical (C7-8) and lumbar (L4-5) spinal cord dorsal horn ipsilateral to forepaw (Upper) or hindpaw (Lower) inflammation. The mean numbers of Fos-labeled neurons from superficial (laminae I/II), deep (laminae V/VI), and whole dorsal horn are shown. The open bars show rats with inflammation only, and the filled bars show rats receiving sucrose and suckling after inflammation. Asterisks indicate significant differences between the two groups. ∗, P < 0.05.
DISCUSSION
These studies demonstrate that the development of persistent pain and hyperalgesia in 10-day-old rats can be attenuated by endogenous pain-modulatory systems activated by taste and orotactile stimulation. They confirm and extend previous behavioral findings on gustatory and orotactile-induced analgesia in response to acute nonnoxious and noxious stimuli. In addition, these studies demonstrate that endogenous pain-modulatory effects are ultimately mediated, at least in part, at the spinal level. Finally, we found evidence for a differential rostrocaudal maturation of these descending systems at the 10th postnatal day. The following discussion centers on the new findings and their implications.
New knowledge of injury-induced changes in the central nervous system was triggered by the development of adult animal models of tissue injury leading to persistent pain and hyperalgesia (19). These behavioral models mimic the persistent response to injury found in humans after surgery, trauma, or disease. The injection of inflammatory agents such as CFA or carrageenan into the hindpaw of the rat produces an intense inflammation characterized by edema, redness, and hyperalgesia that is restricted to the injected paw (20, 21). However, we know very little about the changes in the central nervous system that take place in the immature nervous system following inflammation, particularly at the level of the spinal cord. The present study has presented a model of persistent inflammatory pain and hyperalgesia that mimics pain in human infants to study the development of endogenous pain mechanisms and the ontogeny of pain inhibitory systems that are engaged by peripheral gustatory and orotactile inputs.
Withdrawal of the inflamed hyperalgesic forepaw was significantly inhibited by the combination of intraoral infusion of sucrose solution and nonnutritutive suckling. Future studies will need to analyze the contribution of each component including intraoral sucrose, nonnutritive suckling and maternal contact, and hyperalgesia attenuation. The present result is consistent with that obtained from noninflamed naive animals. Sugar solutions or milk produce antinociception in infant rats and enable them to better cope with pain and stress (15, 22). Sucrose produces pain-reducing effects in human newborns (13). Nonnutritive suckling also induces analgesia (7). These studies suggest that there is an interaction between the gustatory and orotactile afferent inputs and pain-modulating circuits in the brain.
The expression and distribution of Fos-LI has been used as a measure of neuronal activity, particularly in the spinal dorsal horn (16). There is a rapid and robust elevation in the number of dorsal horn neurons that express Fos-LI in their nuclei after nociceptive primary afferent activation by inflammation or other stimuli (23, 24). Morphine or other opioids given systemically or intraventricularly to adult rats reduce noxious stimulus-induced Fos-LI in the dorsal horn via descending mechanisms (25, 26).
Newborn rats exhibit Fos-LI after noxious stimulation from postnatal day 1 (27, 28). We now report that Fos expression in 10-day-old rats is selective and robust after ipsilateral paw inflammation. Moreover, inflammation-induced Fos expression at the cervical, but not the lumbar level, was significantly reduced by intraoral sucrose and suckling. This outcome parallels the results from behavioral studies and indicates that at least one target of these antinociceptive effects is at the level of the spinal cord.
There is considerable evidence that spinal nociceptive information is modulated by descending pain control systems in adult animals (see refs. 29–32 for reviews). Primary sites are located in the brain stem and include the midbrain periaqueductal gray and parabrachial area, pontine structures such as the locus coeruleus/subcoeruleus, and medullary structures such as nucleus raphe magnus and the lateral reticular nucleus. Gustatory and descending pathways converge at a number of their brain stem sites. The nucleus tractus solitarius (NTS) and parabrachial area are important components of gustatory pathways and also are sites of origin of descending inhibitory input (33, 34). The NTS has connections with nucleus raphe magnus (35). Electrical stimulation of NTS produces descending inhibition, and local anesthetic block of the NTS blocks inhibition produced by stimulation of the vagus nerve (36). Thus, gustatory and orotactile inputs may engage descending pain-modulating pathways resulting in inhibition of nociceptive transmission at the spinal level.
Failure of suckling-sucrose to completely inhibit Fos induction is of interest, because the behavioral treatments completely blocked the hyperalgesia induced by the inflammation. Thus, spinal mechanisms may not solely account for the behavioral effects. The role of supraspinal sites is supported by intracerebroventricular (i.c.v.) injections of β-casomorphin affecting escape responding, and especially by the blockade of systemic β-casomorphin effects on forepaw latency through i.c.v. injection of naloxone (37).
An important finding from the present study is that the forepaw and hindpaw hyperalgesia are differentially modulated by gustatory and orotactile inputs in 10-day-old rats. Although there was a clear inhibition of forepaw response and Fos induction by sucrose and suckling, the hindpaw withdrawal response and Fos expression remained unchanged. The absence of sucrose-induced effects on hindpaws suggests a rostrocaudal developmental difference in anatomy and physiology of descending pathways.
Most of the descending pathways originating in the brain stem and traveling via the dorsolateral funiculus in the spinal cord are in place as early as neonatal day 6 and are comparable to the adult pattern (38). Electrophysiology studies, however, reveal that activation of these pathways did not affect lumbar spinal dorsal horn neurons until neonatal day 10, with patterns similar to the adult profile not appearing until neonatal days 22–24 (38). The delay in functional activation of descending pathways may be due in part to delayed maturation of spinal interneurons or to insufficient levels of serotonin or norepinephrine in descending axons (38). The maturation of the serotonin projection follows a rostral-to-caudal gradient with the adult pattern appearing at 14 days in the cervical cord and 21 days in the thoracic and lumbar cord (39). Norepinephrine also appears early in the spinal cord and steadily increases until postnatal day 14 and then decreases (40, 41). The density in the cervical spinal cord is greater than in the lumbar cord at all stages (40, 41). Because spinal monoamines are significantly involved in descending inhibition of nociception, the insufficiency of these neurotransmitters at the caudal spinal level in 10-day-old rats would impair inhibitory mechanisms, thus sparing the effects of sucrose and suckling on hindpaw sensitization.
In summary, these findings support the hypothesis that gustatory- and orotactile-induced analgesia in rat pups is mediated, in part, via supraspinal descending pathways that modulate hyperexcitability in the spinal dorsal horn. The endogenous descending pathways are not fully developed in the newborn, and differential analgesic effects are demonstrable across spinal segments in early postnatal life. This model can be used for further studies on developmental modulation of nociception in newborn rats. In addition, these studies will lead to a better understanding of the development of analgesic mechanisms and provide new clinical approaches for engaging endogenous analgesic mechanisms in premature, newborn, and young infants.
Acknowledgments
We thank Mrs. E. Wade for technical assistance. This research was supported by National Institutes of Health Grants DE11964, DA10275, and HD28245, and Research Scientist Award MH00524.
Footnotes
Abbreviations: CFA, complete Freund’s adjuvant; Fos-LI, Fos-like immunoreactivity.
References
- 1.Schechter N L. Pediatr Clin North Am. 1989;36:781–794. doi: 10.1016/s0031-3955(16)36721-9. [DOI] [PubMed] [Google Scholar]
- 2.Purcell-Jones G, Dormon F, Sumner E. Pain. 1988;33:181–187. doi: 10.1016/0304-3959(88)90089-9. [DOI] [PubMed] [Google Scholar]
- 3.Blass E M. Acta Paediatr (Stockholm) Suppl. 1994;397:71–76. doi: 10.1111/j.1651-2227.1994.tb13268.x. [DOI] [PubMed] [Google Scholar]
- 4.Blass, E. M. (1996) Regul. Pept., in press. [DOI] [PubMed]
- 5.Blass E M, Fitzgerald E, Kehoe P. Pharmacol Biochem Behav. 1987;26:483–489. doi: 10.1016/0091-3057(87)90153-5. [DOI] [PubMed] [Google Scholar]
- 6.Blass E M, Fitzgerald E. Pharmacol Biochem Behav. 1988;29:9–13. doi: 10.1016/0091-3057(88)90266-3. [DOI] [PubMed] [Google Scholar]
- 7.Blass E M, Shide D J, Zaw-Mon C, Sorrentino J. Behav Neurosci. 1995;109:342–353. doi: 10.1037//0735-7044.109.2.342. [DOI] [PubMed] [Google Scholar]
- 8.Shide D J, Blass E M. Behav Neurosci. 1989;103:1168–1175. doi: 10.1037//0735-7044.103.6.1168. [DOI] [PubMed] [Google Scholar]
- 9.Barr R G, Quek V, Cousineau D, Oberlander T F, Brian J A, Young S N. Dev Med Child Neurol. 1994;36:608–618. doi: 10.1111/j.1469-8749.1994.tb11898.x. [DOI] [PubMed] [Google Scholar]
- 10.Blass, E. M. (1996) Pediatrics, in press.
- 11.Blass E M, Ciaramitaro V. Monogr Soc Res Child Dev. 1994;59:1–81. [PubMed] [Google Scholar]
- 12.Blass E M, Hoffmeyer L B. Pediatrics. 1991;88:1287–1288. [PubMed] [Google Scholar]
- 13.Blass E M, Shah A. Chem Senses. 1995;20:29–35. doi: 10.1093/chemse/20.1.29. [DOI] [PubMed] [Google Scholar]
- 14.Smith V A, Fillion T J, Blass E M. Dev Psychol. 1990;26:731–737. [Google Scholar]
- 15.Blass E M, Shide D J. Chem Senses. 1994;19:239–249. doi: 10.1093/chemse/19.3.239. [DOI] [PubMed] [Google Scholar]
- 16.Hunt S P, Pini A, Evan G. Nature (London) 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- 17.Dubner R. In: Textbook of Pain. Melzack R, Wall P D, editors. Edinburgh: Churchill Livingstone; 1994. pp. 293–302. [Google Scholar]
- 18.Hall W G. J Comp Physiol Psychol. 1979;93:977–1000. doi: 10.1037/h0077635. [DOI] [PubMed] [Google Scholar]
- 19.Dubner R. In: Proceedings of the VIth World Congress on Pain. Bond M R, Charlton J E, Woolf C J, editors. Amsterdam: Elsevier; 1991. pp. 264–276. [Google Scholar]
- 20.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 21.Iadarola M J, Brady L S, Draisci G, Dubner R. Pain. 1988;35:313–326. doi: 10.1016/0304-3959(88)90141-8. [DOI] [PubMed] [Google Scholar]
- 22.Blass E M, Jackson A M, Smotherman W P. Behav Neurosci. 1991;105:677–686. doi: 10.1037/0735-7044.105.5.667. [DOI] [PubMed] [Google Scholar]
- 23.Menétrey D, Gannon A, Levine J D, Basbaum A I. J Comp Neurol. 1989;285:177–195. doi: 10.1002/cne.902850203. [DOI] [PubMed] [Google Scholar]
- 24.Abbadie C, Besson J-M. Brain Res. 1993;607:195–204. doi: 10.1016/0006-8993(93)91507-o. [DOI] [PubMed] [Google Scholar]
- 25.Presley R W, Menétrey D, Levine J D, Basbaum A I. J Neurosci. 1990;10:323–335. doi: 10.1523/JNEUROSCI.10-01-00323.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gogas K R, Presley R W, Levine J D, Basbaum A I. Neuroscience. 1991;42:617–628. doi: 10.1016/0306-4522(91)90031-i. [DOI] [PubMed] [Google Scholar]
- 27.Williams S, Evan G, Hunt S P. Neurosci Lett. 1990;109:309–314. doi: 10.1016/0304-3940(90)90013-y. [DOI] [PubMed] [Google Scholar]
- 28.Yi D K, Barr G A. Pain. 1995;60:257–265. doi: 10.1016/0304-3959(94)00119-y. [DOI] [PubMed] [Google Scholar]
- 29.Fields H L, Basbaum A J. Annu Rev Physiol. 1978;40:217–248. doi: 10.1146/annurev.ph.40.030178.001245. [DOI] [PubMed] [Google Scholar]
- 30.Gebhart G F. In: Spinal Afferent Processing. Yaksh T L, editor. New York: Plenum; 1986. pp. 391–416. [Google Scholar]
- 31.Besson J-M, Chaouch A. Physiol Rev. 1987;67:67–186. doi: 10.1152/physrev.1987.67.1.67. [DOI] [PubMed] [Google Scholar]
- 32.Stamford J A. Br J Anaesth. 1995;75:217–227. doi: 10.1093/bja/75.2.217. [DOI] [PubMed] [Google Scholar]
- 33.Hayes R L, Katayama Y, Watkins L R, Becker D P. Brain Res. 1984;311:267–280. doi: 10.1016/0006-8993(84)90089-1. [DOI] [PubMed] [Google Scholar]
- 34.Lewis J W, Baldrighi G, Akil H. Brain Res. 1987;424:65–70. doi: 10.1016/0006-8993(87)91193-0. [DOI] [PubMed] [Google Scholar]
- 35.Beitz A J. Neuroscience. 1982;7:2753–2768. doi: 10.1016/0306-4522(82)90098-7. [DOI] [PubMed] [Google Scholar]
- 36.Ren K, Randich A, Gebhart G F. J Neurophysiol. 1990;63:971–986. doi: 10.1152/jn.1990.63.5.971. [DOI] [PubMed] [Google Scholar]
- 37.Blass E M, Blom J. Pediatr Res. 1996;39:199–203. doi: 10.1203/00006450-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Fitzgerald M, Koltzenburg M. Dev Brain Res. 1986;24:261–270. doi: 10.1016/0165-3806(86)90194-x. [DOI] [PubMed] [Google Scholar]
- 39.Bregman B S. Dev Brain Res. 1987;34:245–263. doi: 10.1016/0165-3806(87)90213-6. [DOI] [PubMed] [Google Scholar]
- 40.Commissiong J W. Brain Res. 1983;264:197–208. doi: 10.1016/0006-8993(83)90817-x. [DOI] [PubMed] [Google Scholar]
- 41.Commissiong J W. Dev Brain Res. 1983;11:75–92. doi: 10.1016/0165-3806(83)90203-1. [DOI] [PubMed] [Google Scholar]