Abstract
Gonadal steroid hormones regulate sexually dimorphic development of brain functions and behaviors. Their nuclear receptors offer the opportunity to relate molecular events in neurons to simple instinctive mammalian behaviors. We have determined the role of estrogen receptor (ER) activation by endogenous estrogen in the development of male-typical behaviors by the use of transgenic estrogen-receptor-deficient (ERKO) mice. Surprisingly, in spite of the fact that they are infertile, ERKO mice showed normal motivation to mount females but they achieved less intromissions and virtually no ejaculations. Aggressive behaviors were dramatically reduced and male-typical offensive attacks were rarely displayed by ERKO males. Moreover, ER gene disruption demasculinized open-field behaviors. In the brain, despite the evident loss of functional ER protein, the androgen-dependent system appears to be normally present in ERKO mice. Together, these findings indicate that ER gene expression during development plays a major role in the organization of male-typical aggressive and emotional behaviors in addition to simple sexual behaviors.
Keywords: sexual behavior, aggression, open-field behavior, androgen receptors, aromatization
It is well established that gonadal steroid hormones, by acting on the central nervous system, regulate various neuroendocrine events related to reproduction and reproductive behaviors in both sexes. In males, testosterone is a major hormone that facilitates both sexual and aggressive behaviors. The mechanisms underlying the behavioral effects of testosterone, however, are complicated by the fact that endogenous testosterone can act not only through the androgen receptor (AR) as can testosterone metabolite (5α-reduced dihydrotestosterone), but also can act through estrogen receptors (ER) after being aromatized to estradiol in the target tissues including the brain. The relative importance of AR and ER in the facilitation of male sexual behaviors by testosterone has been studied (1, 2), but a clear role for ER gene expression needs to be determined.
Aggressive behaviors in male mice can be regulated in adulthood by both AR- and ER-dependent mechanisms depending on the genotype of the animals (3). Some evidence for the involvement of estrogen-dependent mechanisms in male aggressive behaviors has been obtained by comparing relative efficiencies of testosterone, dihydrotestosterone, and estrogen (4, 5) and by concurrent injection of an aromatase inhibitor with testosterone (6, 7). These findings, however, do not provide direct evidence for the role of the ER gene product itself. Furthermore, the antiestrogen tamoxifen gave inconsistent behavioral effects; both inhibitory (8) and facilitatory (9) effects on aggression in male mice have been reported. Until recently, there was never a direct way to manipulate endogenous steroid receptor function to determine the behavioral role of ER.
This became possible through the development of ER-deficient mutant mice (ERKO) by the use of homologous recombination techniques (10–12) .
Using ERKO mice, we have assessed more directly the role of ER gene-dependent brain mechanisms in the development and expression of male-typical sexual and aggressive behaviors. We also tested the same animals for their open-field behaviors. Because sexual dimorphisms in open-field behaviors assessed in adulthood (13–15) are determined by estrogen in the neonatal period, not by hormonal conditions at the time of the testing (16, 17), we hypothesized that the ERKO mice might produce female-type open-field behaviors.
MATERIALS AND METHODS
Mice.
Male transgenic ER-deficient mice (ERKO) and their wild-type (WT) and heterozygous (HZ) littermates were used. Mice are in mixed background of C57BL/6J and 129. Details of ER gene disruption and production of subsequent ER-deficient mice are described elsewhere (10). Mice were maintained in the breeding colony at the National Institute on Environmental Health Sciences by crosses of heterozygotes, which produced all three genotypes with the ratio matched to that expected from a Mendelian cross. Between 14 and 25 weeks of age mice were transferred to the Rockefeller University in five different shipments over the 18-month period. They were then individually housed in plastic cages (30 cm × 20 cm × 13 cm) throughout the studies and maintained on a 12-hr light/12-hr dark cycle with constant temperature (22°C). Food and water were available ad libitum.
The same mice were used for a number of different behavioral tests, which were done in the order of (i) open-field behaviors, (ii) test 1 of 30-min sexual behavior tests, (iii) test 1 of resident–intruder paradigm aggression tests, (iv) test 2 of 30-min sexual behavior tests, (v) test 2 of resident–intruder paradigm aggression tests. After the completion of these tests, some animals were also tested for sexual behaviors for 3 hr or aggression in homogeneous set paradigm. All behavioral tests were done under white light during the dark phase of a light/dark cycle starting at 4 hr after lights off and videotaped for further analysis.
Male Sexual Behavior Tests.
Male sexual behaviors were tested twice (with an interval of 6–12 days) between 16 and 28 weeks of age. Male sexual behavior was measured during a 30-min behavioral test with a Swiss–Webster female mouse [(SW)fBR purchased from Taconic Farms) in the male’s home cage. All females were ovariectomized and primed with 10 μg of estradiol benzoate (for at least 48 hr) and 500 μg of progesterone (for at least 4 hr) to ensure high sexual receptivity. For each male, the numbers and latencies of mounts, intromissions, and ejaculations were recorded. After the completion of two 30-min sexual behavioral tests, some animals (WT, n = 12; ERKO, n = 11) mice were tested once more for 3 hr to examine whether ERKO mice might show ejaculation.
Aggression Tests with a Resident–Intruder Paradigm.
Aggressive behaviors were tested twice (with an interval of 6–12 days) between 16 and 28 weeks of age in a resident–intruder paradigm. Each male was tested in its home cage (as a resident) against a group-housed (4–5 mice per cage) olfactory bulbectomized male intruder mouse (Swiss–Webster) for 15 min. Expression of aggression in mice is mainly regulated by olfactory cues, and therefore olfactory bulbectomized intruders rarely show aggression. However, since their gonads are intact, they can elicit aggressive behaviors from resident mice (18, 19). By testing against olfactory bulbectomized intruder mice, therefore, aggressive behaviors of resident animals, which were not influenced by any defeated experience, were measured. For each experimental male, cumulative duration of aggressive bouts were scored. An aggressive bout was defined as a continuous series of behavioral interactions including at least one aggressive behavioral act (see below). Three seconds was the maximum amount of time that could elapse between aggressive behavioral acts to be considered part of the same aggressive bout: if intervals between the occurrences of two behavioral aggressive acts exceeded 3 sec, the two behavioral acts were scored as two separate aggressive bouts. Chasing, boxing, tail rattling, biting, offensive attack (often accompanied by biting and wrestling), previously shown to be typical for intermale (male vs. male) aggression (18, 20) were defined as aggressive behavior acts. Because some mice never showed offensive attacks, aggressive bouts with and without offensive attacks were counted separately.
Homogeneous Set Tests for Aggressive Behaviors.
Pairs of body-weight matched males from the same genotype were tested in a clean neutral cage (30 cm × 20 cm × 13 cm) for 2 consecutive days. They were first placed in either side of the test cage, which was separated by a transparent acrylic board in the center. After a 5-min adaptation period, the divider was removed and males were tested for aggression for 15 min. For each pair, cumulative durations of aggressive bouts with or without offensive attacks (see above) were scored.
Open-Field Behavior Tests.
Animals were tested for 5 min for 3 consecutive days in an open-field apparatus (90 cm × 60 cm, 50-cm high wall), which was illuminated with two white lights from the top. The floor of the apparatus was divided into 54 squares (10 cm × 10 cm) by black lines. An area consisting of the inner 28 squares, which did not attach to the wall of the apparatus, was designated as the center area. At the beginning of the test, a mouse was placed gently in a corner square with his head facing the corner. The number of line crossings (counted as one if all four paws crossed a line), the number of line crossings in the center area, the number of leanings (mice that stood up on their hindlegs against the wall) or rearings (mice that stood up on their hindlegs without touching the wall), and the number of defecations (boluses) and urinations (pools) were measured.
Immunocytochemistry for ER and AR and Aromatase.
Mice were deeply anesthetized and perfused transcardially with (i) 100 mM of phosphate-buffered saline (PBS) containing 0.1% heparin, pH 7.2 and (ii) 4% paraformaldehyde in 100 mM of phosphate-buffer (PB), pH 7.2. The brains and spinal cords were removed, postfixed in 4% paraformaldehyde in PB, and stored for 24 hr at 4°C in PB containing 30% sucrose. Brain tissues were cut at 30 μm and spinal cord tissue blocks containing the lumber and sacral segments were cut at 50 μm on a freezing microtome. Free-floating sections were incubated in (i) either anti-ER antibody, ER21 (gift of G. Greene, University of Chicago), anti-AR antibody, PG21 (Affinity BioReagent, Neshanic Station, NJ), or anti-aromatase (gift of N. Harada, Fujita Health University) in 50 mM Tris-buffered saline (TBS), pH 7.2, containing 0.5% Triton X-100 and 4% normal goat serum (Vector Laboratories) for 48–72 hr at 4°C; (ii) a 1:200 dilution of the biotinylated horse anti-rabbit secondary antibody (Vector Laboratories) in TBS containing 0.5% Triton X-100 and 4% of normal goat serum for 120 min at room temperature; and (iii) the avidin–biotin complex (Vectastain ABC Elite kit, Vector Laboratories) in TBS containing 0.5% Triton X-100 for 60 min at room temperature. Sections were treated with 0.05% diaminobenzidine and 0.03% hydrogen peroxide in TBS (pH 7.8). Control conditions involved either preabsorption of antibody with antigen protein or omitting the primary antibody from the staining procedure.
Statistics.
Data were analyzed by a two-way ANOVA for repeated measurements for the main effects of genotype and test day and their interaction, followed by post hoc one-way ANOVAs on each test day, if necessary. Data of nonrepeated measurements were analyzed by one-way ANOVAs. Tukey’s test was used for post hoc pairwise comparisons at α = 0.05. Differences in the percentage of animals showing certain behavior were tested with χ2 test.
RESULTS
Male Sexual Behaviors.
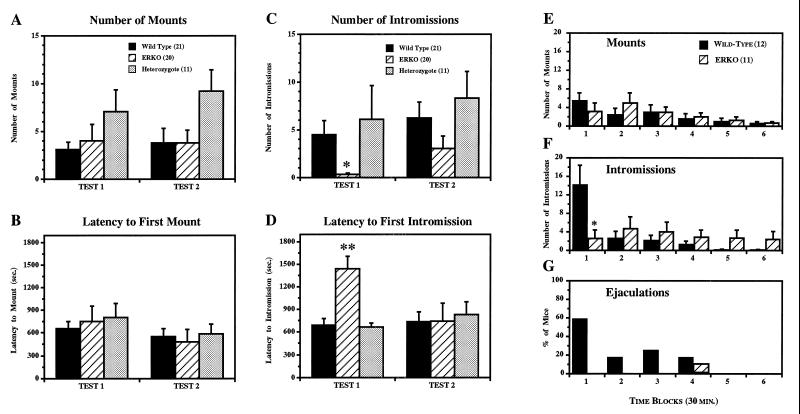
ER gene disruption differentially affected the expression of three discrete components of male sexual behaviors, i.e., mounts, intromissions, and ejaculations. Under our conditions 67%, 50%, and 82% of WT, ERKO, and HZ mice, respectively, showed mounts at least once during two 30-min tests. The mean number of mounts (Fig. 1A) and the mean latency to the first mounts (Fig. 1B) were also not different among the three groups in either test. On the other hand, intromissions were reduced in ERKO mice (especially in the first test) both according to frequency (Fig. 1C) and the latency to the first intromission (Fig. 1D). The percentage of animals that showed intromissions were also much lower in ERKO mice (25%) compared with WT (62%) and HZ (73%) mice [χ2(2) = 8.463, P < 0.05]. Moreover, ejaculations were never observed in ERKO mice in any of the tests, in contrast to the 24% and 38% of WT mice which ejaculated in the first and second tests, respectively. This ERKO male failure may be related to the fact that in ERKO mice sometimes showed extravaginal pelvic thrust patterns: they could stimulate the female’s flanks with their forepaws to induce a strong lordosis posture by females but they could not hold their hind legs tightly to the female’s rump during thrusting movements.
Figure 1.
Effects of ER gene disruption on male sexual behaviors. During 30-min sexual behavior tests, there were no differences in mean number of mounts (A) and mean latency to the first mounts (B; which included only the mice that showed the behavior) between three genotypes. In contrast, there were overall genotype differences in mean number of intromissions [C; F(2,49) = 3.930, P < 0.05] and ERKO mice showed significantly fewer intromissions compared with HZ, but not with WT, mice at α = 0.05 (∗). Mean latency to the first intromissions (D; which included only the mice that showed the behavior) of ERKO mice was significantly longer compared with both WT and HZ mice in Test 1 [F(2,15) = 11.473, P < 0.001; ∗∗, significantly different from both WT and HZ at α = 0.05], but not in Test 2. Temporal changes of mean number of mounts (E), mean number of intromissions (F), and percentage of mice ejaculated (G) during 3-hr tests were analyzed for six time blocks of 30 min each. ERKO mice showed intromissions continuously during the entire 3-hr test period but did not ejaculate, whereas WT mice showed a high number of intromissions (∗, P < 0.05) and ejaculated in the first 30 min. Total numbers of mounts (WT vs ERKO; mean ± SEM, 13.92 ± 5.60 vs. 14.82 ± 5.49) and intromissions (20.08 ± 5.87 vs. 19.00 ± 8.53) during the 3-hr test period were not different between WT and ERKO mice. Vertical bars represent SEM.
The absence of ejaculation in ERKO mice was confirmed in additional sexual behavioral tests, which were done for an extended 3-hr period (Fig. 1 E–G). In these tests, ERKO mice continued to show both mounts (Fig. 1E) and intromissions (Fig. 1F) for the entire 3-hr test period. Nevertheless, ERKO mice rarely ejaculated (Fig. 1G): only one mouse (out of seven ERKO mice that showed at least one mount during 3-hr tests) ejaculated for a short duration (only 14 sec) with a long latency (118 min and 30 sec). In contrast, WT mice showed most intromissions during the first 30 min (Fig. 1F), which resulted in ejaculation in 60% of animals (Fig. 1G). Overall, 83% of WT mice ejaculated at least once and 40% of WT mice ejaculated twice during the 3-hr tests.
Aggressive Behaviors.
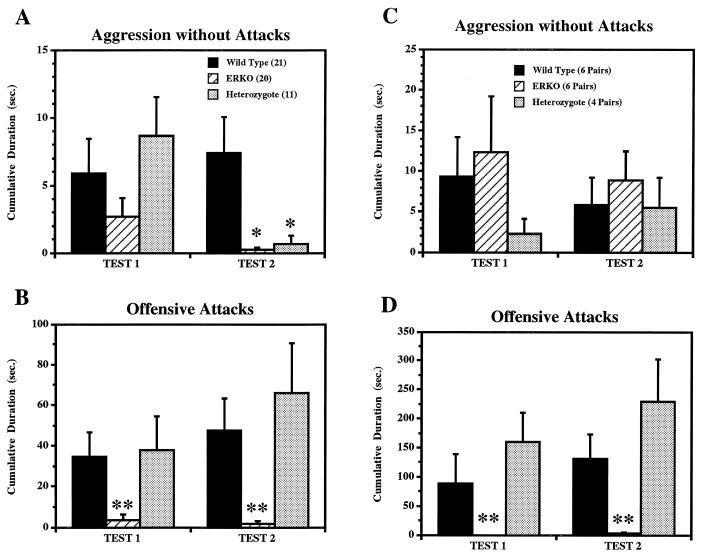
Aggressive behaviors in the resident–intruder paradigm were greatly reduced in ERKO mice compared with WT and HZ mice (Fig. 2 A and B). ERKO mice rarely showed offensive attacks toward the intruders (Fig. 2B) and showed very few aggressive behaviors even without offensive attacks (Fig. 2A; HZ mice also showed significantly lower levels of aggression in the second test compared with WT mice due to their increase of vigorous offensive attacks). Instead of aggression, some ERKO mice showed attempted mounts toward opponent male mice (data not shown). In contrast, both WT and HZ mice showed vigorous offensive attacks toward olfactory bulbectomized intruder mice (Fig. 2B), which themselves were never aggressive.
Figure 2.
Effects of ER gene disruption on aggressive behaviors tested in two different paradigms, resident–intruder tests (A and B) and homogeneous set tests (C and D). Cumulative durations of aggressive bouts with (B and D) and without (A and C) offensive attacks are shown separately with different scales. ERKO mice showed very few aggressive behaviors even without attacks in resident–intruder tests (A; ∗, significantly different from WT mice at α = 0.05), whereas in the homogeneous set tests there were no differences between three genotypes (C). On the other hand, in both tests, cumulative duration of offensive attacks (B and D) were greatly reduced in ERKO mice compared with both WT and HZ mice (∗∗, α = 0.05).
Reduced levels of aggression were also observed in “homogeneous set” aggression tests, which were performed after the completion of all the other behavioral tests (Fig. 2 C and D). Compared with the resident–intruder paradigm, more social behavioral interactions, as well as aggressive behaviors (note differences of y-axis scale between Fig. 2 A and B vs. C and D) were observed in all three genotype groups. Cumulative duration of aggression without offensive attacks shown by ERKO mice in this test paradigm was equivalent to those shown by WT and HZ mice (Fig. 2C). ERKO mice mainly showed “lunge and bite” or “chase and bite” in contrast to tail rattling or boxing typically seen in WT or HZ mice. ERKO mice, however, rarely showed offensive attacks in this testing paradigm (Fig. 2D), similar to the findings in the resident–intruder paradigm (Fig. 2B). Prolonged aggressive behavioral interactions consisting of vigorous offensive attacks, which were often seen in both WT or HZ pairs, were never observed in ERKO pairs.
Open-Field Behaviors.
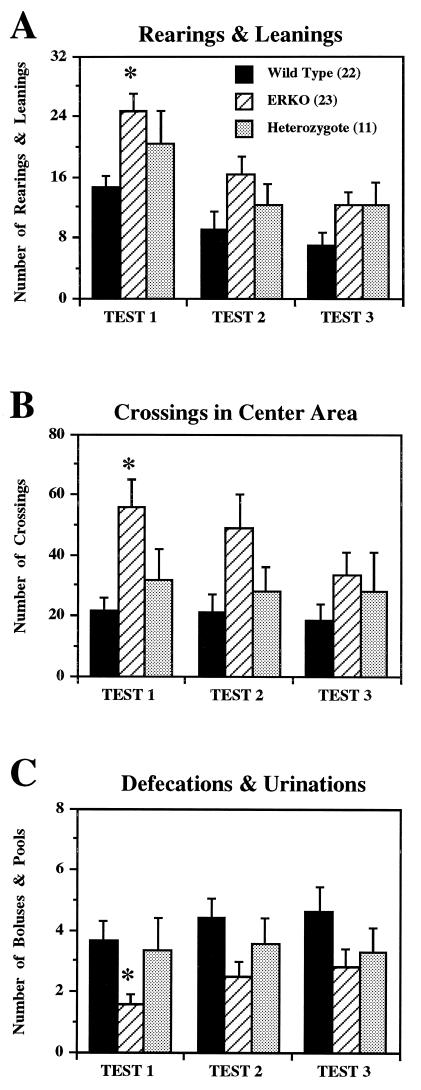
ER gene disruption significantly modified open-field behaviors. In comparison with WT male mice, ERKO male mice exhibited female-type open-field behaviors in all three measurements. First, ERKO male mice were more active than WT male mice as revealed by a significantly higher number of rearing and leaning behaviors, compared with WT mice (Fig. 3A). The number of total line crossings also tended to be higher in ERKO mice compared with WT mice (data not shown). Second, ERKO mice entered the center area and crossed lines significantly more often compared with WT mice (Fig. 3B). Third, ERKO mice showed fewer defecations/urinations compared with WT mice (Fig. 3C). The mean number of these three behaviors in HZ mice were between ERKO and WT mice and were not significantly different from either genotype.
Figure 3.
Effects of ER gene disruption on open-field behaviors in 5-min tests over 3 consecutive days. There were overall significant genotype differences in the mean number of rearings and leanings [A; F(2,53) = 3.731, P < 0.05], mean number of crossings in the center area [B; F(2,53) = 3.840, P < 0.05], and mean number of defecations and urinations [C; F(2,53) = 3.828, P < 0.05]. ERKO mice showed female-type open-field behaviors compared with WT mice (∗, significantly different from WT, but not HZ, mice at α = 0.05), i.e., higher number of rearings and leanings, entered more often to the center area, and defecated less. In these three measurements, there was also a significant day effect but no interaction with genotype: in all three genotype groups, the number of rearings/leanings and the number of crossings in the center area deceased, and the number of defecations/urinations increased over the 3 days of tests.
Distribution of ER–Immunoreactive (IR) Cells.
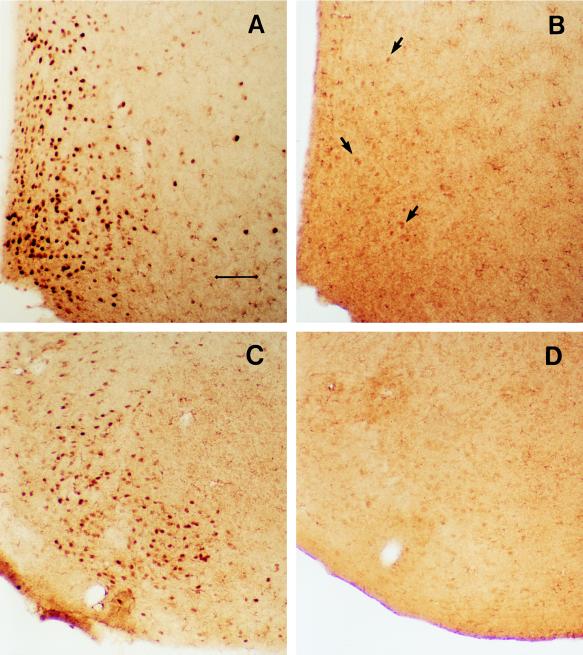
Conventional strong nuclear ER-like immunostaining was not apparent in ERKO mice in any brain region in which abundant ER positive cells were found in WT mice (Fig. 4 and Table 1). Immunostaining with ER21 antibody (a rabbit polyclonal antiserum directed at the N terminal of the ER protein), however, was detected in a small number of cells in the medial preoptic area (MPOA) of ERKO mice; the total number of ER–IR cells there being greatly reduced in ERKO mouse MPOA compared with WT mouse MPOA (Fig. 4 A and B). Because this low level of immunostaining detected in ERKO mouse MPOA tissues could be blocked by preabsorbing the ER21 antibody with ER protein, these immunoreactive cells may have contained residual levels of ER-like protein derived from splicing variants (see below). In contrast to ER–IR cells, the distributions of AR–IR (Table 1) and aromatase–IR cells (data not shown) were very similar between WT and ERKO mice.
Figure 4.
Photomicrographs showing the ER–IR cells (stained with polyclonal ER21 antibody) in the medial preoptic area and the ventromedial nucleus of hypothalamus of WT (A and C) and ERKO (B and D) mouse brains. The number of ER–IR cells was greatly reduced in ERKO mouse brains but not completely eliminated in the medial preoptic area as indicated with arrows in B. (Scale bar = 75 μm.) Despite the evident loss of ER–IR cells, there was no overall difference on the distribution of AR–IR cells between ERKO and WT mice (see Table 1).
Table 1.
Distribution of ER–IR and AR–IR cells
| ER
|
AR
|
|||
|---|---|---|---|---|
| WT | ERKO | WT | ERKO | |
| Medial preoptic area | + | ± | + | + |
| Ventromedial N. | + | − | + | + |
| Arcuate N. | + | − | + | + |
| Paraventricular N. | + | − | + | + |
| Anterior hypothalamus | + | − | + | + |
| Lateral hypothalamus | − | − | + | + |
| Ventral premammillary N. | − | − | + | + |
| Lateral septum | − | − | + | + |
| Bed N. stria terminalis | + | − | + | + |
| Medial N. amygdala | + | − | + | + |
| Cortical N. amygdala | + | − | − | − |
| Midbrain central gray | + | − | + | + |
| Dorsal lumbar spinal cord | + | − | − | − |
| Ventral lumbar spinal cord | − | − | + | + |
+, Present; −, absent; ±, residual binding?
DISCUSSION
Behavior.
This study has revealed a number of unexpected behavioral effects of ER gene disruption. First, despite the fact that ERKO mice are infertile (21), male sexual behaviors were far from abolished in ERKO mice. Many ERKO mice showed frequencies and latencies of mounts equivalent to WT mice. Furthermore, during 3-hr sexual behavior tests, we found that some ERKO males continuously mounted, thus displaying sexual motivation equivalent to WT males, and intromitted during the entire 3 hr, but these behaviors rarely resulted in ejaculation. We infer that ER gene disruption more strongly affected the efficiency of intromissions and the induction of ejaculation itself than it affected the motivational component of sexual behaviors. Tests of olfactory responses (to receptive female urine) in ERKO males also suggest a normal motivational component (22). Moreover, our findings that both intact (in this study) as well as dihydrotestosterone propionate-treated (unpublished observation) ERKO male mice often showed extravaginal pelvic thrusting provide a mechanism for the failure of ejaculation.
This pattern of findings in ERKO males is more complex than with ERKO females, who simply never show lordosis behavior (23). Surprisingly, ER gene expression appears to be necessary for full expression of both female and male sex behavior repertoires.
Second, aggressive behaviors were profoundly affected by ER gene disruption. In two different testing paradigms, it was found that ERKO mice were much less aggressive compared with both WT and HZ mice. Importantly, ER gene disruption not only decreased the occurrence of aggressive behaviors, but also modified the behavioral pattern of aggression. Aggressive behavioral patterns of ERKO male mice observed in homogeneous set tests were mild and short lasting, in contrast to male-typical aggression consisting of prolonged vigorous offensive attacks, including biting and wrestling. These aggressive behavioral patterns of ERKO males (i.e., lunge and bite or chase and bite) are very similar to those exhibited by genetically female mice (24). Taken together, these findings suggest that development and expression of male-typical aggressive behaviors are strongly controlled by the ER-dependent brain mechanisms.
Open-field behaviors of ERKO mice were modified in the expected direction, i.e., toward female type. For all four behavioral measurements, ERKO male mice showed a trend toward behavior typical of WT female mice (unpublished data) in comparison to WT male mice.
Hormones and Receptors.
With ER21 antibody, which has been used for the immunocytochemical detection of ER in neural tissues (25), we detected no ER–IR cells in any brain regions except in the MPOA. The ER21 antibody is specific for an epitope on the N terminal of the ER protein and there is no sequence similarity with the recently described ER–beta isoform (26). Therefore, it is unlikely that immunostaining found in this brain region is ER–beta isoform. Detection of a small amount of protein with antibody ER21 can be due to the E1 splicing variant (27), but also a truncated chimeric-mutant protein form in the ER from the disrupted gene. This chimeric protein would be the translation product of mRNA from the disrupted gene containing the 5′ portion of exon 2 and the inserted Neo protein sequence. This protein would be nonfunctional, but would contain the 5′ region of exon 2 encoding the epitope for the antibody.
It should be noted that reduced levels of sexual (ejaculation) and aggressive behaviors in ERKO mice were not due to the reduced levels of testosterone in these mice. In fact, serum testosterone levels at 20 weeks are slightly elevated in ERKO mice compared with both WT and HZ mice (21), probably due to the lack of negative feedback. Moreover, in spite of the loss of functional ER, the androgen-dependent system would seem normal in ERKO mice. Our studies revealed that there were no obvious differences in the distribution of AR–IR and aromatase–IR cells between WT and ERKO mice. AR–IR cells eliminated by castration were also restored by testosterone propionate or dihydrotestosterone propionate to the same extent in both WT and ERKO mice (as in our preliminary studies). In addition, it was found that sexual dimorphic spinal nucleus of bulbocavernosus, which is known to be regulated by AR-mediated action of testosterone (28), exists in ERKO mice, as well as WT and HZ mice.
Together these findings suggest that behavioral changes found in this study in ERKO mice were indeed consequences of ER gene disruption. It has not been determined, however, whether behavioral modifications were due to the lack of ER activation at the time of the testing in adulthood or due to the interruption of male-typical brain development during the neonatal period.
Genetic Background.
This study was done over an 18-month period on five separate groups of animals derived from the colony at the National Institute of Environmental Health Sciences, which were maintained by crosses of heterozygotes in each generation. Detailed analysis of the behavioral data revealed that the levels of sexual behaviors varied across generations. For example, the number of intromissions, decreased in both WT and ERKO mice from the latest generations, compared with the earlier generations. Since this trend was not obvious in HZ mice, it cannot be simply due to environmental variations. There was also an indication that the genetic background in ER-deficient mice may affect the behavioral phenotype reported here. We have tested 11 ERKO and 7 WT mice derived from the colony at University of Missouri–Columbia, which originated from the mixture of the same population as used in this study and the colony of the University of North Carolina, and was maintained thereafter as a separate colony in which back-crossing heterozygotes to the parental C57BL/6J strain has been performed every 6 months. We have found that all three components of male sexual behaviors, mounts, intromissions, and ejaculations were reduced in ERKO mice compared with WT mice, although both mounts and intromissions were not absent in ERKO mice. These findings show that in multigenically determined traits, such as instinctive behaviors, the genetic background can play a significant role, even when the gene disruption is as physiologically powerful as the transcription factor ER.
Acknowledgments
We are thankful to Dr. Geoffrey Greene for his generous gifts of ER antibody and peptides and to Dr. Nobuo Harada for the aromatase antibody. We are also indebted to Drs. L. M. Kow and C. Pavlides for critical reading of the manuscript and Mr. Todd Washburn at the National Institute of Environmental Health Sciences for his administration and maintenance of the ERKO animal colony. Support for this work was provided by the Harry Frank Guggenheim Foundation (S.O.), University of Missouri–Columbia Molecular Biology Program (D.B.L.), and National Institutes of Health Grant HD-05751 (D.W.P.).
Footnotes
Abbreviations: AR, androgen receptor; ER, estrogen receptor; ERKO, ER-deficient mutant mice; WT, wild type; HZ, heterozygous; IR, immunoreactive; MPOA, medial preoptic area.
References
- 1.Meisel R L, Sachs B D. In: Physiology of Reproduction. 2nd Ed. Knobil E, Neill J D, editors. New York: Raven; 1994. pp. 3–105. [Google Scholar]
- 2.Olsen K L. In: Sexual Differentiation. Arnold G A, Howard M, Ingeborg W L, editors. New York: Plenum; 1992. pp. 1–40. [Google Scholar]
- 3.Simon N G, Whalen R E. Aggressive Behav. 1986;12:255–266. [Google Scholar]
- 4.Brain P F, Haug M, Kamis A B. In: Hormones and Behavior in Higher Vertebrates. Balthazart J, Prove E, Gilles R, editors. Berlin: Springer; 1983. pp. 290–304. [Google Scholar]
- 5.Nyby J, Matochik J A, Barfield R J. Horm Behav. 1992;26:24–45. doi: 10.1016/0018-506x(92)90029-u. [DOI] [PubMed] [Google Scholar]
- 6.Bowden N J, Brain P F. Physiol Behav. 1978;20:543–546. doi: 10.1016/0031-9384(78)90244-5. [DOI] [PubMed] [Google Scholar]
- 7.Clark C R, Nowell N W. Horm Behav. 1979;12:205–210. doi: 10.1016/0018-506x(79)90002-3. [DOI] [PubMed] [Google Scholar]
- 8.Hasan S A, Brain P F, Castano D. Horm Behav. 1988;22:178–185. doi: 10.1016/0018-506x(88)90064-5. [DOI] [PubMed] [Google Scholar]
- 9.Simon N G, Perry M. Physiol Behav. 1988;30:829–833. doi: 10.1016/0091-3057(88)90107-4. [DOI] [PubMed] [Google Scholar]
- 10.Lubahn D B, Moyer J S, Golding T S, Couse J F, Korach K S, Smithies O. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korach K S. Science. 1994;266:1524–1527. doi: 10.1126/science.7985022. [DOI] [PubMed] [Google Scholar]
- 12.Korach K S, Couse J F, Curtis S W, Washburn T F, Lindzey J, Kimbro K S, Eddy E M, Migliaccio S, Snedeker S M, Lubahn D B, Schomberg D W, Smith E P. Rec Prog Horm Res. 1996;51:159–186. [PubMed] [Google Scholar]
- 13.Masur J, Schutz M T, Boerngen R. Dev Psychobiol. 1980;13:107–110. doi: 10.1002/dev.420130202. [DOI] [PubMed] [Google Scholar]
- 14.Nagy Z M, Glaser H D. Psychon Sci. 1970;19:143–145. [Google Scholar]
- 15.DeFries J C. J Hered. 1964;55:289–295. doi: 10.1093/oxfordjournals.jhered.a107353. [DOI] [PubMed] [Google Scholar]
- 16.McCarthy M M, Schlenker E H, Pfaff D W. Endocrinology. 1993;133:433–439. doi: 10.1210/endo.133.2.8344188. [DOI] [PubMed] [Google Scholar]
- 17.Blizard D A, Lippman H R, Chen J J. Physiol Behav. 1975;14:601–608. doi: 10.1016/0031-9384(75)90188-2. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa S, Robbins A, Kumar N, Pfaff D W, Sundaram K, Bardin C W. Horm Behav. 1996;30:74–84. doi: 10.1006/hbeh.1996.0011. [DOI] [PubMed] [Google Scholar]
- 19.Denenberg V H, Gaulin-Kremer E, Gandelman R, Zarrow M X. Anim Behav. 1973;21:590–598. doi: 10.1016/s0003-3472(73)80021-1. [DOI] [PubMed] [Google Scholar]
- 20.Maxson S C, Didier-Erickson A, Ogawa S. Behav Neural Biol. 1989;52:251–259. doi: 10.1016/s0163-1047(89)90369-5. [DOI] [PubMed] [Google Scholar]
- 21.Eddy E M, Washburn T F, Bunch D O, Goulding E H, Gladen B C, Lubahn D B, Korach K S. Endocrinology. 1996;137:4796–4805. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]
- 22.Ogawa, S., Gordan, J., Taylor, J. A., Lubahn, D. B., Korach, K. S. & Pfaff, D. W. (1996) Horm. Behav., in press. [DOI] [PubMed]
- 23.Ogawa S, Taylor J A, Lubahn D B, Korach K S, Pfaff D W. Neuroendocrinology. 1996;64:467–470. doi: 10.1159/000127154. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa S, Makino J. Behav Neural Biol. 1984;40:195–204. doi: 10.1016/s0163-1047(84)90303-0. [DOI] [PubMed] [Google Scholar]
- 25.Blaustein J D. Endocrinology. 1992;131:1336–1342. doi: 10.1210/endo.131.3.1380440. [DOI] [PubMed] [Google Scholar]
- 26.Kuiper G G J M, Enmark E, Peltohuikko M, Nilsson S. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Couse J F, Curtis S W, Washburn T F, Lindzey J, Golding T S, Lubahn D B, Smithies O, Korach K S. Mol Endocrinol. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 28.Breedlove M S, Arnold A P. J Neurosci. 1983;3:417–423. doi: 10.1523/JNEUROSCI.03-02-00417.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]