Abstract
While evidence has accumulated in favor of cAMP-associated genomic involvement in long-term synaptic plasticity, the mechanisms downstream of the activated nucleus that underlie these changes in neuronal function remain mostly unknown. Dendritic spines, the locus of excitatory interaction among central neurons, are prime candidates for long-term synaptic modifications. We now present evidence that links phosphorylation of the cAMP response element binding protein (CREB) to formation of new spines; exposure to estradiol doubles the density of dendritic spines in cultured hippocampal neurons, and concomitantly causes a large increase in phosphorylated CREB and in CREB binding protein. Blockade of cAMP-regulated protein kinase A eliminates estradiol-evoked spine formation, as well as the CREB and CREB binding protein responses. A specific antisense oligonucleotide eliminates the phosphorylated CREB response to estradiol as well as the formation of new dendritic spines. These results indicate that CREB phosphorylation is a necessary step in the process leading to generation of new dendritic spines.
The nuclear mechanisms underlying long-term synaptic plasticity, known to require protein synthesis, are beginning to emerge in a recent series of studies which links plasticity-producing stimuli to the phosphorylation of nuclear cAMP response element binding protein (CREB) (1–3). cAMP-dependent activation of protein kinase A (PKA) has been shown to be critical for the maintenance of the late phase of long-term potentiation (LTP) (1, 3) and extensive synaptic stimulation which yields LTP in cultured neurons leads to calcium dependent phosphorylation of CREB (2). Central to the study of long-term neuronal plasticity are dendritic spines, which are the primary targets of excitatory synaptic inputs and have been intimately associated with long-term morphological modifications seen during LTP and behavioral plasticity (4–7). Changes in spine morphology may affect dendritic integration of synaptic potentials (8). Despite an apparently pivotal role in neuronal plasticity, little is known about the molecular events that regulate formation of dendritic spines.
In an earlier study, we found that estradiol produces a 2-fold increase in dendritic spine density in cultured hippocampal neurons (9), qualitatively similar to its effects in vivo (10). As such, it is a convenient stimulus for the analysis of biological mechanisms regulating dendritic spine formation. We also found that the effect of estradiol is mediated by activation of a serine/threonine kinase. Because estradiol activates cAMP-dependent PKA (11) and estradiol may affect CREB (12), we explored the possible mediation by CREB of the action of estradiol on dendritic spine formation in cultured hippocampal neurons. To this end, we correlated CREB phosphorylation, CREB binding protein (CBP), and spine formation. CBP binds specifically to the PKA-phosphorylated form of CREB, and thus augments the ability of phosphorylated CREB (pCREB) to activate transcription of cAMP responsive genes (13, 14). CBP has been observed to be recruited only in the presence of pCREB (13, 15). Thus, changes in CBP may indicate that CREB is activated to the extent that it triggers a downstream nuclear response.
MATERIALS AND METHODS
Hippocampal Cultures.
Hippocampal cultures were prepared as described (16). Briefly, 19-day-old embryos were taken out of anesthetized Wistar rats. The hippocampus was mechanically disaggregated, dissociated cells (500,000 cells/well) were plated onto 12-mm glass coverslips coated with poly-l-lysine (15 μg/ml). The plating medium was Eagle’s minimal essential medium containing 10% heat-inactivated horse serum, 5% fetal calf serum, 2 mM glutamine, 0.6% glucose, and 15 μg/ml gentamicin. Cells were incubated at 37°C with 5% CO2. The first change of medium, 4–6 days after plating, included 50 μg/ml uridine and 20 μg/ml deoxyuridine to prevent glial cell overgrowth. The cultures were fed thereafter 1–2 times a week, with Eagle’s minimal essential medium and 10% horse serum. Cells were used for experiments when they were 2.5–3 weeks old, when spine density is at a peak (16).
Cell Dosing.
Experimental solutions were prepared in hippocampal growth media on the day of dosing and placed in the incubator to reach the appropriate temperature and pH. Half the media was removed from wells and replaced with experimental media to give the appropriate final desired concentration. Unless otherwise indicated, 17-β-Estradiol (Sigma) was used at 0.1 μg/ml. The PKA antagonist RP-adenosine 3′,5′-cyclic monophosphothioate triethylamine (RP-cAMP[S], H89), and the PKA agonist SP-adenosine 3′,5′-cyclic monophosphothioate triethylamine (SP-cAMP[S]), were obtained from Research Biochemicals (Natick, MA) and used at 10 μM. 2-Aminophosphonovalerate (2-APV, Research Biochemicals) was used at 50 μM from frozen stock solutions, bis(2-aminophenoxy)ethane-N,N,N′,N′-tetra-acetate (BAPTA-AM; Molecular Probes) was dissolved in dimethyl sulfoxide at 10 mM and used at 50 μM. Cells were incubated with drug solutions for up to 24 h and subsequently fixed for immunostaining.
Immunohistochemistry and Imaging.
Cultures were fixed in 4% paraformaldehyde in PBS for 1 h at room temperature. Cultures were then incubated in 5% goat serum with 0.1% saponin for 30 min, and subsequently inverted over 10-μl drops of primary antibody (rabbit polyclonal anti-pCREB or anti-CBP, Upstate Biotechnology, Lake Placid, NY) for 1 h. Coverslips were then rinsed in PBS and incubated with fluorescein isothiocyanate-labeled antibodies. Coverslips were mounted in Vectashield (Vector Laboratories), sealed, and viewed on a confocal laser scanning microscope with a ×100, 1.4 N.A. oil immersion objective. Random fields were imaged and stored for later analysis. Active nuclei of neurons and glia were clearly separable and counted for each treatment group, and the number of active nuclei was calculated as a percent of the total number of nuclei seen. Additionally, the net fluorescence intensity (with background subtracted, and in an arbitrary scale of 256 levels) of the nuclei of all the cells in the imaged fields were measured using NIH image software. This yielded a more quantitative estimate of the treatment effects and allowed a better statistical analysis of the data. The two methods of measurement yielded similar results and were used together to validate the observed effects.
Spine Counts.
Cultures were fixed with 4% paraformaldehyde in PBS for 1 h at room temperature. Medium-sized pyramidal shaped cells were labeled with a microdrop of oil containing a saturated solution of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (DiI) as detailed elsewhere (9, 16, 17). The dye was allowed to diffuse throughout the dendritic arbors for 4–6 h, and the cells were imaged at ×100 with ×2 zoom on the confocal laser scanning microscope. Serial optical sections were taken through secondary dendrites of isolated cells and stored for later analysis. For each cell, 3–4 fields were scanned, and a total of 8–10 cells were sampled in each coverglass. Spines were counted from averaged z-sections for a measured length of dendrite. No corrections were made for hidden spines, but all visible spines (defined as small protrusions, at right angle to the parent dendrite, measuring up to 3–4 μm in length) were counted on clearly focused dendrites. Aspiny, probably GABAergic neurons (see ref. 16) were not included in the analysis.
Antisense Treatments.
The growth medium was replaced in 2- week-old cultures with a N2 medium (18), containing no serum. An antisense oligonucleotide sequence to the CREB gene (5′-TGGTCATCTAGTCACCGGTG-3′, bp 27–46 of GenBank (as in ref. 19) was used. A sense oligonucleotide (having no sequence similarity to known oligonucleotides in the GenBank database) and N2 medium alone served as controls. The oligonucleotides were purified on a NAP-10 sephadex column before use, and added to a final concentration of 5 μM in the N2 medium. They were added again 24 h after the first application. Cultures were fixed at 24–36 h after onset of exposure to the antisense/estradiol for the immunohistochemistry, and at 48–52 h for the spine analysis. These time points were found to be at the peak response for the histochemical and morphological effects, respectively.
Data Analysis.
Experiments were always conducted with treatment and control groups run simultaneously. While we did not adapt a double blind procedure, some of the experiments were conducted independently in the two collaborating institutes and yielded similar observations. Statistical comparisons were made with the Student’s t test.
RESULTS
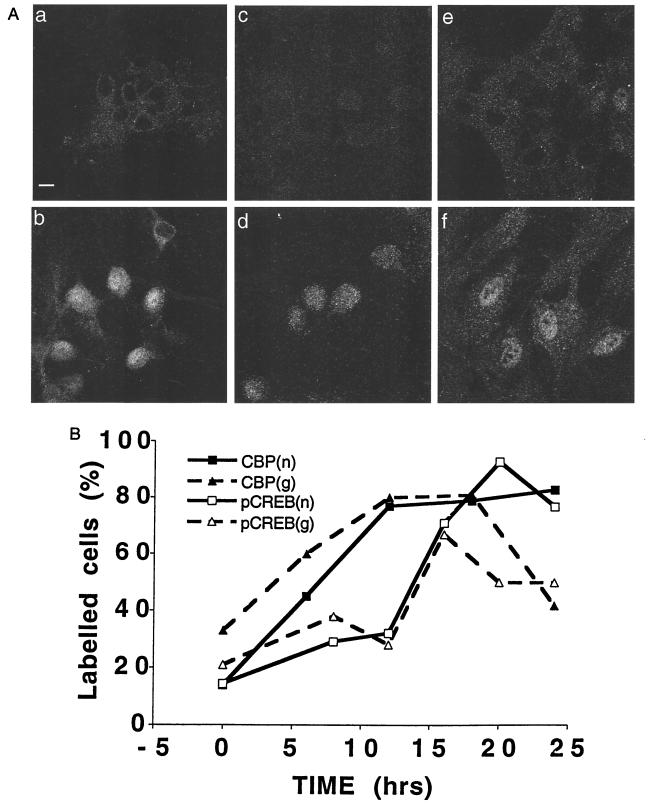
Estradiol produced a slow onset, long lasting increase in immunoreactivity of nuclear pCREB and CBP (Fig. 1). Baseline immunofluorescence of the cells for CBP was about 15 fluorescence units, and only 14% of the cells displayed active nuclei. Fluorescence intensity began increasing at 6 h and reached a peak after 24 h of exposure to estradiol, after which time 83% of the nuclei were active. Similarly, pCREB staining was seen in only 13% of the cells in rest conditions, and the density of cells stained increased to a peak of 93% after 20 h of exposure to estradiol. Interestingly, a higher proportion of glial cells, also present in the culture, were CBP and pCREB positive at rest, and their numbers also increased transiently with exposure to estradiol, returning to rest level faster than the neurons.
Figure 1.
Estradiol causes a rise in pCREB and CBP immunoreactivity in nuclei of cultured hippocampal neurons, in a slow time dependent manner. (A) Cultured neurons stained for CBP and pCREB in control and following estradiol treatment (0.1 μg/ml for 24 h) (a, c, and e) Controls. (b, d, and f) Estradiol-treated. (a and b) CBP staining in neurons. (c and d) pCREB staining in neurons. (e and f) CBP staining in glial cells. (Bar = 10 μm.) (B) Time course for CBP and pCREB changes in both neurons (n) and glia (g) following exposure to 0.1 μg/ml estradiol (abscissa). The total number of neuronal nuclei counted in each group are (from left to right) for CBP: 33, 55, 90, 168, 120; and for pCREB: 149, 71, 45, 84, 118, 62. The fluorescently labeled nuclei are expressed as a percentage of the total nuclei on the ordinate. The time-dependent changes in proportion of labeled nuclei was highly significant for both the CBP and the pCREB groups (χ2 tests, P < 0.001).
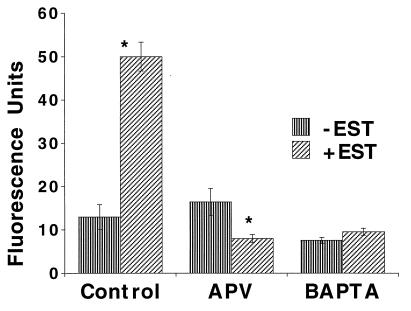
Previous studies have implicated the activation of the N-methyl-d-aspartate (NMDA) receptor in spine forming action of estradiol both in vivo (20) and in our in vitro experiments (9). Whether the NMDA receptor lies upstream or downstream of the CREB activation can be determined by measuring the effect of blocked NMDA receptor on the CREB response. Cultures were exposed to estradiol for 24 h in the presence of 50 μM 2-APV. Estradiol-exposed cells displayed an enhanced CBP fluorescence intensity from 13 ± 2.8 (n = 26 cells) to 50 ± 3.3 (n = 44) fluorescence units, corresponding to an increase from 31.7% to 79.7% active nuclei. This effect was blocked by 2-APV [down from 16.4 ± 3.1 (n = 40) to 8 ± 0.9, (n = 51); Fig. 2], corresponding to 29% total active nuclei. The α-amino-3-hydroxy-5-methylisoxazol-4-propionic acid (AMPA) receptor antagonist 6,7-dinitroquinoxaline-2,3-dione (DNQX) was ineffective (data not shown).
Figure 2.
The CBP response of cells to the presence of estradiol is dependent on activation of NMDA receptors and a rise of intracellular calcium concentration. Estradiol increased fluorescence intensity significantly from control and this effect was completely blocked by 2-APV. BAPTA-AM alone caused a significant reduction in mean fluorescence intensity, and also blocked the response to estradiol. Asterisks (∗) in this and the following figures indicate a significant change from control at P < 0.01.
If NMDA receptor activation is related to CREB phosphorylation by allowing a rise of intracellular calcium concentration ([Ca]i), then reducing [Ca]i should block the CREB response. This can be achieved by the use of membrane permeant calcium buffer BAPTA-AM (2, 21). Indeed, BAPTA-AM, which by itself caused a reduction in mean CBP fluorescence intensity of the measured neurons (down to 7.6 ± 0.7 units, n = 26 cells, 11% of cells were active), also blocked the response to estradiol (9.6 ± 0.8, n = 47 cells, only 6% of cells were active; Fig. 2).
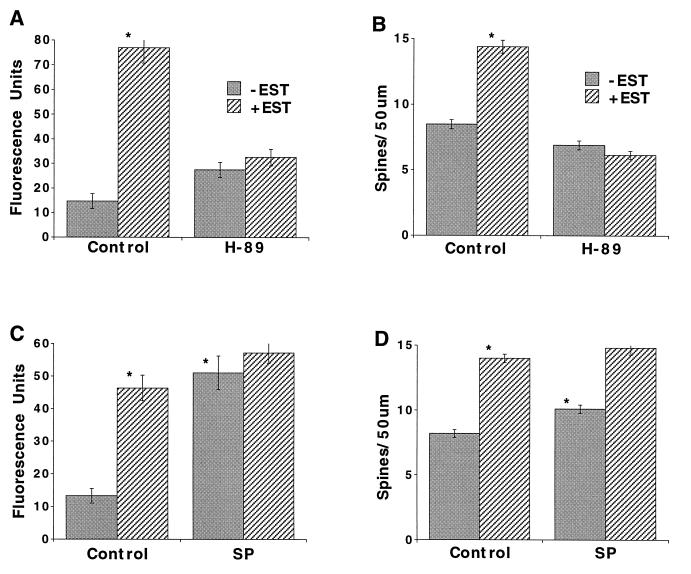
If phosphorylation of CREB is predicated by cAMP-dependent PKA, then antagonists or agonists of PKA should block or induce the activation of CREB, respectively. Estradiol caused a rise in CBP fluorescence [from 14.7 ± 3.1 to 77 ± 6.3 (n = 28 and 27 cells, respectively), corresponding to an increase from 24.6% of active cells to 72.3%], which was blocked by H89, a specific antagonist of PKA [27.5 ± 3.1 and 32.6 ± 3.4 fluorescence units (n = 37 and 17 cells, respectively), 22% and 19.7%]. The specific agonist of PKA, SP-cAMP[S], caused a rise in fluorescence that mimicked the estradiol response {control, 13.3 ± 2.2 fluorescence units (n = 22 cells); estradiol, 46.4 ± 3.9 (n = 44); SP-cAMP[S], 51.1 ± 5.1 (n = 45); SP-cAMP[S] plus estradiol, 57.2 ± 3.1 (n = 51); Fig. 3 A and C}. The corresponding counts of active nuclei were 17%, 81%, 66% and 70%, respectively. The effects of estradiol and SP-cAMP[S] on CBP were not additive.
Figure 3.
cAMP and PKA mediate the CBP response and the spine-forming actions of estradiol. Cultures were exposed to estradiol (0.1 μg/ml) for 24 h (A and C) or 48 h (B and D) in the presence of the PKA antagonist H89 (A and B) or the cAMP analog SP-cAMP[S] (SP) (C and D) and subjected to immunofluorescence for CBP (A and C) or to a morphological analysis of spine density (B and D). Estradiol caused a rise in CBP fluorescence that was blocked by H89, and mimicked by SP-cAMP[S]. Spine density increased by 69% following exposure to estradiol, and this effect was totally blocked by H89. SP-cAMP[S] alone produced a significant (P < 0.001) 23% increase in spine density and did not interact with estradiol.
To verify that activation of PKA is a necessary step in the production of new spines, the effects of H89 on the spine-forming action of estradiol were measured using the membrane dye DiI (16, 17). As seen before, spine density increased following exposure to estradiol by 69% (Fig. 3B) or 71% (Fig. 3D) in two separate experiments. This effect was totally blocked by H89 (Fig. 3B).
Correspondingly, the cAMP analog SP-cAMP[S], which activates PKA and CREB formation, may induce formation of new spines. Indeed, SP-cAMP[S] in itself produced a significant (P < 0.001) albeit smaller (23%) effect than that produced by estradiol in the same experiment (Fig. 3D). This indicates that cAMP is necessary but not sufficient to induce spine formation following exposure to estradiol.
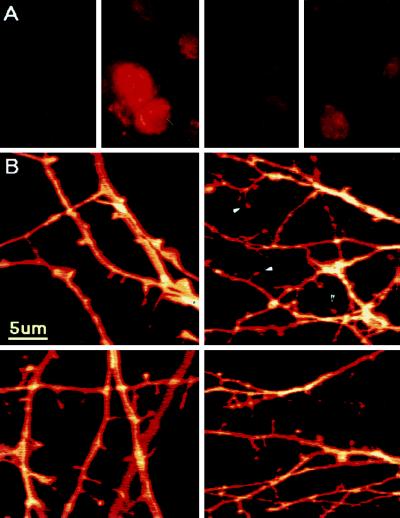
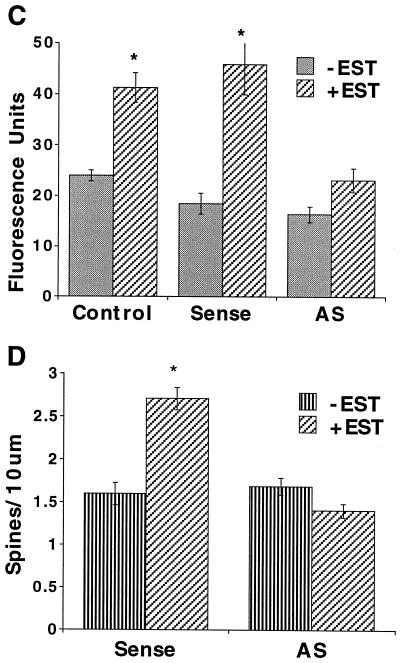
Lastly, we studied the effects of exposing the cultures to a specific antisense oligonucleotide to CREB to determine if the estradiol-induced pCREB and subsequent increase in spine density would be eliminated by a nonpharmacological, supposedly more selective nuclear probe. At the concentration used (5 μM) the antisense oligonucleotide did not affect viability of the cells. Higher (20–50 μM) concentrations, tested in preliminary experiments, were toxic to a large proportion of the cells. Cells treated with antisense for 36 h in the presence of estradiol displayed significantly less pCREB fluorescence response to estradiol in comparison to sense and medium controls (Fig. 4 A and C). Cells treated with antisense for 52 h failed to produce a significant increase in dendritic spine density in response to estradiol when compared with sense controls (Fig. 4 B and D). Spine density, measured in DiI-stained cells, was increased by 69% in the sense control culture after exposure to estradiol, while it was not affected by the hormone in the antisense-treated cells (Fig. 4D).
Figure 4.
CREB antisense oligonucleotide blocks the pCREB response and the spine forming action of estradiol. (A) pCREB immunstaining in cultured neurons treated for 36 h with, from left to right, sense control (S), sense plus estradiol (SE), antisense control (AS), and antisense plus estradiol (ASE). Note the nuclear fluorescence in SE and not in the ASE-treated cells. (B) DiI-stained dendrites and spines of cells treated for 52 h with (from left to right and top to bottom) S, AS, SE, and ASE. Arrowheads indicate mature spines. (C) Antisense (AS) blocked the estradiol-induced CREB fluorescence response, while in the presence of the sense oligonucleotide or the N2 medium control, estradiol produced the typical increase in pCREB fluorescence. (D) Spine density was increased by 69% in the sense control culture after exposure to estradiol, while it was not affected by the hormone in antisense treated cells (AS). The difference in spine density between the groups was highly significant.
DISCUSSION
In previous experiments we have demonstrated that estradiol increases the density of dendritic spines in cultured rat hippocampal neurons. Spines have been suggested as the morphological substrates for long-term changes in synaptic efficacy in that they are the primary sites of excitatory synaptic inputs to the neuron. It has been shown previously that CREB phosphorylation may be associated with synaptic plasticity (1, 2) and with long term potentiation-producing stimuli in CA1 of the hippocampus (3). In the present experiments, we demonstrated that estradiol causes an increase in pCREB in cultured hippocampal neurons, that this increase is correlated with a rise in CBP immunocytochemistry, and that these are necessary steps in the pathway to an increase in dendritic spine density in response to estradiol. Taken together, we propose that activated CREB and production of CBP are necessary steps in the cellular pathways leading to plasticity-related spine formation.
The time course of estradiol effect on pCREB and CBP (20–24 h) is somewhat slow in comparison to other reported immediate early genes. This time course is consistent with the slow time course of spine formation following exposure to estradiol, which peaks 48 h after onset of exposure to the drug (9). This is also consistent with in vivo studies on c-Fos expression in the brain, which occurs anywhere from 12 to 48 h of exposure to estradiol (22). This slow time course also suggests that the effects of estradiol in stimulating CREB activation may be indirect, acting through a series of cellular cascades.
Previous studies have implicated the activation of NMDA receptors in the spine-forming action of estradiol both in vivo and in vitro (9, 20). The effects of estradiol on CBP expression were eliminated in the presence of an NMDA receptor antagonist, 2-APV, while the AMPA receptor antagonist DNQX was ineffective. These results indicate that the effects of estradiol are mediated in part by activation of NMDA receptors, which leads to the CREB response and eventually to spine formation. Estradiol may involve NMDA activation by a rapid membrane effect on NMDA receptors, as intracellular recordings have shown that estradiol increases the duration of excitatory postsynaptic potentials in CA1 neurons (23). This enhancement of NMDA currents may affect intracellular calcium levels. These hypotheses await further experiments.
It has been previously suggested that NMDA receptor activation is related to plasticity via a rise in intracellular calcium concentration leading to activation of CREB (2, 24). One can therefore expect to reduce pCREB in cells where intracellular calcium is reduced to low levels. This was achieved with the use of the calcium buffer BAPTA-AM (2, 21). Indeed, cells exposed to estradiol in presence of membrane permeable BAPTA-AM [in conditions similar to those used to reduce reactivity to stimulation which increases spine formation (21)], did not express a CBP response. This is consistent with CREB and CBP being activated by a rise of intracellular calcium concentration. It is possible that BAPTA-AM may have caused its effect by reducing calcium dependent transmitter release in a manner that is only indirectly related to activated NMDA receptors, thus, a presynaptic locus of action of BAPTA-AM cannot be ruled out at the present time.
CREB is activated by a rise in cAMP (25). If the effects of estradiol are mediated by activation of cAMP-dependent PKA, then its blockade by PKA antagonists should reduce the CREB response to estradiol. Indeed, in the presence of H89, a selective PKA antagonist, the CBP response to estradiol was totally eliminated. To verify that activation of PKA is a necessary step in the production of new spines, the effects of H89 on spine formation in response to estradiol was studied in individual cells stained with the membrane dye, DiI. As seen before, estradiol caused a nearly 80% increase in dendritic spine density in the neurons, while H89, by itself causing a small reduction in spine density, eliminated the response to estradiol.
Correspondingly, cAMP analogs that traverse the membrane and activate PKA should induce CREB activation. Indeed, the cAMP analog SP-cAMP[S] caused a marked rise in CBP production in neurons, saturating the ability of estradiol to cause a further rise in CBP fluorescence. However, activation of CREB by cAMP is necessary but apparently not sufficient to mimic the estradiol-evoked rise in spine density, while SP-cAMP[S] caused a significant rise in spine density, it was not of the magnitude produced by estradiol. This indicates that spine formation evoked by estradiol may require other steps coincident with, or downstream of, CREB activation which are not yet identified.
The present study demonstrates that estradiol may trigger formation of new dendritic spines by activation of a cAMP-regulated CREB phosphorylation. Induction of the CREB response requires activation of NMDA receptors, increased intracellular calcium concentrations and cAMP-activated PKA. These systems together then contribute to the CREB response, which in turn leads to the morphological changes seen with estradiol—i.e., spine formation. The biochemical and cellular routes leading from activated CREB to the morphological change in dendritic spine density are still uncharted.
Acknowledgments
We would like to thank Drs. T. Reese and S. B. Andrews of the Laboratory of Neurobiology, National Institutes of Health, and the Light Imaging Facility, National Institute of Neurological Disorders and Stroke, National Institutes of Health. We also thank Dr. A. Fine for comments on the manuscript. Lastly, thanks to Ms. V. Greenberger for culture preparations, and N. B. Cole for assistance with cell staining. This work was supported in part by a U.S.–Israel Binational Science Foundation grant.
Footnotes
Abbreviations: CREB, cAMP response element binding protein; PKA, protein kinase A; CBP, CREB binding protein; pCREB, phosphorylated CREB; H89 (RP-cAMP[S]), RP-adenosine 3′,5′-cyclic monophosphothioate triethylamine; SP-cAMP[S], SP-adenosine 3′,5′-cyclic monophosphothioate triethylamine; 2-APV, 2-aminophosphonovalerate; BAPTA-AM, bis(2-aminophenoxy)ethane-N,N,N′,N′-tetra-acetate; DiI, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine; NMDA, N-methyl-d-aspartate; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropidnic acid; DNQX,6,7-dinitroquinoxaline-2,3-dione.
References
- 1.Nguyen P V, Kandel E R. J Neurosci. 1996;16:3189–3198. doi: 10.1523/JNEUROSCI.16-10-03189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deisseroth K, Bito H, Tsien R. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- 3.Impey S, Mark M, Villacres E C, Poser S, Chavkin C, Storm D R. Neuron. 1996;16:973–982. doi: 10.1016/s0896-6273(00)80120-8. [DOI] [PubMed] [Google Scholar]
- 4.Chang F, Greenough W T. Brain Res. 1984;309:35–36. doi: 10.1016/0006-8993(84)91008-4. [DOI] [PubMed] [Google Scholar]
- 5.Fifkova E, Van Harreveld A. J Neurocytol. 1977;6:211–230. doi: 10.1007/BF01261506. [DOI] [PubMed] [Google Scholar]
- 6.Geinisman Y, Morrell F, de Toledo-Morrell L. Proc Natl Acad Sci USA. 1988;85:3260–3264. doi: 10.1073/pnas.85.9.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee A K S, Schottler F, Oliver M, Lynch G. J Neurophysiol. 1980;44:247–258. doi: 10.1152/jn.1980.44.2.247. [DOI] [PubMed] [Google Scholar]
- 8.Harris K M, Kater S B. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- 9.Murphy D D, Segal M. J Neurosci. 1996;16:4059–4068. doi: 10.1523/JNEUROSCI.16-13-04059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woolley C S, Gould E, Frankfurt M, McEwen B S. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spaulding S W. Endocr Rev. 1993;14:632–650. doi: 10.1210/edrv-14-5-632. [DOI] [PubMed] [Google Scholar]
- 12.Gu G, Rojo A A, Zee M C, Yu J, Simerly R B. J Neurosci. 1996;16:3035–3044. doi: 10.1523/JNEUROSCI.16-09-03035.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwok R P, Lundblad J R, Chrivia J C, Richards J P, Bachinger H P, Brennan R G, Roberts S G, Green M R, Goodman R H. Nature (London) 1994;370:177. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- 14.Chrivia J C, Kwok R P, Lamb N, Hagiwara M, Montminy M R, Goodman R H. Nature (London) 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 15.Kwok R P, Laurance M E, Lundblad J R, Goldman P S, Shih H, Connor L M, Marriott S J, Goodman R H. Nature (London) 1996;380:642–646. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- 16.Papa M, Bundman M C, Greenberger V, Segal M. J Neurosci. 1995;15:1–11. doi: 10.1523/JNEUROSCI.15-01-00001.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosokawa T, Rusakov D A, Bliss T V, Fine A. J Neurosci. 1995;15:5560–5573. doi: 10.1523/JNEUROSCI.15-08-05560.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banker G, Goslin K. Culturing Nerve Cells. Cambridge MA: MIT Press; 1991. pp. 189–191. [Google Scholar]
- 19.Konradi C, Heckers S. Neuroscience. 1995;65:1051–1061. doi: 10.1016/0306-4522(94)00546-h. [DOI] [PubMed] [Google Scholar]
- 20.Woolley C, McEwen B. J Neurosci. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papa M, Segal M. Neuroscience. 1996;71:1005–1011. doi: 10.1016/0306-4522(95)00490-4. [DOI] [PubMed] [Google Scholar]
- 22.Insel T R. Endocrinology. 1990;126:1849–1853. doi: 10.1210/endo-126-4-1849. [DOI] [PubMed] [Google Scholar]
- 23.Wong M, Moss R L. J Neurosci. 1992;12:3217–3225. doi: 10.1523/JNEUROSCI.12-08-03217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bading H, Segal M M, Suche N J, Dudek H, Lipton S A, Greenberg M E. Neuroscience. 1995;64:653–664. doi: 10.1016/0306-4522(94)00462-e. [DOI] [PubMed] [Google Scholar]
- 25.Hagiwara M, Brindle P, Hartounian A, Armstrong R, Rivier J, Vale W, Tsien R Y, Montminy R. Mol Cell Biol. 1994;13:4852–4859. doi: 10.1128/mcb.13.8.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]