Abstract
Current theories on the encoding and storage of information in the brain commonly suppose that a short-term memory is converted into a lasting one; thus, it becomes consolidated over time. Within a finite period after training, such a short-term memory can be reinforced by behavioral and humoral stimuli. We have found that, long-term potentiation (LTP), a likely candidate for a memory-encoding mechanism at the cellular level, displays similar features. LTP in the dentate gyrus of freely moving rats was reinforced after its induction by appetitive and aversive stimuli. The efficacy of these stimuli terminates about 1 h after tetanization, which may reflect the time constants of the mechanisms underlying the consolidation that takes place. The reinforcement by appetitive and aversive stimulation was blocked by the β-adrenergic antagonist propranolol, implicating norepinephrine in the underlying cellular processes.
Keywords: motivation, water deprivation, footshock, norepinephrine
It has been extensively documented that memory of a recent experience is fragile—i.e., memory traces become consolidated over time (1–3) and this consolidation is manipulable by a variety of treatments (4–6). Within a finite time window after a learning event such treatments markedly affect retention, thus indicating a permissive influence over the neuronal processes which underlie the consolidation of a memory trace. Available evidence indicates that retention can be altered both by the administration of endogenous substances such as hormones or transmitters after training and by treatments that change the functioning of systems in which such compounds are involved (4–7).
Long-term potentiation (LTP), an activity-dependent, long-lasting increase in synaptic strength observed at monosynaptic junctions in the mammalian forebrain, has many features that make it a plausible cellular mechanism for many types of learning and memory (8–11). The demonstration of both correlations and interactions between hippocampal LTP and hippocampus-dependent learning has provided further support for this idea (7, 8, 11). If LTP-like processes are involved in learning they may be anticipated to display some of the characteristics that have been attributed to the consolidation of memory traces such as: (i) the consolidation is subject to both retrograde impairment and reinforcement; (ii) modulating stimuli may affect the encoding of information only within a distinct time after training; and (iii) consolidation can be affected by a variety of different stimuli.
Here we provide evidence that LTP in the freely moving rat may be reinforced after its induction by appetitive and aversive behavioral stimulation, features well known for the retrograde modulation of the consolidation of memory traces. The susceptibility of LTP to these treatments was strongly time-dependent. Since the reinforcement by appetitive and aversive stimulation could be selectively prevented by application of the β-adrenergic antagonist propranolol, β-adrenergic pathways appear to be involved in the underlying molecular processes.
MATERIALS AND METHODS
Subjects and Surgery.
Adult male Wistar rats (8–10 weeks old; 230–320 g) were prepared while they were under Nembutal anesthesia (40 mg/kg, i.p.) as previously described (12). A monopolar recording electrode (coordinates AP −2.8, L 1.8 from bregma) and a bipolar stimulation electrode (coordinates AP −6.9, L 4.1) were implanted stereotaxically into the granule cell layer of the dentate gyrus and into the perforant path, respectively, in the right hemisphere. The electrodes were adjusted such that the population spike (PS) amplitude (PS amplitude: difference between the first positive and negative deflections) was maximal. All animals were given 6–8 days to recover from surgery, during which period they had free access to food and water.
Recording.
At the start of the experiment the rat was placed into an experimental box (40 × 40 × 40 cm) and the electrodes were connected by a flexible cable. The recorded responses were fed through a differential amplifier (Inhvers+, Science Products, Hochheim, Germany), filtered by band-pass filters at 0.1 Hz and 5 kHz, transformed by an analog/digital interface (CED 1401, Cambridge Electronic Design, Cambridge, U.K.) and stored on-line on a personal computer. The stimulus intensity which evoked 40% of the maximum of PS amplitude (assessed by input/output curves) was used as standard for tetanization and the following recordings. The slope of the initial ongoing part of the recorded PS was taken as a measure of the field excitatory postsynaptic potential (fEPSP) slope. During baseline recording, five single responses were averaged every 5 min (10-s interpulse interval). Once a stable baseline was attained, average responses were recorded every 15 min up to 8 h after tetanization. Control measurements were carried out 24 h after the tetanization. An “unsaturated” LTP was induced by three bursts of 15 pulses, 200 Hz, 0.2-ms pulse width each stimulus, interburst interval 10 s (weak tetanus), resulting in a potentiation that decayed within 4–7 h to pretetanus values. For clarity, the data after tetanization are given as 1-h values.
Appetitive Stimulation.
In the water-deprivation experiments the animals were water deprived 23 h/day on four consecutive days. The morning after each deprivation period the animals were transferred to the experimental chamber for 4 h to familiarize the animals with the test conditions. Thirty to 60 min afterwards, water was provided for 1 h according to a randomized schedule.
On the day of the experiment the animals received the water either before tetanization (30 min or 5 min), immediately after the tetanus, or 30 min and 60 min after the tetanus. After access to water the animals started immediately to drink for a few minutes, followed by a short grooming bout. Subsequently, the behavior was indistinguishable from controls that were subjected to the same time schedule but had free access to water both in their home cage and in the test chamber.
Because we have found that the repetition of the weak tetanization with a 1-week interval did not modify the time course of the second potentiation (data not shown), in these experiments each animal served as its own control—i.e., in the first week the animals were subjected to the weak tetanization protocol under control conditions (water ad libitum). One week later they received the same protocol after water deprivation.
To test the effect of “spontaneous drinking,” i.e., drinking without preceding water deprivation, undeprived animals were placed into the same test chamber as used by the deprived animals. As soon as they started to drink, a weak tetanus was delivered. Statistical comparisons were performed with the Wilcoxon matched pairs signed rank test.
Aversive Stimulation.
All experiments were carried out with different groups (independent samples)—i.e., each animal was used only once, either in the control or in one of the experimental groups. On three consecutive days before the experiment the animals were allowed to habituate to the experimental chamber each morning for about 2 h. The experimental chamber was the same as used for appetitive stimulation. It was equipped with a metal-grid floor allowing the application of the electrical footshock. The controls were treated as the footshock group except that they did not receive a footshock. The footshock, which consisted of three stimuli of 800 μA (duration 2 s with a 10-s interstimulus interval), was delivered according to the same experimental schedule as described above. Group differences were evaluated by the U test.
Temperature Recordings.
For temperature measurements a small-bed thermistor (111–802EAJ-B01, 0.5 mm diameter, Fenwal Electronics, Milford, MA) was implanted stereotaxically into the granule cell layer of the dentate gyrus under Nembutal anesthesia. The leads were connected to a socket fixed to the skull with dental acrylic for chronic recordings. Rats were allowed at least 1 week to recover. Before implantation the calibration curve of each thermistor was determined in a water bath by using a precision thermometer. During the experiment the thermistor resistance was measured every 10 s and digitized by an analog/digital converter, and the temperature was calculated and stored on-line on a personal computer. The values were normalized as temperature change from baseline (Δt).
Drug Application.
For drug application a brass cannula was chronically inserted in the right lateral ventricle. All experiments were carried out with different groups—i.e., independent samples. Rats were allowed 8–10 days to recover from surgery. Water and footshock, respectively, were given 30 min after tetanization. Five minutes after tetanus a recording was taken and immediately thereafter propranolol (2 μg in 5 μl) or NaCl was infused. Group differences were evaluated by the U test.
RESULTS
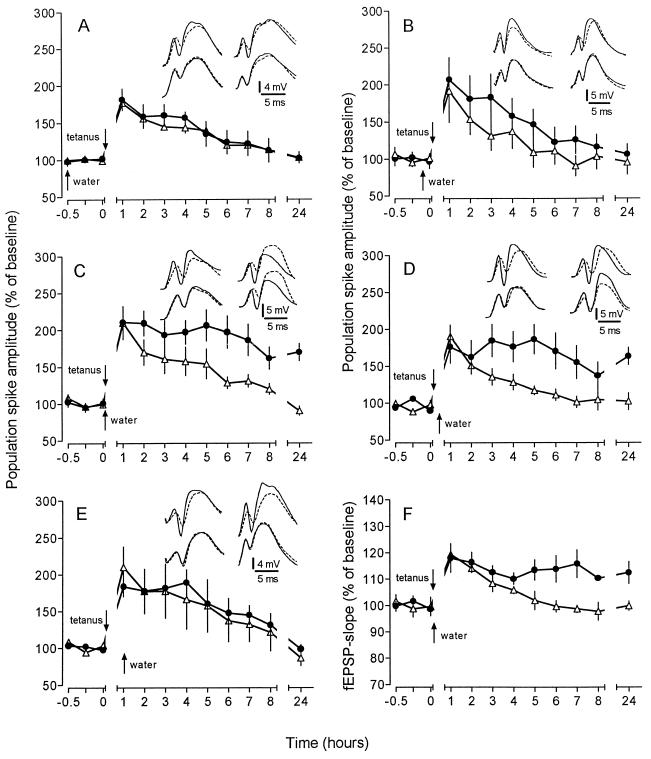
In the first set of experiments, we examined whether an appetitive stimulation, consisting of drinking after water deprivation (deprivation-induced drinking, DID), could affect such an “unsaturated” LTP. Tetanization immediately after the start of DID resulted in a marked protraction of LTP, with potentiated values even after 24 h (Fig. 1C and F). This effect represents a specific reinforcement of LTP, as shown by the lack of any effect on baseline recordings without tetanization (see Fig. 3A). The reinforcement was still obvious when the water was given 30 min after tetanization but faded thereafter, since application of water 60 min after tetanus was unable to produce a significant prolongation of LTP (Fig. 1 D and E). The reinforcement caused by DID, if occurring within 30 min after tetanization, did not result in overt changes of field responses. This was shown by separate experiments employing the recording of single responses with a 30-s interstimulus interval during the critical time period from 15 min before until 45 min after LTP induction (data not shown). In contrast to the time-dependent post-tetanic efficacy of DID, water access 30 min and 5 min before tetanization did not affect a subsequent potentiation (Fig. 1 A and B).
Figure 1.
Effect of “deprivation-induced drinking” (DID) on an “unsaturated” LTP induced by weak tetanic stimulation. Comparison of animals under nondeprived conditions (controls, ▵) and after water deprivation 1 week later (•). Water was provided at different times relative to tetanization. Data are plotted as average change from baseline response (mean ± SEM). Insets display representative analogue traces of a control animal (Left Insets) and a DID subject (Right Insets). For comparison, baseline traces (broken line) are superimposed on recordings taken 15 min (upper traces) and 24 h (lower traces) after weak tetanization. (A) Water access 30 min before tetanization did not affect LTP. Potentiation was sustained for 4 h significantly above baseline (P < 0.05; n = 7). (B) Application of water 5 min prior to tetanization had no influence on the subsequent potentiation (n = 6). (C) DID at the same time as the weak tetanic stimulus markedly reinforced the “unsaturated” LTP, resulting in a potentiation that lasted for more than 24 h (170% ± 11% at 24 h; P < 0.01; n = 7). (D) DID 30 min after tetanization was still effective in protracting an “unsaturated” LTP. The potentiation persisted for at least 24 h (163% ± 12%; P < 0.05; n = 7). (E) DID 1 h after tetanization was unable to reinforce LTP. Potentiation of controls and experimental group showed no difference (n = 6). (F) The potentiation of the slope of the fEPSP was prolonged in a similar way to the potentiation of the PS amplitude (112% ± 4% at 24 h; P < 0.02; n = 7).
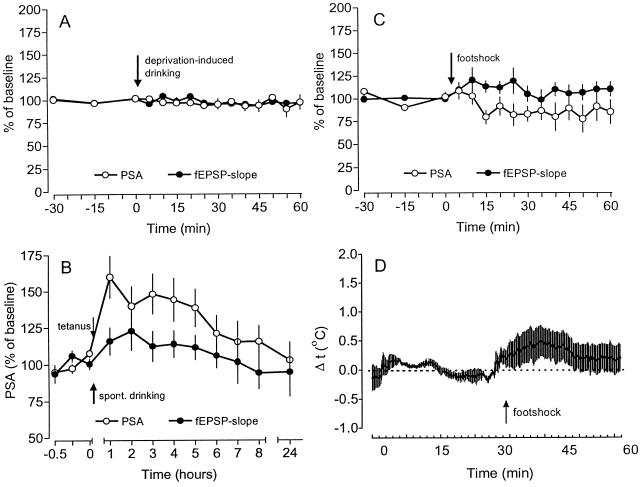
Figure 3.
(A) The water access and the DID had no effect on baseline recordings of PS amplitude (PSA) and fEPSP slope (n = 5). (B) A tetanization given after undeprived animals had started to drink (spontaneous drinking) did not lead to a reinforcement of the potentiation of PSA and fEPSP slope (n = 5). (C) A weak intermittent footshock resulted in slight changes of PSA and fEPSP slope, that were, however, relatively short lasting and not statistically significant (n = 5). (D) The delivery of footshock resulted only in a slight short-term increase of the intrahippocampal temperature of about 0.4°C (n = 5). Data are plotted as average change from baseline (mean ± SEM).
Water deprivation in itself—i.e., without drinking—was unable to reinforce LTP. If the tetanus was delivered to thirsty animals that either received water 1 h post-tetanus (Fig. 1E) or were deprived of access to water during the whole experiment (data not shown), tetanization resulted in an LTP which did not differ from the potentiation obtained in undeprived controls. Vice versa, drinking without previous deprivation was also ineffective. As shown in Fig. 3B, a tetanization given after undeprived animals had started to drink (spontaneous drinking) resulted only in a decremental potentiation which did not differ from controls.
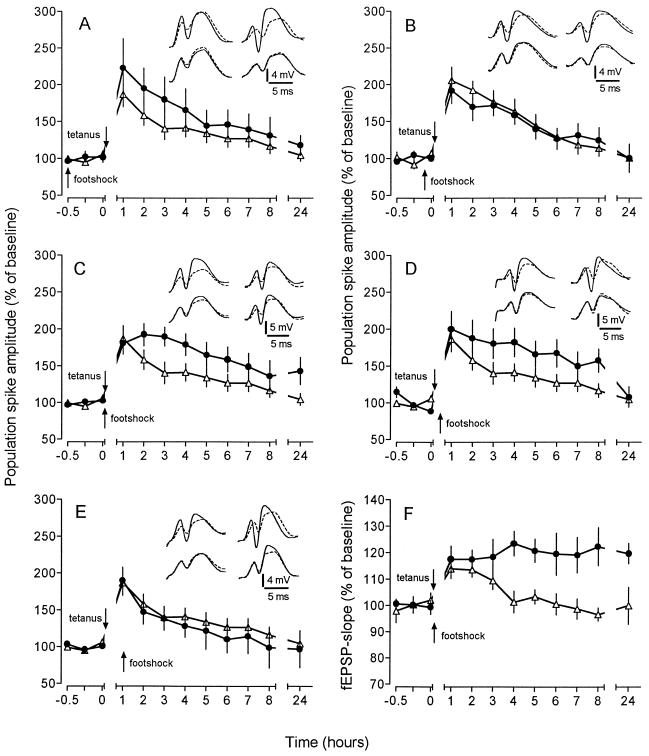
In the second series of experiments we addressed the question of whether the observed reinforcement was specific to a post-tetanic appetitive stimulation, or whether even an aversive stimulation could produce similar effects. To test this, a footshock was applied as an aversive stimulus to naive animals. Since weak and strong aversive stimulations were found to have opposite effects on memory (4, 6) and a strong intermittent tailshock was reported to produce a depression of LTP (13, 14), we used a mild footshock, administered at the same times as the water in the previous experiments. If the footshock was applied immediately after the tetanus, the maintenance of LTP was markedly improved, as seen with the appetitive stimulation (Fig. 2 C and F). An identical footshock delivered 30 min post-tetanus produced only a temporary enhancement of potentiation which was no longer detectable 24 h after tetanus (Fig. 2D). Virtually no effect could be observed if the footshock was applied either before (30 or 5 min) or 60 min after tetanization (Fig. 2 A, B, and E).
Figure 2.
Effect of a mild footshock on an “unsaturated” LTP induced by weak tetanic stimulation. The experimental groups (•) received the footshock according to the same schedule as used for the examination of the effects of water deprivation. Insets depict representative analogue traces obtained from an animal from the control group (Left Insets) and footshock group (Right Insets). For comparison, baseline traces (broken line) are superimposed on recordings collected 15 min (upper traces) and 24 h (lower traces) after weak tetanization. (A) Footshock delivered 30 min before weak tetanization did not affect an “unsaturated” LTP. Potentiation in both groups declined to baseline after 4–5 h (n = 7). (B) Application of footshock 5 min prior to the tetanus also had no effect on potentiation (n = 8). (C) When the footshock was applied immediately after tetanization the potentiation was significantly improved, persisting for at least 24 h (143% ± 18%; P < 0.02; n = 7). (D) Administration of the footshock 30 min after tetanic stimulation produced only a slight reinforcement of LTP that decayed after 8 h (157% ± 16%; P < 0.02; n = 7). Twenty-four hours after tetanization the difference between controls and the “footshock” group had disappeared (108% ± 14%, P > 0.05). (E) No prolongation could be observed when the footshock was delivered 1 h after tetanization (n = 6). (F) The potentiation of the fEPSP slope was affected in a way similar to that of the population spike amplitude. The concomitant administration of footshock and tetanization protracted the potentiation up to 24 h (120% ± 4%; P < 0.05; n = 7).
The administration of the weak footshock alone during baseline recording produced a delayed increase of the fEPSP slope and a slight decline of the PS amplitude (Fig. 3C). Since the animals displayed an increased locomotor activity during and shortly after the delivery of the footshock, these behavioral changes might have induced a slight increase in brain temperature (15), which in turn could have caused the observed changes in the hippocampal recordings. However, intrahippocampal temperature measurements before and after delivery of the footshock revealed only a slight short-term increase of the temperature (Fig. 3D), ruling out changes of brain temperature as being responsible for the prolongation of LTP.
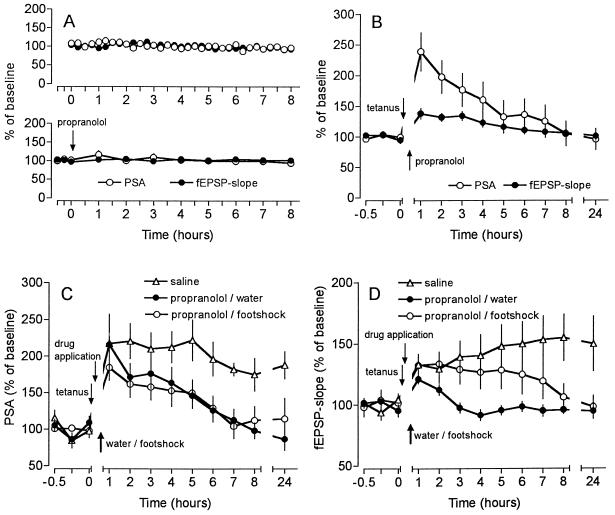
Next we addressed which neurotransmitter may be involved in this type of behavioral reinforcement of LTP. Since the application of norepinephrine was found to reinforce memory (6, 7, 16, 17) and to facilitate the induction and maintenance of LTP (18) via activation of β-adrenergic receptors (19, 20), we applied the β-adrenergic antagonist propranolol intracerebroventricularly (i.c.v.) 5 min after induction of LTP (i.e., 25 min before water access to deprived animals and application of the footshock, respectively). This treatment abolished the reinforcement of LTP (Fig. 4C and D). The application of propranolol to animals that did not receive water or footshock was without effect (Fig. 4B). Therefore, β-adrenergic mechanisms seem to be involved in this type of reinforcement of LTP.
Figure 4.
Effect of an intracerebroventrical infusion of the β-adrenergic antagonist propranolol (2 μg in 5 μl) on baseline recordings and on weak and reinforced LTP. (A Upper) The quality of the preparation allowed stable long-term recordings of PS amplitude (PSA) and fEPSP slope (n = 8). (A Lower) The infusion of propranolol did not affect the baseline recordings (n = 7). (B) Propranolol, given 5 min after tetanization (n = 7) did not influence an “unsaturated” LTP in comparison to saline controls (n = 6). (C and D) Application of the β-adrenergic antagonist propranolol 5 min post-tetanically (25 min before water access (n = 6) or footshock (n = 7) prevented the reinforcement of LTP of PSA and fEPSP slope, respectively. Data were plotted as average change from baseline response (mean ± SEM).
DISCUSSION
Since the first descriptions of LTP-like phenomena in mammals (21) considerable efforts have been centered on whether LTP or LTP-like mechanisms do participate in the neural mechanisms of learning and memory. Resolution of this issue would have important implications because mechanisms already identified as underlying LTP may also be relevant for learning. In this study we demonstrate that LTP in the intact hippocampus—i.e., in the awake, freely moving rat—may be strengthened after its induction by behavioral reinforcers, features well known for the retrograde modulation of the consolidation of memory traces (1, 4, 6).
As the data show, drinking after water deprivation (DID) represents a very effective reinforcer of a weak LTP. The susceptibility of LTP to such a behavioral reinforcement is restricted to a finite time window which appears to be predominantly post-tetanic because water access 30 min or 5 min before tetanization did not yield any effect. Since the effectiveness of the reinforcement terminated about 1 h after tetanization, it can be reckoned that the extension of the critical time window for reinforcement is in the range of 30–60 min.
It is generally accepted that the withdrawal of water (and/or of food) generates an increased motivation, which leads to a promotion of learning in a variety of tasks (22, 23). Recently, it was described that water deprivation resulted in both a significantly greater potentiation of fEPSP slope and a significantly larger decrease in PS peak latency (24). This finding suggests that, in water-deprived animals, the changed motivational state may initiate an enhancement of potentiation. However, in our experiments, high motivation induced by water deprivation in itself was unable to reinforce LTP. If the tetanus was given to animals that that were highly motivated (thirsty), but did not receive access to water during the critical time window, no reinforcement could be observed. Since tetanization of undeprived animals during spontaneous drinking (i.e., normal drinking) also failed to induce reinforcement, our data strongly suggest that both high motivation and drinking during a finite time window are required to trigger reinforcement.
Although the reinforcement induced by footshock was not as efficient as that induced by water access, the properties of reinforcement were very similar—i.e., the critical time window was post-tetanically situated and the efficiency of reinforcement was higher, the closer the tetanus and the footshock were paired. Therefore, in agreement with previous findings on learning paradigms (1, 2, 4, 6), the time gradient for a retrograde modulation of LTP in the intact hippocampus seems to vary with the type and the strength of the stimulus. However, since only one level of footshock was tested, the lower reinforcing efficacy of the footshock may not always be the case. The similarity of the effects gained with such different types of behavioral stimulation suggests that both stimuli trigger the reinforcement by more general mechanisms [such as the generation of a certain level of arousal (5)] rather than by pathways specific to each of the stimuli used.
Among the neurotransmitters and signal transduction pathways that could be suggested to be involved in the reinforcement of LTP (4, 6, 7, 16, 25–27), we considered norepinephrine (NE) as the most prominent candidate. NE may be released directly into the hippocampus from ascending terminals of the locus ceruleus upon arousal and stress, which in turn may induce a specific long-lasting potentiation (LLP) of the evoked potentials per se, or a facilitation of the induction and maintenance of LTP (18, 19, 28–30). The NE-induced LLP is confined to the medial perforant path–granule cell synapse (31, 32) and is contingent upon activation of β-adrenergic receptors (19, 31, 32). NE has been repeatedly shown to be involved in the reinforcement of memory by a variety of behavioral and emotional stimuli (4, 6, 16, 17, 20). Since the β-adrenergic antagonist propranolol was capable of blocking the reinforcement by DID and by footshock in our experiments, β-adrenergic mechanisms appear to be involved. Although norepinephrine is likely to play a crucial role in mediating the effect, the learning studies imply that other transmitters such as dopamine, acetylcholine, and opioids may also participate (4, 5, 7, 33).
Evaluated together, the features of the reinforcement of a weak LTP by appetitive and aversive stimuli which we have described resemble the characteristics reported for the post-training reinforcement of memory. Since LTP in the dentate gyrus is likely to represent one element in a complex sequence of events which lead to the formation of a particular memory, our results indicate how a particular synaptic portion of a memory trace which was only weakly generated by the initial cue may be consolidated by an associative behavioral activation during a critical time window. The ability to associate the direct activation of a synaptic input (weak tetanus) and a behavioral reinforcement at the cellular level would seem to be a key requirement for behavioral associative memory.
Acknowledgments
We thank T. V. P. Bliss and R. G. M. Morris for helpful comments on the manuscript. The technical help of K. Krautwald, U. Stolze, R. Brown, and T. Wagner is gratefully acknowledged.
Footnotes
Abbreviations: LTP, long-term potentiation; PS, population spike; fEPSP, field excitatory postsynaptic potential; DID, deprivation-induced drinking.
References
- 1.McGaugh J L. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- 2.Squire L R, Knowlon B, Musen G. Annu Rev Psychol. 1993;44:453–495. doi: 10.1146/annurev.ps.44.020193.002321. [DOI] [PubMed] [Google Scholar]
- 3.Rosenzweig M R. Annu Rev Psychol. 1996;47:1–32. doi: 10.1146/annurev.psych.47.1.1. [DOI] [PubMed] [Google Scholar]
- 4.McGaugh J L. Annual Rev Neurosci. 1989;12:255–287. doi: 10.1146/annurev.ne.12.030189.001351. [DOI] [PubMed] [Google Scholar]
- 5.Flood J F, Rosenzweig M R, Jarvik M E. Science. 1978;199:324–326. doi: 10.1126/science.619461. [DOI] [PubMed] [Google Scholar]
- 6.Gold P E. In: Brain and Memory: Modulation and Mediation of Neuroplasticity. McGaugh J L, Weinberger N W, Lynch G, editors. New York: Oxford Univ. Press; 1995. pp. 41–74. [Google Scholar]
- 7.Izquierdo I, Medina J H. Neurobiol Learning Memory. 1995;63:19–32. doi: 10.1006/nlme.1995.1002. [DOI] [PubMed] [Google Scholar]
- 8.Morris R G M, Anderson E, Lynch G S, Baudry M. Nature (London) 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 9.Bliss T V P, Collingridge G L. Nature (London) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 10.Nicoll R A, Wyllie D J A, Watanabe T, Perkel D J. In: Cellular and Molecular Mechanisms Underlying Higher Neural Functions. Selverston A I, Ascher P, editors. Chichester, United Kingdom: Wiley; 1994. pp. 189–202. [Google Scholar]
- 11.Martinez J L, Derrick B E. Annu Rev Psychol. 1996;47:173–203. doi: 10.1146/annurev.psych.47.1.173. [DOI] [PubMed] [Google Scholar]
- 12.Seidenbecher T, Balschun D, Reymann K G. Physiol Behav. 1995;57:1001–1004. doi: 10.1016/0031-9384(94)00352-6. [DOI] [PubMed] [Google Scholar]
- 13.Foy M R, Stanton M E, Levine S, Thompson R F. Behav Neural Biol. 1987;48:138–149. doi: 10.1016/s0163-1047(87)90664-9. [DOI] [PubMed] [Google Scholar]
- 14.Shors T J, Seib T B, Levine S, Thompson R F. Science. 1989;244:224–226. doi: 10.1126/science.2704997. [DOI] [PubMed] [Google Scholar]
- 15.Moser E, Mathiesen I, Andersen P. Science. 1993;259:1324–1326. doi: 10.1126/science.8446900. [DOI] [PubMed] [Google Scholar]
- 16.McGaugh J L, Liang K C, Bennett C, Sternberg D B. In: Neurobiology of Learning and Memory. Lynch G, McGaugh J L, Weinberger N M, editors. New York: Guilford; 1984. pp. 313–332. [Google Scholar]
- 17.Wilson D A, Pham T-C, Sullivan R M. Behav Neurosci. 1994;108:1053–1058. doi: 10.1037//0735-7044.108.6.1053. [DOI] [PubMed] [Google Scholar]
- 18.Hopkins W F, Johnston D. Science. 1984;226:350–351. doi: 10.1126/science.6091272. [DOI] [PubMed] [Google Scholar]
- 19.Stanton P, Sarvey J M. Brain Res. 1985;361:276–283. doi: 10.1016/0006-8993(85)91299-5. [DOI] [PubMed] [Google Scholar]
- 20.Cahill L, Prins B, Weber M, McGaugh J L. Nature (London) 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- 21.Bliss T V P, Lomo T. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bindra D. Psychol Rev. 1974;81:199–213. doi: 10.1037/h0036330. [DOI] [PubMed] [Google Scholar]
- 23.Berry S D, Swain R A. Behav Neurosci. 1989;103:71–76. doi: 10.1037//0735-7044.103.1.71. [DOI] [PubMed] [Google Scholar]
- 24.Maren S, DeCola P, Swain R A, Fanselow M S, Thompson R F. Behav Neurosci. 1994;108:44–56. doi: 10.1037//0735-7044.108.1.44. [DOI] [PubMed] [Google Scholar]
- 25.Reymann K G, Brödemann R, Kase H, Matthies H. Brain Res. 1988;461:388–392. doi: 10.1016/0006-8993(88)90274-0. [DOI] [PubMed] [Google Scholar]
- 26.Malenka R C, Nicoll R A. Semin Neurosci. 1990;2:335–343. [Google Scholar]
- 27.Reymann, K. G. (1993) Funct. Neurol.8, Suppl. 5, 7–32.
- 28.Loy R, Koziell D A, Lindsey J D, Moore R Y. J Comp Neurol. 1980;189:699–710. doi: 10.1002/cne.901890406. [DOI] [PubMed] [Google Scholar]
- 29.Bliss T V P, Goddard G V, Riives M. J Physiol. 1983;334:475–491. doi: 10.1113/jphysiol.1983.sp014507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neuman R S, Harley C W. Brain Res. 1983;273:162–165. doi: 10.1016/0006-8993(83)91106-x. [DOI] [PubMed] [Google Scholar]
- 31.Dahl D, Sarvey J M. Proc Natl Acad Sci USA. 1989;86:4776–4780. doi: 10.1073/pnas.86.12.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pelletier M R, Kirkby D, Jones S J, Corcoran M E. Hippocampus. 1994;4:181–188. doi: 10.1002/hipo.450040208. [DOI] [PubMed] [Google Scholar]
- 33.White N W, Packard M G, Seamans J. Behav Neural Biol. 1993;59:230–241. doi: 10.1016/0163-1047(93)90998-w. [DOI] [PubMed] [Google Scholar]